Functional Validation of Endogenous Redox Partner Cytochrome P450 Reductase Reveals the Key P450s CYP6P9a/-b as Broad Substrate Metabolizers Conferring Cross-Resistance to Different Insecticide Classes in Anopheles funestus
Abstract
:1. Introduction
2. Results
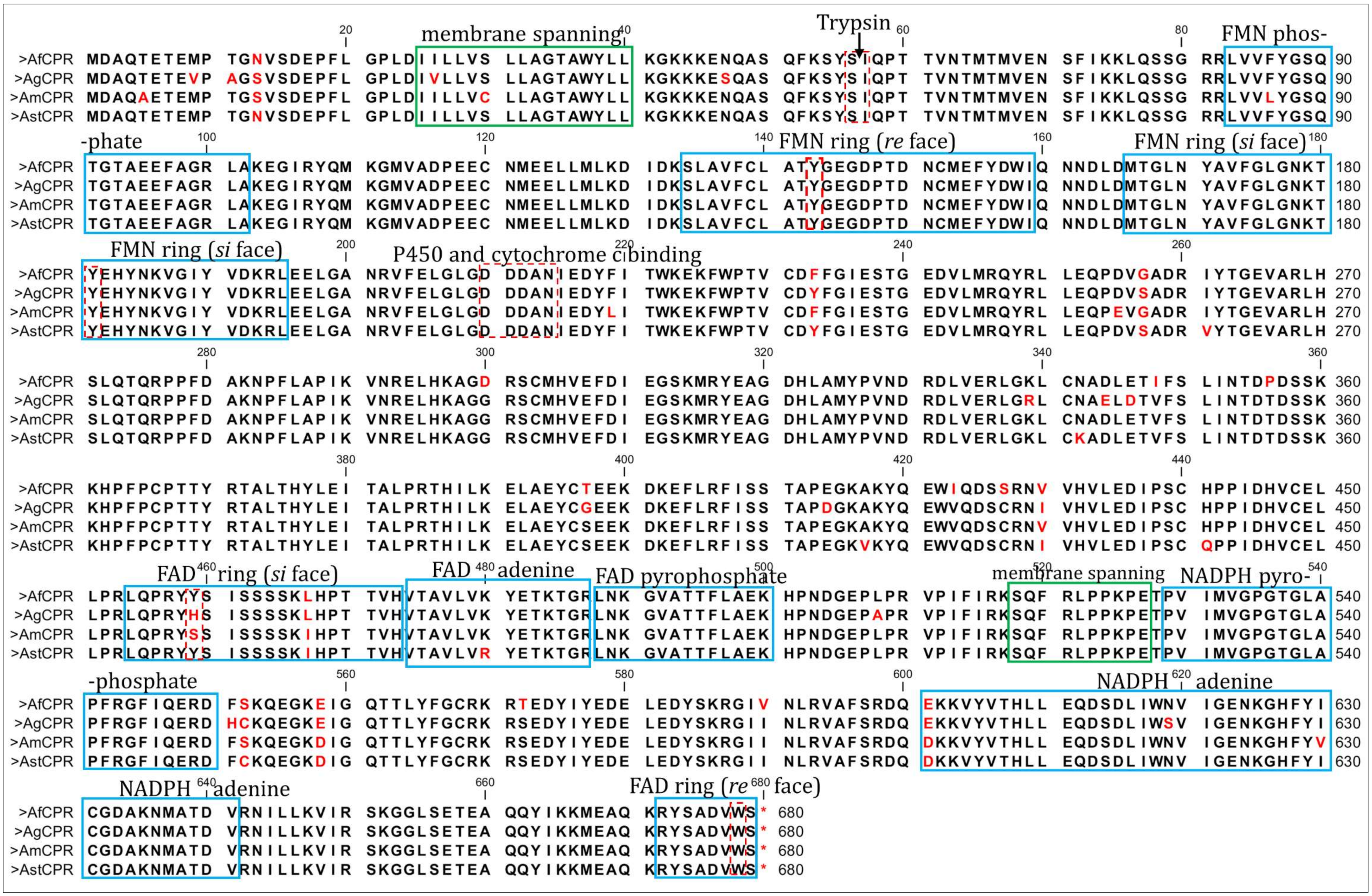
2.1. Patterns of Genetic Variability of AfCPR
2.2. Comparative Sequence Characterisation of Anopheles CPR
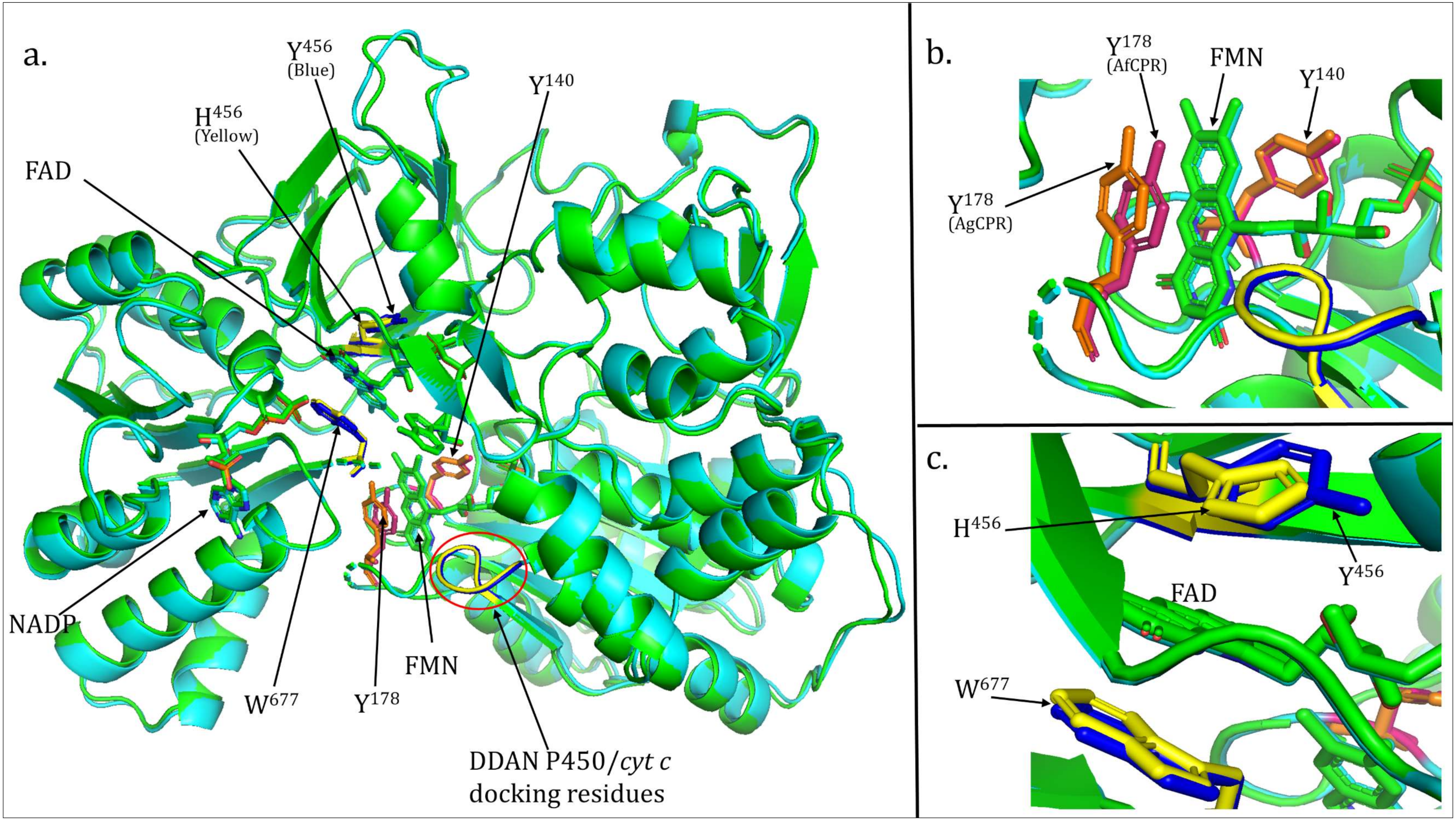
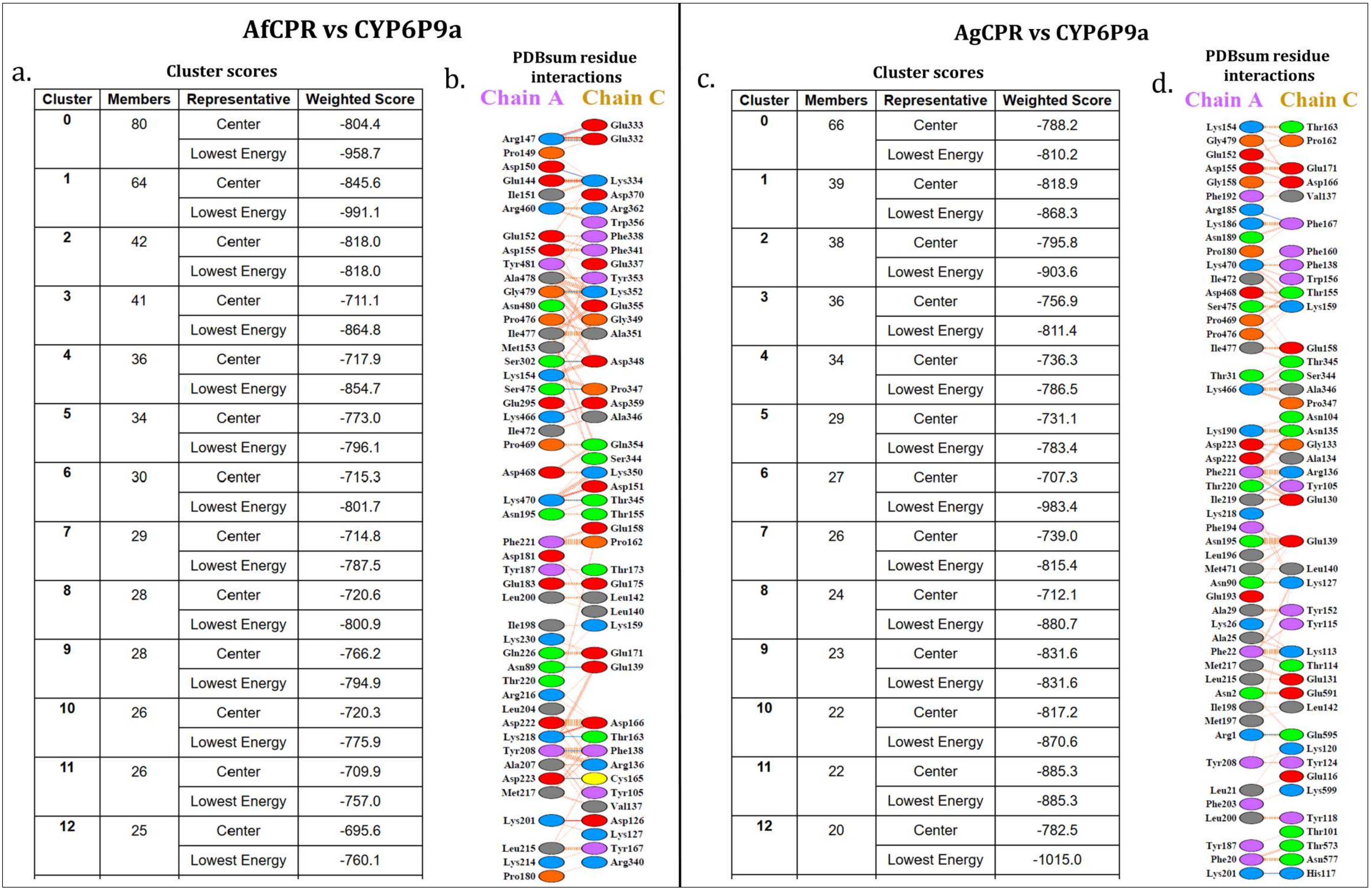
2.3. Comparative Prediction of AfCPR and AgCPR 3D Folding and Interaction with AfCYP6P9a
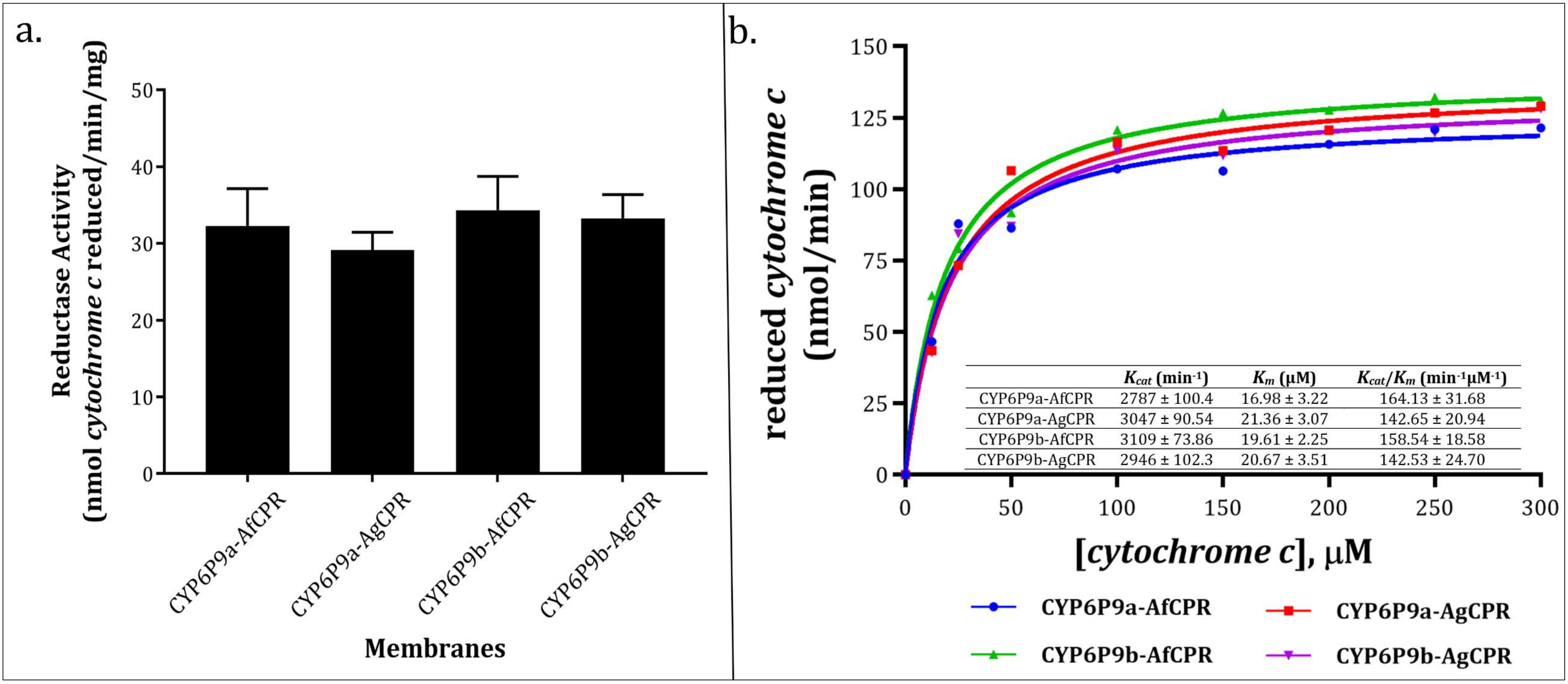
2.4. Comparative Assessment of Cytochrome c Reductase Activity
2.5. Comparative Assessment of the Role of AfCPR on Insecticide Metabolism by Recombinant CYP6P9a/-b
3. Discussion
3.1. Evidence of High Homology in AfCPR Sequences
3.2. Homologous CPR May Not Reconstruct Full Detoxification Potentials of Certain P450s
3.3. Recombinant AfCPR and AgCPR Exhibits Comparable Cytochrome c Reductase Activities
3.4. Endogenous CPR Confers Non-Pyrethroid Metabolising Abilities in CYP6P9a and -b
4. Materials and Methods
4.1. Polymorphism Analysis of Full-Length An. funestus CPR cDNA
4.2. Sequence Characterisation of CPR
4.3. Comparative Prediction of CPR 3D Folding and Interaction with AfCYP6P9a
4.4. Preparation of Recombinant AfCPR for Heterologous Expression
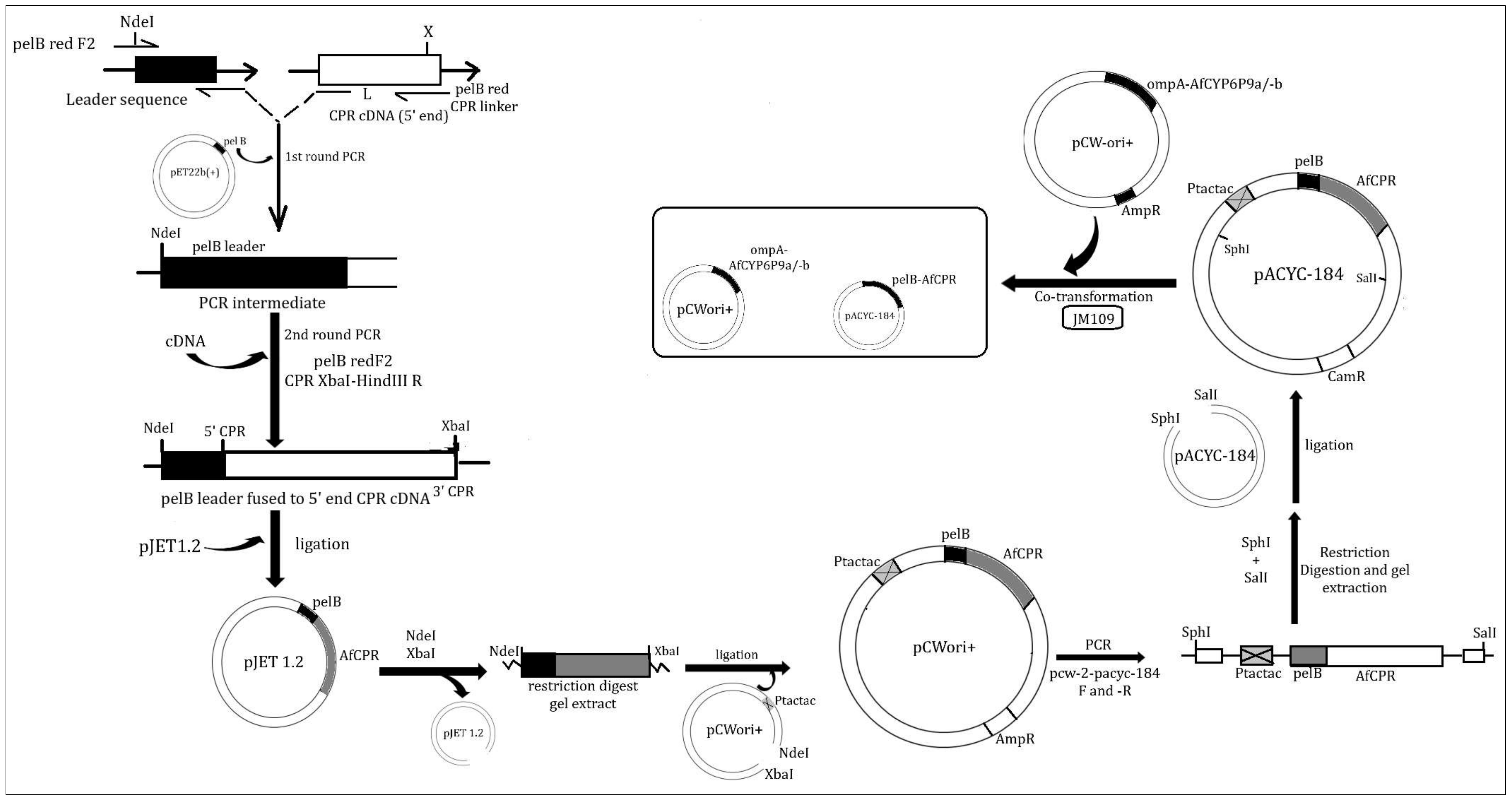
4.4.1. Fusion of AfCPR cDNA to pelB Leader
4.4.2. Cloning of tac-tac Promoter into pelB-AfCPR Construct
4.4.3. Subcloning of tac-tac-pelB-AfCPR Construct into pACYC-184 Expression Vector
4.5. Heterologous Co-Expression of Recombinant AfCYP6P9a/-b with AfCPR and AgCPR
4.6. Comparative Determination of CPR Activities Using Model Substrate Cytochrome c
4.7. Comparative Determination of Insecticides Metabolizing Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-008617-3.
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect P450 inhibitors and insecticides: Challenges and opportunities. Pest. Manag. Sci. 2015, 71, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s—Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nikou, D.; Ranson, H.; Hemingway, J. An adult-specific CYP6 P450 gene is overexpressed in a pyrethroid-resistant strain of the malaria vector, Anopheles gambiae. Gene 2003, 318, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide resistance in malaria vectors: An update at a global scale. In Towards Malaria Elimination—A Leap Forward; IntechOpen: London, UK, 2018. [Google Scholar]
- Weedall, G.D.; Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R.; et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Warr, E.; Stevenson, B.J.; Pignatelli, P.M.; Morgan, J.C.; Steven, A.; Yawson, A.E.; Mitchell, S.N.; Ranson, H.; Hemingway, J.; et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008, 4, e1000286. [Google Scholar] [CrossRef] [PubMed]
- Edi, C.V.; Djogbenou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Ketoh, G.K.; Paine, M.J.; et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef] [PubMed]
- Yunta, C.; Grisales, N.; Nasz, S.; Hemmings, K.; Pignatelli, P.; Voice, M.; Ranson, H.; Paine, M.J. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect Biochem. Mol. Biol. 2016, 78, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.N.; Stevenson, B.J.; Muller, P.; Wilding, C.S.; Egyir-Yawson, A.; Field, S.G.; Hemingway, J.; Paine, M.J.; Ranson, H.; Donnelly, M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA 2012, 109, 6147–6152. [Google Scholar] [CrossRef]
- Adolfi, A.; Poulton, B.; Anthousi, A.; Macilwee, S.; Ranson, H.; Lycett, G.J. Functional genetic validation of key genes conferring insecticide resistance in the major African malaria vector, Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2019, 116, 25764–25772. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, L.A.; Niazi, U.; Bibby, J.; David, J.P.; Vontas, J.; Hemingway, J.; Ranson, H.; Sutcliffe, M.J.; Paine, M.J. Characterization of inhibitors and substrates of Anopheles gambiae CYP6Z2. Insect Mol. Biol. 2008, 17, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Muhammad, A.; Hearn, J.; Weedall, G.D.; Nagi, S.C.; Mukhtar, M.M.; Fadel, A.N.; Mugenzi, L.J.; Patterson, E.I.; Irving, H.; et al. Molecular drivers of insecticide resistance in the Sahelo-Sudanian populations of a major malaria vector Anopheles coluzzii. BMC Biol. 2023, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Wondji, C.S.; Irving, H.; Morgan, J.; Lobo, N.F.; Collins, F.H.; Hunt, R.H.; Coetzee, M.; Hemingway, J.; Ranson, H. Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res. 2009, 19, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Riveron, J.M.; Bibby, J.; Irving, H.; Yunta, C.; Paine, M.J.I.; Wondji, C.S. Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector. PLoS Genet. 2015, 11, e1005618. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Ibrahim, S.S.; Chanda, E.; Mzilahowa, T.; Cuamba, N.; Irving, H.; Barnes, K.G.; Ndula, M.; Wondji, C.S. The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genom. 2014, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Mugenzi, L.M.J.; Tekoh, T.A.; Muhammad, A.; Kouamo, M.; Wondji, M.J.; Irving, H.; Hearn, J.; Wondji, C.S. The duplicated P450s CYP6P9a/b drive carbamates and pyrethroids cross-resistance in the major African malaria vector Anopheles funestus. PLoS Genet. 2023, 19, e1010678. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Ndula, M.; Riveron, J.M.; Irving, H.; Wondji, C.S. The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 2016, 25, 3436–3452. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Amvongo-Adjia, N.; Wondji, M.J.; Irving, H.; Riveron, J.M.; Wondji, C.S. Pyrethroid Resistance in the Major Malaria Vector Anopheles funestus is Exacerbated by Overexpression and Overactivity of the P450 CYP6AA1 Across Africa. Genes 2018, 9, 140. [Google Scholar] [CrossRef]
- Hearn, J.; Djoko Tagne, C.S.; Ibrahim, S.S.; Tene-Fossog, B.; Mugenzi, L.M.J.; Irving, H.; Riveron, J.M.; Weedall, G.D.; Wondji, C.S. Multi-omics analysis identifies a CYP9K1 haplotype conferring pyrethroid resistance in the malaria vector Anopheles funestus in East Africa. Mol. Ecol. 2022, 31, 3642–3657. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Liu, N.; Wen, Z. Insect cytochromes P450: Diversity, insecticide resistance and tolerance to plant toxins. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 121, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect cytochrome P450. Compr. Mol. Insect Sci. 2005, 4, 1–77. [Google Scholar]
- De Montellano, P.R.O. Cytochrome P450: Structure, Mechanism, and Biochemistry; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; Volume 3. [Google Scholar]
- Denisov, I.G.; Makris, T.M.; Sligar, S.G.; Schlichting, I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.J.; Scrutton, N.S.; Munro, A.W.; Gutierrez, A.; Roberts, G.C.; Wolf, C.R. Electron transfer partners of cytochrome P450. In Cytochrome P450; Springer: Berlin/Heidelberg, Germany, 2005; pp. 115–148. [Google Scholar]
- Lycett, G.J.; McLaughlin, L.A.; Ranson, H.; Hemingway, J.; Kafatos, F.C.; Loukeris, T.G.; Paine, M.J. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 2006, 15, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, X.P.; Wang, L.L.; Wei, D.; Feng, Z.J.; Zhang, Q.; Xiao, L.F.; Dou, W.; Wang, J.J. Functional characterization of NADPH-cytochrome P450 reductase from Bactrocera dorsalis: Possible involvement in susceptibility to malathion. Sci. Rep. 2015, 5, 18394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Liu, J.; Li, Y.; Liu, X.; Wu, H.; Ma, E.; Zhang, J. Knockdown of NADPH-cytochrome P450 reductase increases the susceptibility to carbaryl in the migratory locust, Locusta migratoria. Chemosphere 2017, 188, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Boonsuepsakul, S.; Luepromchai, E.; Rongnoparut, P. Characterization of Anopheles minimus CYP6AA3 expressed in a recombinant baculovirus system. Arch. Insect Biochem. Physiol. 2008, 69, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Duangkaew, P.; Pethuan, S.; Kaewpa, D.; Boonsuepsakul, S.; Sarapusit, S.; Rongnoparut, P. Characterization of mosquito CYP6P7 and CYP6AA3: Differences in substrate preference and kinetic properties. Arch. Insect Biochem. Physiol. 2011, 76, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Ibrahim, S.S.; Mulamba, C.; Djouaka, R.; Irving, H.; Wondji, M.J.; Ishak, I.H.; Wondji, C.S. Genome-Wide Transcription and Functional Analyses Reveal Heterogeneous Molecular Mechanisms Driving Pyrethroids Resistance in the Major Malaria Vector Anopheles funestus Across Africa. G3 2017, 7, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Ilias, A.; Vontas, J.; Douris, V. Co-Expression of a Homologous Cytochrome P450 Reductase Is Required for In Vivo Validation of the Tetranychus urticae CYP392A16-Based Abamectin Resistance in Drosophila. Insects 2020, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Nolden, M.; Paine, M.J.I.; Nauen, R. Biochemical profiling of functionally expressed CYP6P9 variants of the malaria vector Anopheles funestus with special reference to cytochrome b5 and its role in pyrethroid and coumarin substrate metabolism. Pestic. Biochem. Physiol. 2022, 182, 105051. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.D.; Kasper, C.B. Coding nucleotide sequence of rat NADPH-cytochrome P-450 oxidoreductase cDNA and identification of flavin-binding domains. Proc. Natl. Acad. Sci. USA 1985, 82, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.; Kim, J.J. Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Porter, T.D.; Wilson, T.E.; Kasper, C.B. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. J. Biol. Chem. 1989, 264, 7584–7589. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Kasper, C.B. Role of acidic residues in the interaction of NADPH-cytochrome P450 oxidoreductase with cytochrome P450 and cytochrome c. J. Biol. Chem. 1995, 270, 27475–27480. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y. Localization of cytochrome c-binding domain on NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1986, 261, 14232–14239. [Google Scholar] [CrossRef] [PubMed]
- Dohr, O.; Paine, M.J.; Friedberg, T.; Roberts, G.C.; Wolf, C.R. Engineering of a functional human NADH-dependent cytochrome P450 system. Proc. Natl. Acad. Sci. USA 2001, 98, 81–86. [Google Scholar] [CrossRef]
- Elmore, C.L.; Porter, T.D. Modification of the nucleotide cofactor-binding site of cytochrome P-450 reductase to enhance turnover with NADH In Vivo. J. Biol. Chem. 2002, 277, 48960–48964. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.Y.; Widdowson, P.; McLaughlin, L.A.; Paine, M.J. Biochemical comparison of Anopheles gambiae and human NADPH P450 reductases reveals different 2′-5′-ADP and FMN binding traits. PLoS ONE 2011, 6, e20574. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Kempna, P.; Hofer, G.; Mullis, P.E.; Fluck, C.E. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol. Endocrinol. 2007, 21, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Van Vliet, G.; Sack, J.; Fluck, C.E.; et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Fluck, C.E.; Pandey, A.V.; Huang, N.; Agrawal, V.; Miller, W.L. P450 oxidoreductase deficiency—A new form of congenital adrenal hyperplasia. Endocr. Dev. 2008, 13, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Fluck, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Rojas Velazquez, M.N.; Noebauer, M.; Pandey, A.V. Loss of Protein Stability and Function Caused by P228L Variation in NADPH-Cytochrome P450 Reductase Linked to Lower Testosterone Levels. Int. J. Mol. Sci. 2022, 23, 141. [Google Scholar] [CrossRef] [PubMed]
- Fluck, C.E.; Tajima, T.; Pandey, A.V.; Arlt, W.; Okuhara, K.; Verge, C.F.; Jabs, E.W.; Mendonca, B.B.; Fujieda, K.; Miller, W.L. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat. Genet. 2004, 36, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Marohnic, C.C.; Panda, S.P.; Martasek, P.; Masters, B.S. Diminished FAD binding in the Y459H and V492E Antley-Bixler syndrome mutants of human cytochrome P450 reductase. J. Biol. Chem. 2006, 281, 35975–35982. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, T.; Li, Q.; Liu, S.; Liu, N. The Central Role of Multiple P450 Genes and Their Co-factor CPR in the Development of Permethrin Resistance in the Mosquito Culex quinquefasciatus. Front. Physiol. 2021, 12, 802584. [Google Scholar] [CrossRef] [PubMed]
- Kaewpa, D.; Boonsuepsakul, S.; Rongnoparut, P. Functional expression of mosquito NADPH-cytochrome P450 reductase in Escherichia coli. J. Econ. Entomol. 2007, 100, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Sarapusit, S.; Pethuan, S.; Rongnoparut, P. Mosquito NADPH-cytochrome P450 oxidoreductase: Kinetics and role of phenylalanine amino acid substitutions at leu86 and leu219 in CYP6AA3-mediated deltamethrin metabolism. Arch. Insect Biochem. Physiol. 2010, 73, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Sarapusit, S.; Lertkiatmongkol, P.; Duangkaew, P.; Rongnoparut, P. Modeling of Anopheles minimus Mosquito NADPH-cytochrome P450 oxidoreductase (CYPOR) and mutagenesis analysis. Int. J. Mol. Sci. 2013, 14, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Murataliev, M.B.; Arino, A.; Guzov, V.M.; Feyereisen, R. Kinetic mechanism of cytochrome P450 reductase from the house fly (Musca domestica). Insect Biochem. Mol. Biol. 1999, 29, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Sem, D.S.; Kasper, C.B. Effect of ionic strength on the kinetic mechanism and relative rate limitation of steps in the model NADPH-cytochrome P450 oxidoreductase reaction with cytochrome c. Biochemistry 1995, 34, 12768–12774. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.M.; Houston, J.B. Substrate depletion approach for determining in vitro metabolic clearance: Time dependencies in hepatocyte and microsomal incubations. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Yunta, C.; Ooi, J.M.F.; Oladepo, F.; Grafanaki, S.; Pergantis, S.A.; Tsakireli, D.; Ismail, H.M.; Paine, M.J.I. Chlorfenapyr metabolism by mosquito P450s associated with pyrethroid resistance identifies potential activation markers. Sci. Rep. 2023, 13, 14124. [Google Scholar] [CrossRef] [PubMed]
- Kusimo, M.O.; Mackenzie-Impoinvil, L.; Ibrahim, S.S.; Muhammad, A.; Irving, H.; Hearn, J.; Lenhart, A.E.; Wondji, C.S. Pyrethroid resistance in the New World malaria vector Anopheles albimanus is mediated by cytochrome P450 CYP6P5. Pestic. Biochem. Physiol. 2022, 183, 105061. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Thiomela, R.F.; Nchoutpouen, E.; Menze, B.D.; Ndo, C.; Achu, D.; Tabue, R.N.; Njiokou, F.; Joel, A.; Wondji, C.S. High efficacy of chlorfenapyr-based net Interceptor((R)) G2 against pyrethroid-resistant malaria vectors from Cameroon. Infect. Dis. Poverty 2023, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Ngufor, C.; Fongnikin, A.; Fagbohoun, J.; Agbevo, A.; Syme, T.; Ahoga, J.; Accrombessi, M.; Protopopoff, N.; Cook, J.; Churcher, T.S.; et al. Evaluating the attrition, fabric integrity and insecticidal durability of two dual active ingredient nets (Interceptor((R)) G2 and Royal((R)) Guard): Methodology for a prospective study embedded in a cluster randomized controlled trial in Benin. Malar. J. 2023, 22, 276. [Google Scholar] [CrossRef] [PubMed]
- Tungu, P.K.; Michael, E.; Sudi, W.; Kisinza, W.W.; Rowland, M. Efficacy of interceptor(R) G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: Experimental hut trials in north-eastern Tanzania. Malar. J. 2021, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Assatse, T.; Tazokong, H.R.; Oruni, A.; Menze, B.D.; Nguiffo-Nguete, D.; Mugenzi, L.M.J.; Kayondo, J.; Watsenga, F.; Mzilahowa, T.; et al. Detection of a reduced susceptibility to chlorfenapyr in the malaria vector Anopheles gambiae contrasts with full susceptibility in Anopheles funestus across Africa. Sci. Rep. 2023, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, B.L.; Edi, C.; Tia, E.; Konan, L.Y.; Akre, M.A.; Koffi, A.A.; Ouattara, A.F.; Tanoh, A.M.; Zinzindohoue, P.; Kouadio, B.; et al. Susceptibility of Anopheles gambiae from Cote d’Ivoire to insecticides used on insecticide-treated nets: Evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar. J. 2020, 19, 454. [Google Scholar] [CrossRef] [PubMed]
- Black, B.C.; Hollingworth, R.M.; Ahammadsahib, K.I.; Kukel, C.D.; Donovan, S. Insecticidal action and mitochondrial uncoupling activity of AC-303,630 and related halogenated pyrroles. Pestic. Biochem. Physiol. 1994, 50, 115–128. [Google Scholar] [CrossRef]
- Oliver, S.V.; Kaiser, M.L.; Wood, O.R.; Coetzee, M.; Rowland, M.; Brooke, B.D. Evaluation of the pyrrole insecticide chlorfenapyr against pyrethroid resistant and susceptible Anopheles funestus (Diptera: Culicidae). Trop. Med. Int. Health 2010, 15, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Agumba, S.; Gimnig, J.E.; Ogonda, L.; Ombok, M.; Kosgei, J.; Munga, S.; Guyah, B.; Omondi, S.; Ochomo, E. Diagnostic dose determination and efficacy of chlorfenapyr and clothianidin insecticides against Anopheles malaria vector populations of western Kenya. Malar. J. 2019, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Fongnikin, A.; Houeto, N.; Agbevo, A.; Odjo, A.; Syme, T.; N’Guessan, R.; Ngufor, C. Efficacy of Fludora(R) Fusion (a mixture of deltamethrin and clothianidin) for indoor residual spraying against pyrethroid-resistant malaria vectors: Laboratory and experimental hut evaluation. Parasites Vectors 2020, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Chabi, J.; Seyoum, A.; Edi, C.V.A.; Kouassi, B.L.; Yihdego, Y.; Oxborough, R.; Gbalegba, C.G.N.; Johns, B.; Desale, S.; Irish, S.R.; et al. Efficacy of partial spraying of SumiShield, Fludora Fusion and Actellic against wild populations of Anopheles gambiae s.l. in experimental huts in Tiassale, Cote d’Ivoire. Sci. Rep. 2023, 13, 11364. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.; Dia, I.; Diedhiou, S.; Samb, B.; Kane Dia, A.; Diagne, M.; Faye, O.; Konate, L. Evaluation of the Efficacy of Fludora((R)) Fusion WP-SB 56.25 (Mixture of Clothianidin and Deltamethrin) against Anopheles coluzzii Laboratory and An. arabiensis Wild Colonies. Trop. Med. Infect. Dis. 2022, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Pambit Zong, C.M.; Coleman, S.; Mohammed, A.R.; Owusu-Asenso, C.M.; Akuamoah-Boateng, Y.; Sraku, I.K.; Attah, S.K.; Cui, L.; Afrane, Y.A. Baseline susceptibility of Anopheles gambiae to clothianidin in northern Ghana. Malar. J. 2024, 23, 12. [Google Scholar] [CrossRef]
- Ashu, F.A.; Fouet, C.; Ambadiang, M.M.; Penlap-Beng, V.; Kamdem, C. Adult mosquitoes of the sibling species Anopheles gambiae and Anopheles coluzzii exhibit contrasting patterns of susceptibility to four neonicotinoid insecticides along an urban-to-rural gradient in Yaounde, Cameroon. Malar. J. 2024, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Assatse, T.; Mugenzi, L.M.J.; Menze, B.D.; Nguiffo-Nguete, D.; Tchapga, W.; Kayondo, J.; Watsenga, F.; Manzambi, E.Z.; Osae, M.; et al. Comparative study of the effect of solvents on the efficacy of neonicotinoid insecticides against malaria vector populations across Africa. Infect. Dis. Poverty 2022, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Assatse, T.; Tchouakui, M.; Mugenzi, L.; Menze, B.; Nguiffo-Nguete, D.; Tchapga, W.; Kekeunou, S.; Wondji, C.S. Anopheles funestus Populations across Africa Are Broadly Susceptible to Neonicotinoids but with Signals of Possible Cross-Resistance from the GSTe2 Gene. Trop. Med. Infect. Dis. 2023, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Wondji, C.S.; Coleman, M.; Kleinschmidt, I.; Mzilahowa, T.; Irving, H.; Ndula, M.; Rehman, A.; Morgan, J.; Barnes, K.G.; Hemingway, J. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc. Natl. Acad. Sci. USA 2012, 109, 19063–19070. [Google Scholar] [CrossRef] [PubMed]
- Mulamba, C.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Barnes, K.G.; Mukwaya, L.G.; Birungi, J.; Wondji, C.S. Widespread Pyrethroid and DDT Resistance in the Major Malaria Vector Anopheles funestus in East Africa Is Driven by Metabolic Resistance Mechanisms. PLoS ONE 2014, 9, e110058. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Matambo, T.S.; Paine, M.J.; Coetzee, M.; Koekemoer, L.L. Sequence characterization of cytochrome P450 CYP6P9 in pyrethroid resistant and susceptible Anopheles funestus (Diptera: Culicidae). Genet. Mol. Res. 2010, 9, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Suwanchaichinda, C.; Brattsten, L.B. Genomic and bioinformatic analysis of NADPH-cytochrome P450 reductase in Anopheles stephensi (Diptera: Culicidae). J. Insect Sci. 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Janson, G.; Paiardini, A. PyMod 3: A complete suite for structural bioinformatics in PyMOL. Bioinformatics 2021, 37, 1471–1472. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. PyMOL User’s Guide; DeLano Scientific LLC: San Carlos, CA, USA, 2004; Available online: https://www.pymol.org/ (accessed on 10 December 2023).
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.D.; Tchouakui, M.; Mugenzi, L.M.J.; Tchapga, W.; Tchoupo, M.; Wondji, M.J.; Chiumia, M.; Mzilahowa, T.; Wondji, C.S. Marked aggravation of pyrethroid resistance in major malaria vectors in Malawi between 2014 and 2021 is partly linked with increased expression of P450 alleles. BMC Infect. Dis. 2022, 22, 660. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Jablonska, J.; Pravda, L.; Varekova, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, M.P.; McLaughlin, L.; Friedberg, T. Establishment of functional human cytochrome P450 monooxygenase systems in Escherichia coli. Methods Mol. Biol. 2006, 320, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, M.P.; Glancey, M.J.; Blake, J.A.; Gilham, D.E.; Burchell, B.; Wolf, C.R.; Friedberg, T. Functional co-expression of CYP2D6 and human NADPH-cytochrome P450 reductase in Escherichia coli. Pharmacogenetics 1998, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.P.; Lin, H.C.; Wang, S.S.; Callaway, J.; Wilcox, G. Characterization of the Erwinia carotovora pelB gene and its product pectate lyase. J. Bacteriol. 1987, 169, 4379–4383. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Cohen, S.N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 1978, 134, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988, 16, 355. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.A.; Comstock, L.J.; Vasser, M. The tac promoter: A functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA 1983, 80, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sato, R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 2009, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Riveron, J.M.; Stott, R.; Irving, H.; Wondji, C.S. The cytochrome P450 CYP6P4 is responsible for the high pyrethroid resistance in knockdown resistance-free Anopheles arabiensis. Insect Biochem. Mol. Biol. 2016, 68, 23–32. [Google Scholar] [CrossRef] [PubMed]

| Population | n | S | Syn | Non syn | h | Hd | π (k) | D (Tajima) | D * (Fu and Li) |
|---|---|---|---|---|---|---|---|---|---|
| MALAWI | 5 | 0 | 0 | 0 | 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| UGANDA | 5 | 9 | 5 | 4 | 2 | 0.60 | 0.0027 | 1.78 | 1.78 * |
| FANG | 5 | 0 | 0 | 0 | 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| All | 15 | 252 | 222 | 33 | 4 | 0.77 | 0.058 | 2.30 * | 1.73 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.S.; Kouamo, M.F.M.; Muhammad, A.; Irving, H.; Riveron, J.M.; Tchouakui, M.; Wondji, C.S. Functional Validation of Endogenous Redox Partner Cytochrome P450 Reductase Reveals the Key P450s CYP6P9a/-b as Broad Substrate Metabolizers Conferring Cross-Resistance to Different Insecticide Classes in Anopheles funestus. Int. J. Mol. Sci. 2024, 25, 8092. https://doi.org/10.3390/ijms25158092
Ibrahim SS, Kouamo MFM, Muhammad A, Irving H, Riveron JM, Tchouakui M, Wondji CS. Functional Validation of Endogenous Redox Partner Cytochrome P450 Reductase Reveals the Key P450s CYP6P9a/-b as Broad Substrate Metabolizers Conferring Cross-Resistance to Different Insecticide Classes in Anopheles funestus. International Journal of Molecular Sciences. 2024; 25(15):8092. https://doi.org/10.3390/ijms25158092
Chicago/Turabian StyleIbrahim, Sulaiman S., Mersimine F. M. Kouamo, Abdullahi Muhammad, Helen Irving, Jacob M. Riveron, Magellan Tchouakui, and Charles S. Wondji. 2024. "Functional Validation of Endogenous Redox Partner Cytochrome P450 Reductase Reveals the Key P450s CYP6P9a/-b as Broad Substrate Metabolizers Conferring Cross-Resistance to Different Insecticide Classes in Anopheles funestus" International Journal of Molecular Sciences 25, no. 15: 8092. https://doi.org/10.3390/ijms25158092





