Transcriptome Reveals the Regulation of Exogenous Auxin Inducing Rooting of Non-Rooting Callus of Tea Cuttings
Abstract
1. Introduction
2. Results
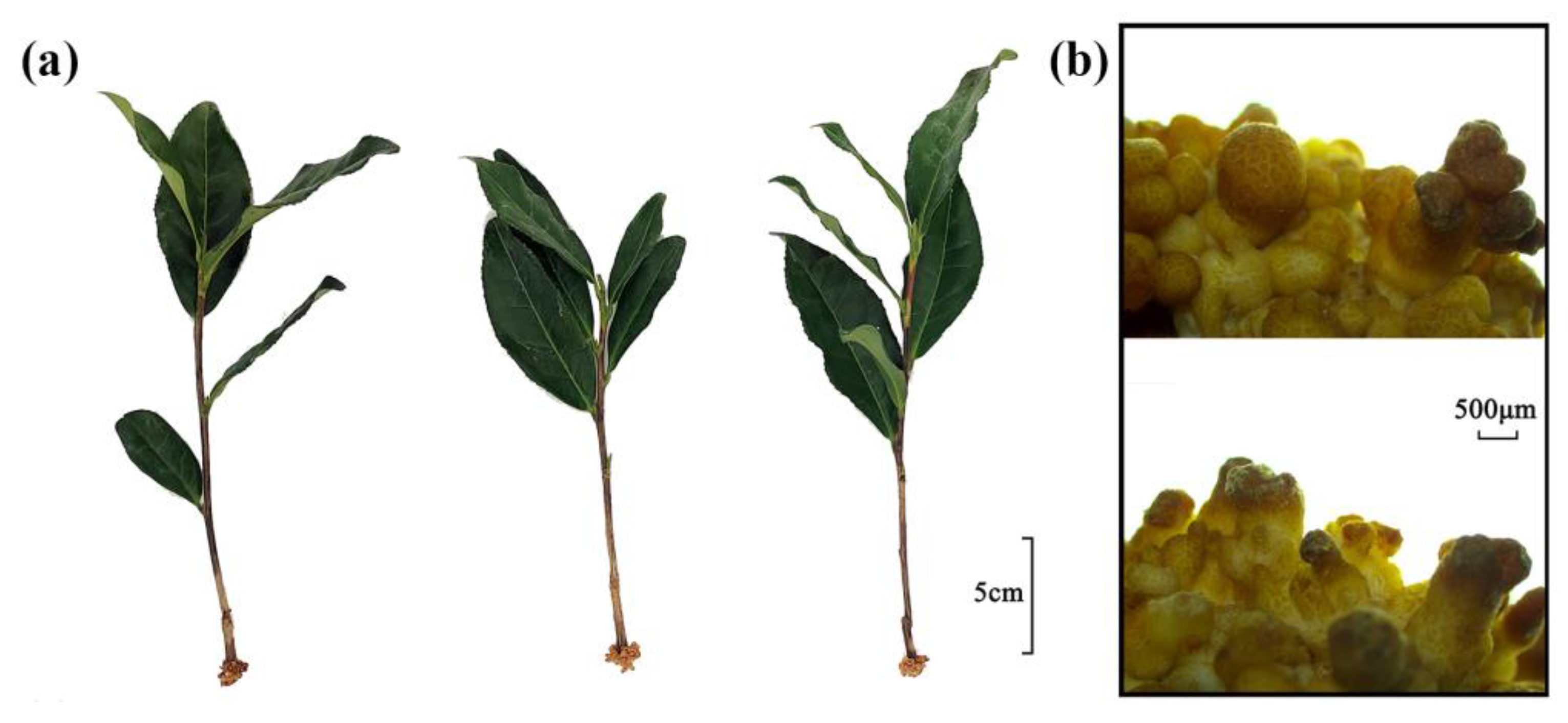
2.1. Phenotypic Changes in the Roots of Cuttings Treated with Exogenous Auxin
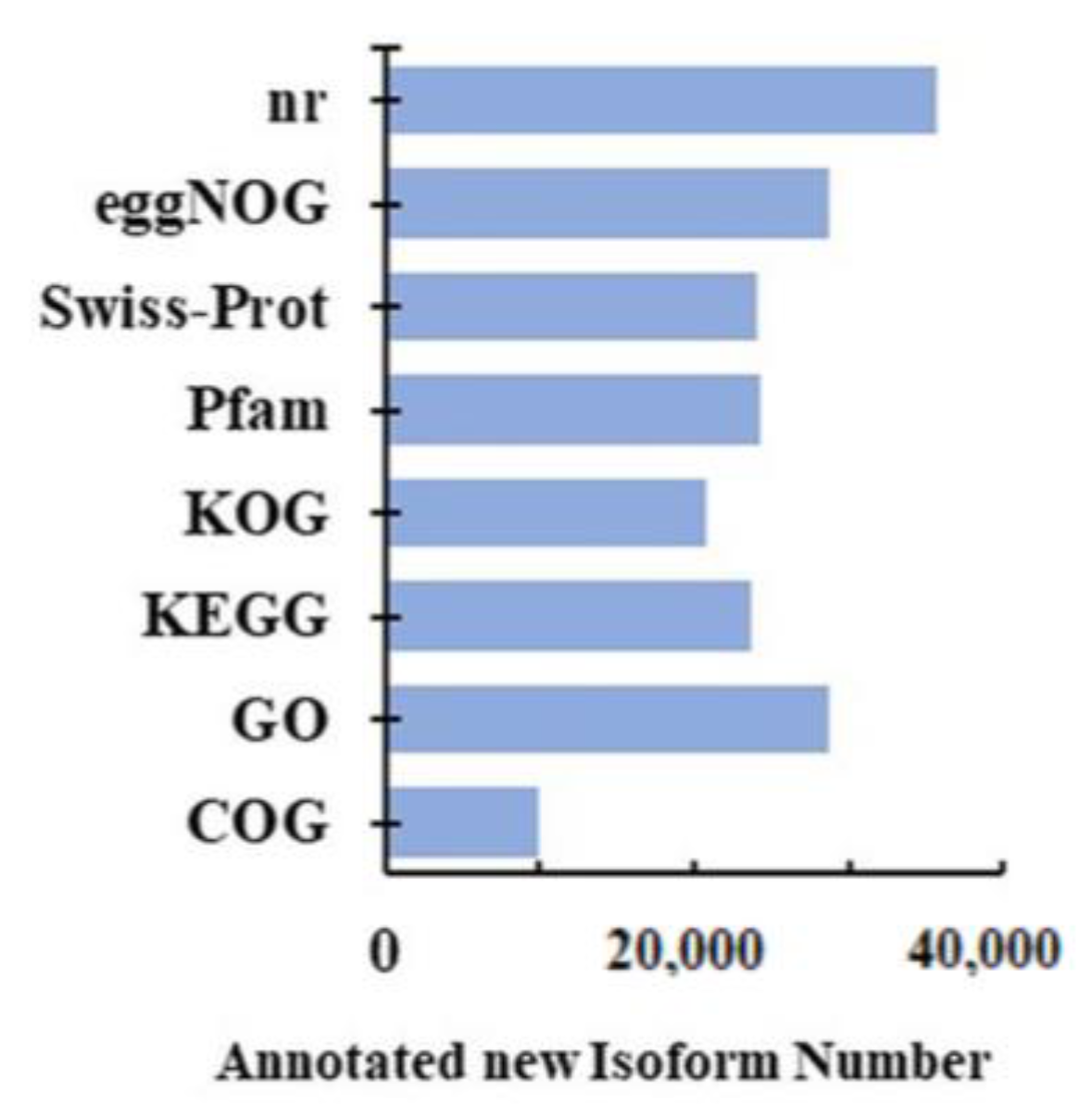
2.2. Full-Length RNA-seq and Functional Annotation
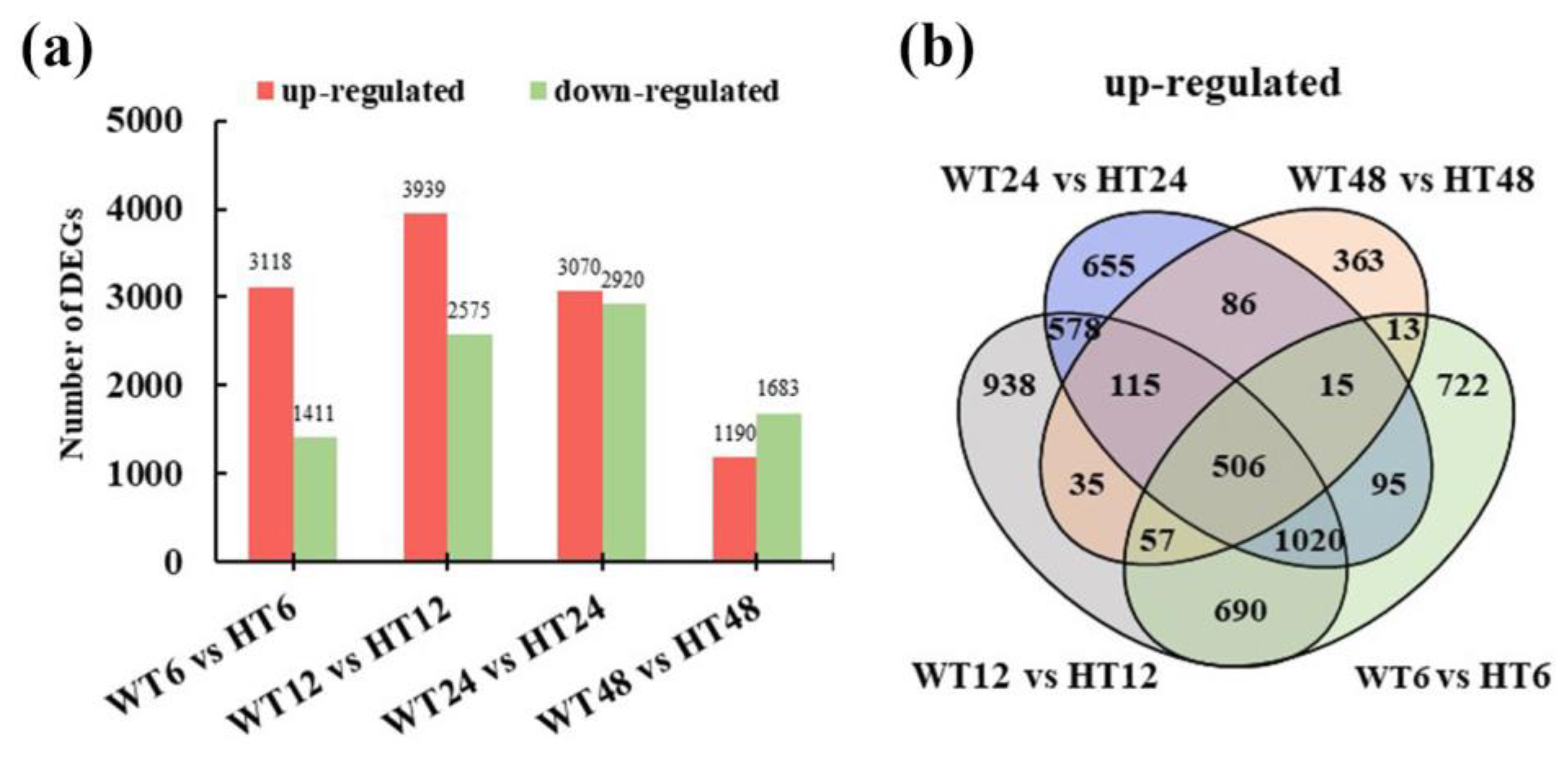
2.3. Comparative Analysis of DEGs
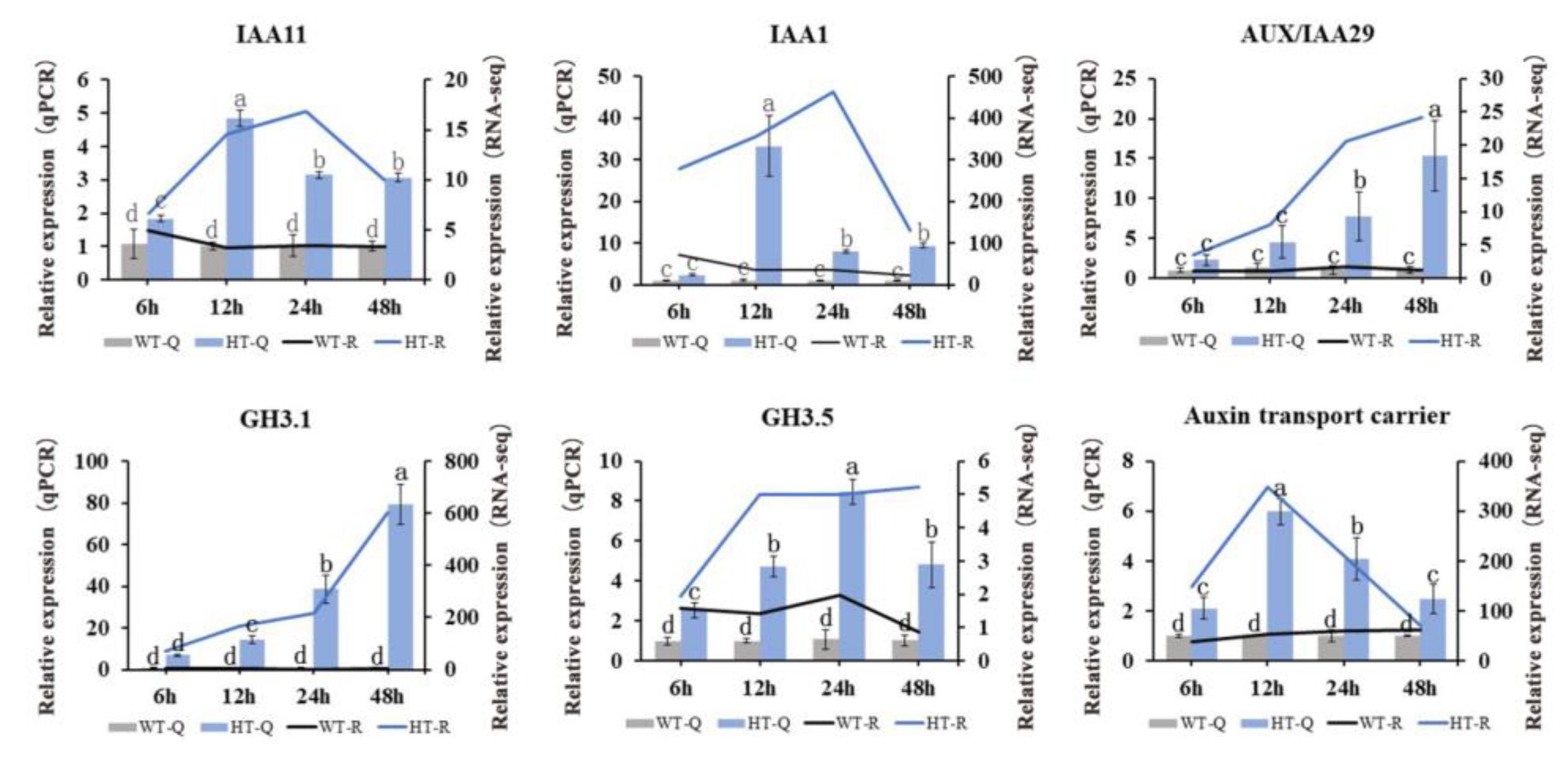
2.4. Identification of Auxin-Related DEGs
2.5. Auxin-Responsive LOB-Domain TFs and SCL Family Genes
2.6. DEGs of TF Families That Affected by Auxin Treatment
2.7. qRT-PCR Verification of Gene Expression
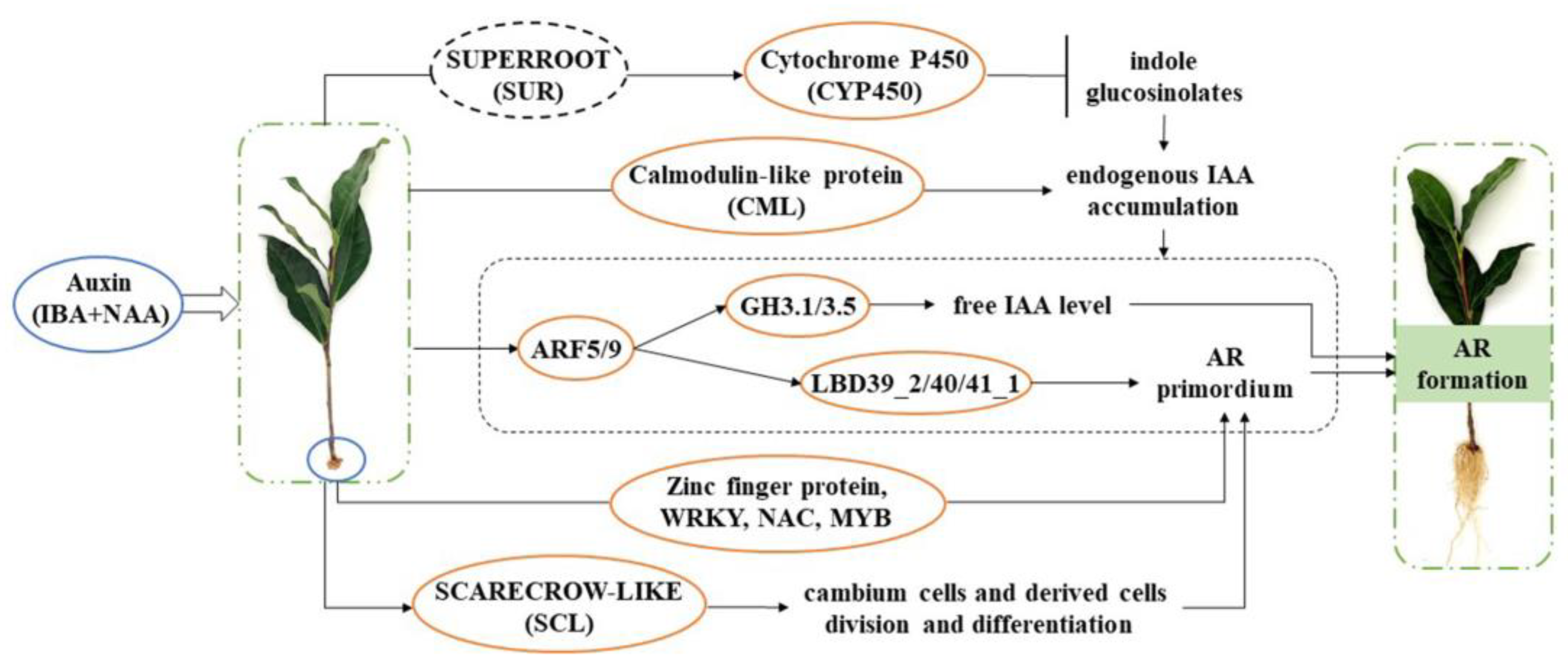
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample
4.2. cDNA Library Construction, ONT RNA-Seq, Quality Control, and Function Annotation
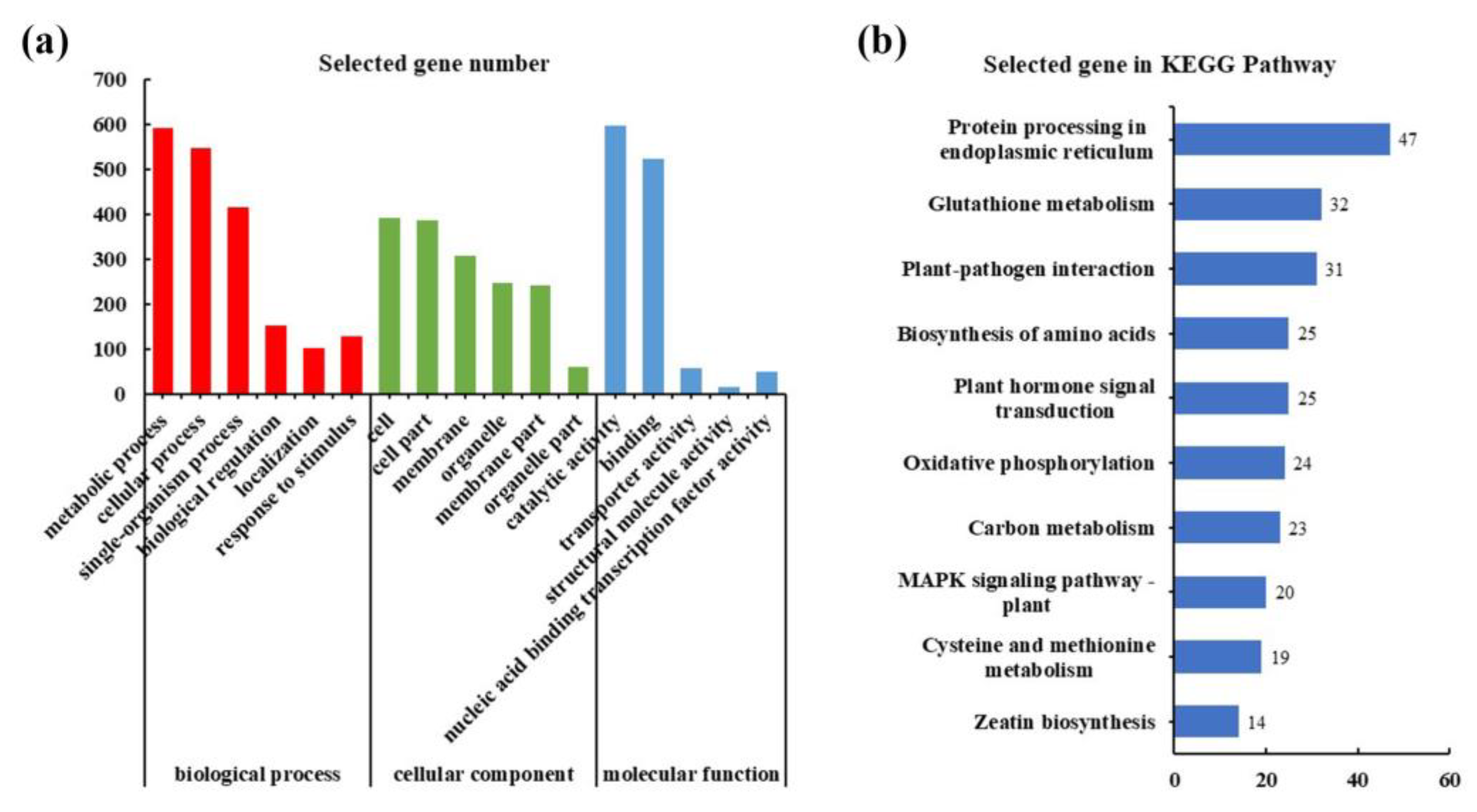
4.3. GO and KEGG Pathway Enrichment Analyses
4.4. Reverse Transcription of cDNA and Gene Expression Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akula, A.; Becker, D.; Bateson, M. High-yielding repetitive somatic embryogenesis and plant recovery in a selected tea clone, ‘TRI-2025’, by temporary immersion. Plant Cell Rep. 2000, 19, 1140–1145. [Google Scholar] [CrossRef]
- Mondal, T.K.; Bhattacharya, A.; Laxmikumaran, M.; Singh Ahuja, P. Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tissue Organ Cult. 2004, 76, 195–254. [Google Scholar] [CrossRef]
- Taryono, S.W. Adventitious Root Characteristics of Some Assamica Tea Clones (Camellia sinensis L. Kuntz). Ilmu Pertan. (Agric. Sci.) 2014, 17, 37–45. [Google Scholar]
- Gao, Y.; Zhao, M.; Wu, X.-H.; Li, D.; Borthakur, D.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L. Analysis of differentially expressed genes in tissues of Camellia sinensis during dedifferentiation and root redifferentiation. Sci. Rep. 2019, 9, 2935. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Hirao, T.; Mishima, K.; Ohira, M.; Hiraoka, Y.; Takahashi, M.; Watanabe, A. Transcriptome dynamics of rooting zone and aboveground parts of cuttings during adventitious root formation in Cryptomeria japonica D. Don. BMC Plant Biol. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Z.A.; Zhan, C.; Liu, M.; Xia, W.; Wang, N. Comprehensive analysis of dynamic gene expression and investigation of the roles of hydrogen peroxide during adventitious rooting in poplar. BMC Plant Biol. 2019, 19, 99. [Google Scholar] [CrossRef]
- Swarup, R.; Parry, G.; Graham, N.; Allen, T.; Bennett, M. Auxin cross-talk: Integration of signalling pathways to control plant development. In Auxin Molecular Biology; Springer: Dordrecht, The Netherlands, 2002; pp. 411–426. [Google Scholar] [CrossRef]
- Raya-González, J.; Oropeza-Aburto, A.; López-Bucio, J.S.; Guevara-García, Á.A.; De Veylder, L.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR18 influences Arabidopsis root architecture, represses auxin signaling and is a critical factor for cell viability in root meristems. Plant J. 2018, 96, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, L.-Y.; Wu, L.-Y.; Zhang, C.-C.; Li, H.-L.; Tan, L.-Q.; Cao, H.-L.; Cheng, H. Transcriptome analysis of indole-3-butyric acid-induced adventitious root formation in nodal cuttings of Camellia sinensis (L.). PLoS ONE 2014, 9, e107201. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zou, M.-H.; Feng, B.-H.; Huang, X.; Zhang, Z.; Sun, G.-M. Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol. Biochem. 2012, 55, 33–42. [Google Scholar] [CrossRef]
- Sorin, C.; Negroni, L.; Balliau, T.; Corti, H.; Jacquemot, M.-P.; Davanture, M.; Sandberg, G.; Zivy, M.; Bellini, C. Proteomic analysis of different mutant genotypes of Arabidopsis led to the identification of 11 proteins correlating with adventitious root development. Plant Physiol. 2006, 140, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Chaiwanon, J.; Wang, Z.-Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr. Biol. 2015, 25, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Peat, T.S.; Böttcher, C.; Newman, J.; Lucent, D.; Cowieson, N.; Davies, C. Crystal structure of an indole-3-acetic acid amido synthetase from grapevine involved in auxin homeostasis. Plant Cell 2012, 24, 4525–4538. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Zimmermann, R. Conserved and diverse mechanisms in root development. Curr. Opin. Plant Biol. 2008, 11, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, T.; Benfey, P.N. Signals that regulate stem cell activity during plant development. Curr. Opin. Genet. Dev. 2005, 15, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Hu, X.; Du, Y.; Zhang, G.; Huang, H.; Scheres, B.; Xu, L. Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development 2017, 144, 3126–3133. [Google Scholar] [CrossRef]
- Wang, D.; Ma, X.; Hao, Z.; Long, X.; Shi, J.; Chen, J. Overexpression of Liriodenron WOX5 in Arabidopsis Leads to Ectopic Flower Formation and Altered Root Morphology. Int. J. Mol. Sci. 2023, 24, 906. [Google Scholar] [CrossRef]
- Wang, S.; Sun, G.; Luo, Y.; Qian, W.; Fan, K.; Ding, Z.; Hu, J. Role of IAA and Primary Metabolites in Two Rounds of Adventitious Root Formation in Softwood Cuttings of Camellia sinensis (L.). Agronomy 2022, 12, 2486. [Google Scholar] [CrossRef]
- Cui, H.; Jiang, X.; Zhang, T.; Wang, Z.; Long, R.; Yang, Q.; Kang, J. Progress in the plant CYP450family. J. Chin. Grassl. 2020, 42, 173–180. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W. Molecular bases for the regulation of adventitious root generation in plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, U.C.; DiFazio, S.P.; Brunner, A.M.; Tuskan, G.A. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Poutrain, P.; Guirimand, G.; Glévarec, G.; Courdavault, V.; Pichon, O. Molecular characterization of an Aux/IAA of Catharanthus roseus. J. Plant Growth Regul. 2011, 30, 235–241. [Google Scholar] [CrossRef]
- Gan, D.; Zhuang, D.; Ding, F.; Yu, Z.; Zhao, Y. Identification and expression analysis of primary auxin-responsive Aux/IAA gene family in cucumber (Cucumis sativus). J. Genet. 2013, 92, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Han, S.; Lee, S.; Nam, H.; Lim, H.; Lee, G.; Cho, H.S.; Dang, T.V.T.; Choi, S.; Lee, M.M.; et al. CPR5-mediated nucleo-cytoplasmic localization of IAA12 and IAA19 controls lateral root development during abiotic stress. Proc. Natl. Acad. Sci. USA 2023, 120, e2209781120. [Google Scholar] [CrossRef]
- Mandal, D.; Datta, S.; Raveendar, G.; Mondal, P.K.; Chaudhuri, R.N. RAV1 mediates cytokinin signaling for regulating primary root growth in Arabidopsis. Plant J. 2023, 113, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; Djordjevic, M.A.; Imin, N. Global gene expression analysis of in vitro root formation in Medicago truncatula. Funct. Plant Biol. 2010, 37, 1117–1131. [Google Scholar] [CrossRef]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef]
- Moison, M.; Pacheco, J.M.; Lucero, L.; Fonouni-Farde, C.; Rodríguez-Melo, J.; Mansilla, N.; Christ, A.; Bazin, J.; Benhamed, M.; Ibañez, F. The lncRNA APOLO interacts with the transcription factor WRKY42 to trigger root hair cell expansion in response to cold. Mol. Plant 2021, 14, 937–948. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, J.; Chen, L.; Zhang, G.; Huang, H.; Zhang, Y.; Xu, L. Auxin-independent NAC pathway acts in response to explant-specific wounding and promotes root tip emergence during de novo root organogenesis in Arabidopsis. Plant Physiol. 2016, 170, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Păcurar, D.I.; Perrone, I.; Jobert, F. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef]
- Tao, G.-Y.; Xie, Y.-H.; Li, W.-F.; Li, K.-P.; Sun, C.; Wang, H.-M.; Sun, X.-M. LkARF7 and LkARF19 overexpression promote adventitious root formation in a heterologous poplar model by positively regulating LkBBM1. Commun. Biol. 2023, 6, 372. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, C.; Sharma, N.; Khurana, P. Genome-wide identification of Aux/IAA and ARF gene families in bread wheat (Triticum aestivum L.). Protoplasma 2023, 260, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bao, Y.; Wang, B.; Liu, L.; Chen, J.; Dai, L.; Peng, D. Identification and expression of Aux/IAA, ARF, and LBD family transcription factors in Boehmeria nivea. Biol. Plant. 2016, 60, 244–250. [Google Scholar] [CrossRef]
- Fan, M.; Xu, C.; Xu, K.; Hu, Y. LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012, 22, 1169–1180. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, C.; Pandey, S.K.; Park, Y.; Kim, M.-J.; Kim, J. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 2019, 19, 46. [Google Scholar] [CrossRef]
- An, H.; Zhang, J.; Xu, F.; Jiang, S.; Zhang, X. Transcriptomic profiling and discovery of key genes involved in adventitious root formation from green cuttings of highbush blueberry (Vaccinium corymbosum L.). BMC Plant Biol. 2020, 20, 182. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; He, W.; Su, H.; Wang, Y.; Hong, G.; Xu, P. Structural and functional insights into the LBD family involved in abiotic stress and flavonoid synthases in Camellia sinensis. Sci. Rep. 2019, 9, 15651. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Naur, P.; Halkier, B.A. Arabidopsis mutants in the C–S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004, 37, 770–777. [Google Scholar] [CrossRef]
- Xiao, Z.a.; Zhang, Y.; Liu, M.; Zhan, C.; Yang, X.; Nvsvrot, T.; Yan, Z.; Wang, N. Coexpression analysis of a large-scale transcriptome identified a calmodulin-like protein regulating the development of adventitious roots in poplar. Tree Physiol. 2020, 40, 1405–1419. [Google Scholar] [CrossRef]
- Levesque, M.P.; Vernoux, T.; Busch, W.; Cui, H.; Wang, J.Y.; Blilou, I.; Hassan, H.; Nakajima, K.; Matsumoto, N.; Lohmann, J.U. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006, 4, e143. [Google Scholar] [CrossRef]
- Xu, J.; Hofhuis, H.; Heidstra, R.; Sauer, M.; Friml, J.i.; Scheres, B. A molecular framework for plant regeneration. Science 2006, 311, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Solé, A.; Sánchez, C.; Vielba, J.M.; Valladares, S.; Abarca, D.; Díaz-Sala, C. Characterization and expression of a Pinus radiata putative ortholog to the Arabidopsis SHORT-ROOT gene. Tree Physiol. 2008, 28, 1629–1639. [Google Scholar] [CrossRef]
- Xuan, L.; Xu, M.; Chen, C.; Yang, C.; Xu, L.a.; Huang, M. Identification and characterization of three PeSHRs and one PeSCR involved in adventitious root development of Populus. Plant Cell Tissue Organ Cult. 2014, 117, 253–264. [Google Scholar] [CrossRef]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Vielba, J.M.; Díaz-Sala, C.; Ferro, E.; Rico, S.; Lamprecht, M.; Abarca, D.; Ballester, A.; Sánchez, C. CsSCL1 is differentially regulated upon maturation in chestnut microshoots and is specifically expressed in rooting-competent cells. Tree Physiol. 2011, 31, 1152–1160. [Google Scholar] [CrossRef]
- Stevens, M.E.; Woeste, K.E.; Pijut, P.M. Localized gene expression changes during adventitious root formation in black walnut (Juglans nigra L.). Tree Physiol. 2018, 38, 877–894. [Google Scholar] [CrossRef]
- Ding, S.; Song, D.; Fang, F.; Zhang, Y.; Sun, X.; Wang, H. The propagation technique of non-woven fabric in tea tree. Anhui Agric. Bull. 2018, 24, 33–34+74. [Google Scholar]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.M.; Ball, C.; Blake, J.; Botstein, D.D.; Sherlock, G. Gene Ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.A.; Li, G.; Abdullah, M.; Wang, H.; Han, W.; Zhang, Y.; Wang, X.; Zhao, Y.; Feng, X.; Jin, Q.; et al. Genome-wide investigation and comparative analysis of MATE gene family in Rosaceae species and their regulatory role in abiotic stress responses in Chinese pear (Pyrus bretschneideri). Physiol. Plant. 2021, 173, 1163–1178. [Google Scholar] [CrossRef]
- Manzoor, M.A.; Sabir, I.A.; Shah, I.H.; Wang, H.; Yu, Z.; Rasool, F.; Mazhar, M.Z.; Younas, S.; Abdullah, M.; Cai, Y. Comprehensive Comparative Analysis of the GATA Transcription Factors in Four Rosaceae Species and Phytohormonal Response in Chinese Pear (Pyrus bretschneideri) Fruit. Int. J. Mol. Sci. 2021, 22, 12492. [Google Scholar] [CrossRef]
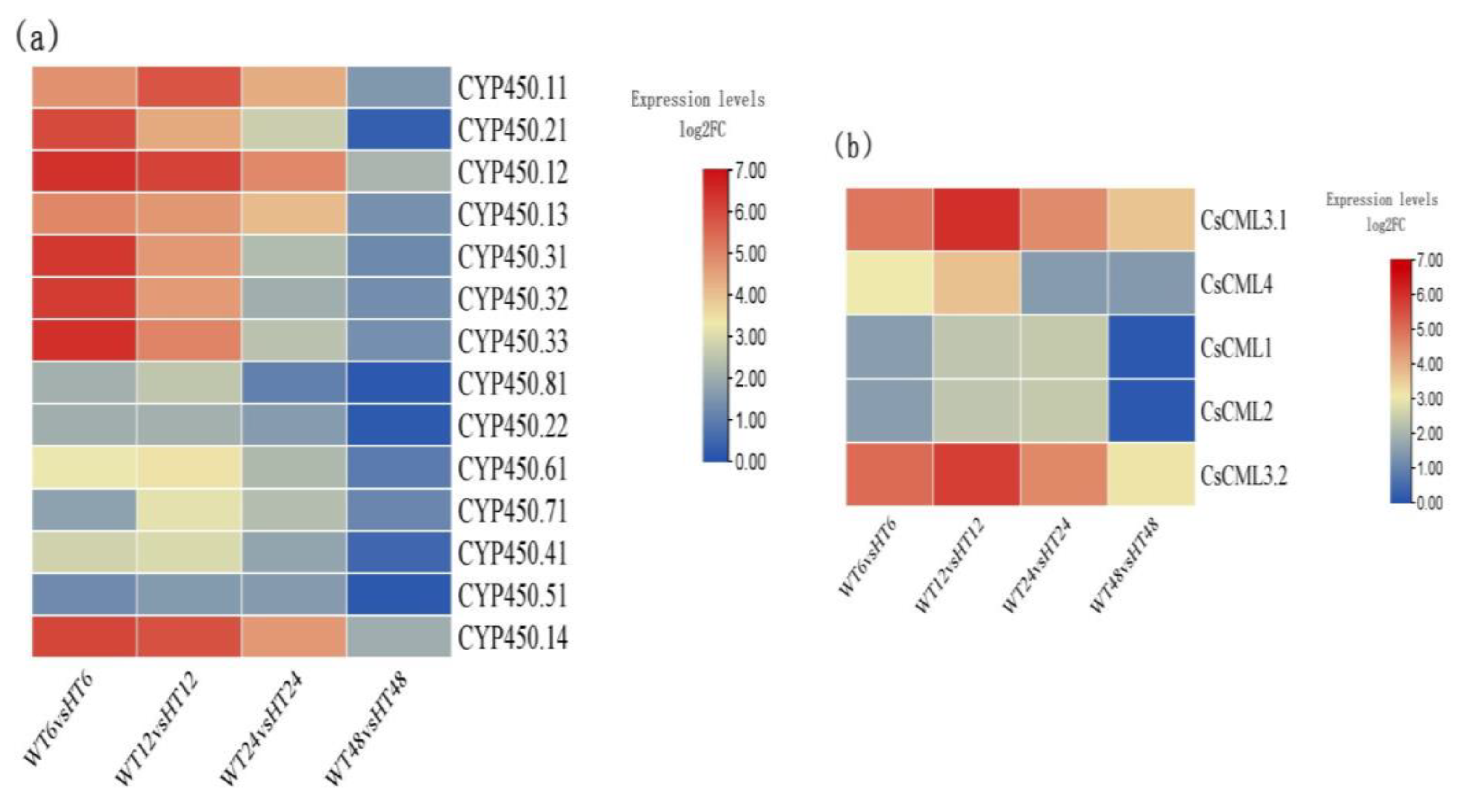
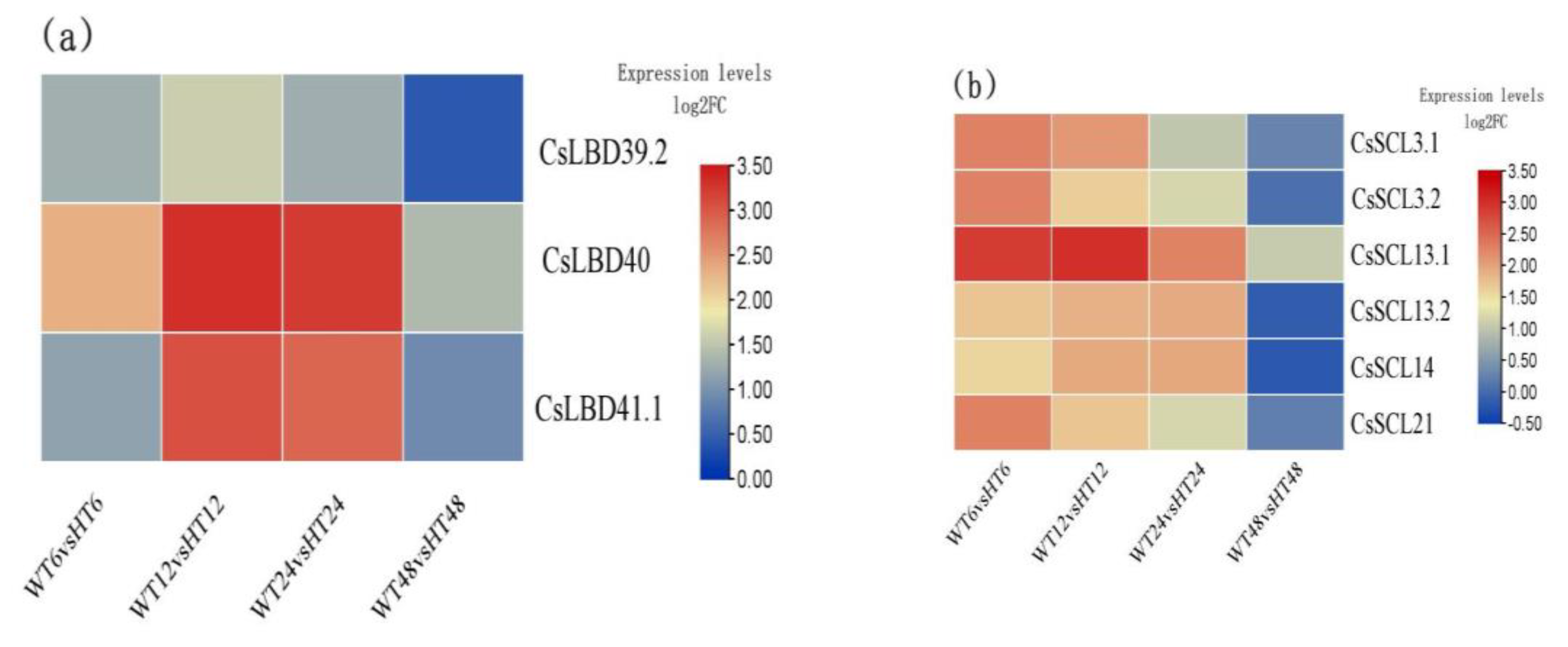
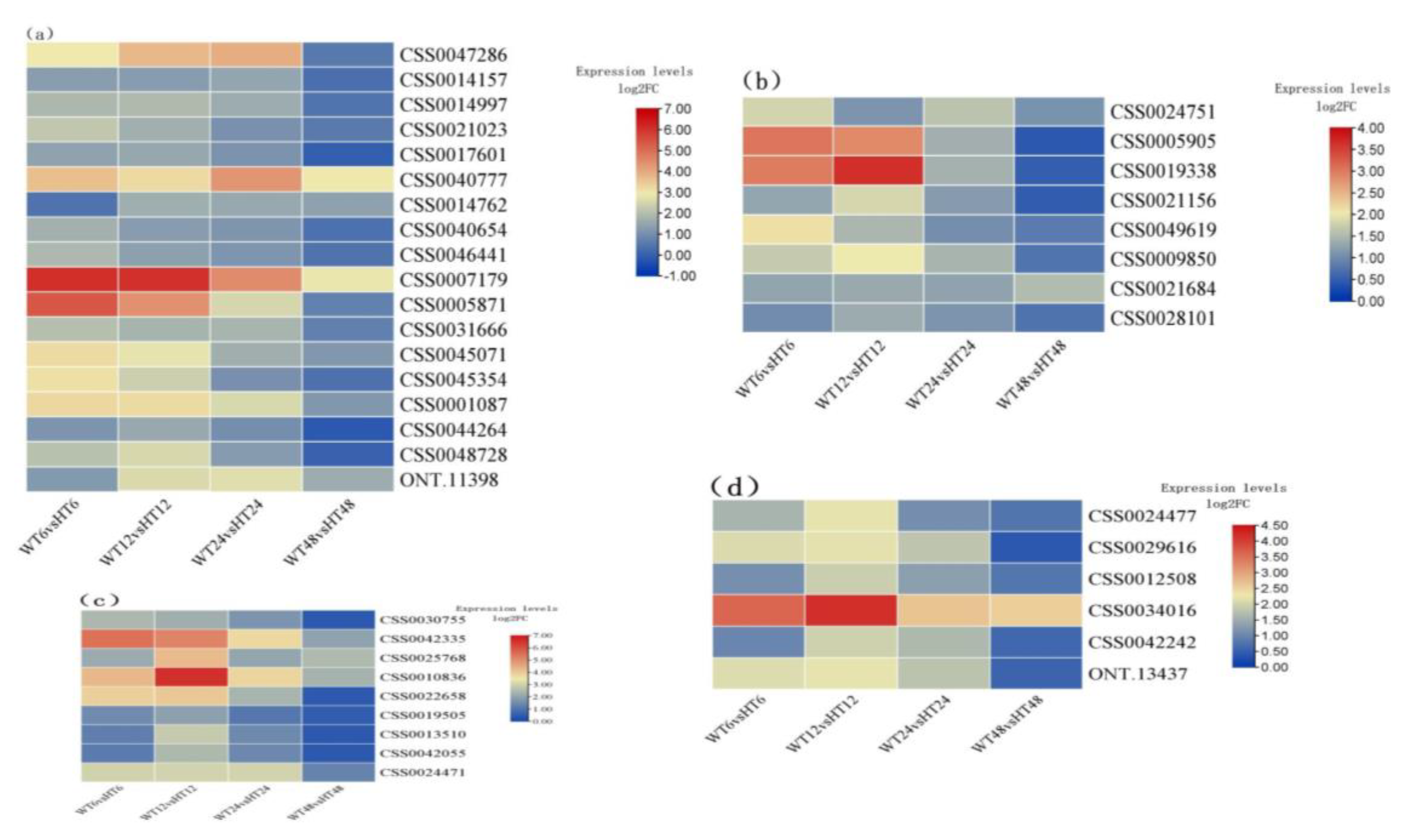
| #ID | log2FC | NR Annotation | |||
|---|---|---|---|---|---|
| WT6 vs. HT6 | WT12 vs. HT12 | WT24 vs. HT24 | WT48 vs. HT48 | ||
| CSS0016015 | 2.10 | 2.76 | 3.37 | 2.84 | auxin-induced protein 22D |
| CSS0008143 | 2.46 | 3.95 | 4.11 | 2.58 | auxin-responsive protein IAA1 |
| ONT.21529 | 0.87 | 2.71 | 2.62 | 1.56 | auxin-responsive protein IAA11 |
| CSS0017030 | 2.11 | 3.33 | 3.29 | 2.65 | auxin early response protein AUX/IAA4 |
| CSS0019988 | 2.11 | 3.30 | 3.78 | 4.00 | auxin early response protein AUX/IAA29 |
| CSS0014406 | 4.08 | 5.73 | 5.67 | 6.80 | GH3 auxin-responsive promoter |
| CSS0050062 | 4.17 | 5.34 | 5.61 | 6.66 | GH3 auxin-responsive promoter |
| CSS0046770 | 4.74 | 7.50 | 6.57 | 6.24 | auxin early response protein GH3.1 |
| CSS0025132 | 5.15 | 7.90 | 6.68 | 6.34 | auxin early response protein GH3.1 |
| CSS0028487 | 0.76 | 2.34 | 1.66 | 2.27 | auxin early response protein GH3.5 |
| ONT.9850 | 7.48 | 6.66 | 4.69 | 1.44 | auxin response factor 5 |
| CSS0019949 | 1.17 | 1.53 | 1.27 | −0.27 | auxin response factor 9 |
| CSS0041860 | 2.46 | 3.27 | 2.16 | 0.27 | auxin transport carrier |
| CSS0050423 | 1.13 | 1.66 | 1.46 | 0.95 | auxin transport carrier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wu, H.; Zhang, Y.; Sun, G.; Qian, W.; Qu, F.; Zhang, X.; Hu, J. Transcriptome Reveals the Regulation of Exogenous Auxin Inducing Rooting of Non-Rooting Callus of Tea Cuttings. Int. J. Mol. Sci. 2024, 25, 8080. https://doi.org/10.3390/ijms25158080
Wang S, Wu H, Zhang Y, Sun G, Qian W, Qu F, Zhang X, Hu J. Transcriptome Reveals the Regulation of Exogenous Auxin Inducing Rooting of Non-Rooting Callus of Tea Cuttings. International Journal of Molecular Sciences. 2024; 25(15):8080. https://doi.org/10.3390/ijms25158080
Chicago/Turabian StyleWang, Shuting, Huanran Wu, Yazhao Zhang, Guodong Sun, Wenjun Qian, Fengfeng Qu, Xinfu Zhang, and Jianhui Hu. 2024. "Transcriptome Reveals the Regulation of Exogenous Auxin Inducing Rooting of Non-Rooting Callus of Tea Cuttings" International Journal of Molecular Sciences 25, no. 15: 8080. https://doi.org/10.3390/ijms25158080
APA StyleWang, S., Wu, H., Zhang, Y., Sun, G., Qian, W., Qu, F., Zhang, X., & Hu, J. (2024). Transcriptome Reveals the Regulation of Exogenous Auxin Inducing Rooting of Non-Rooting Callus of Tea Cuttings. International Journal of Molecular Sciences, 25(15), 8080. https://doi.org/10.3390/ijms25158080






