Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions
Abstract
1. Introduction
2. Properties of Stigmasterol as a Component of Plant Membranes
3. Stigmasterol in Different Organisms
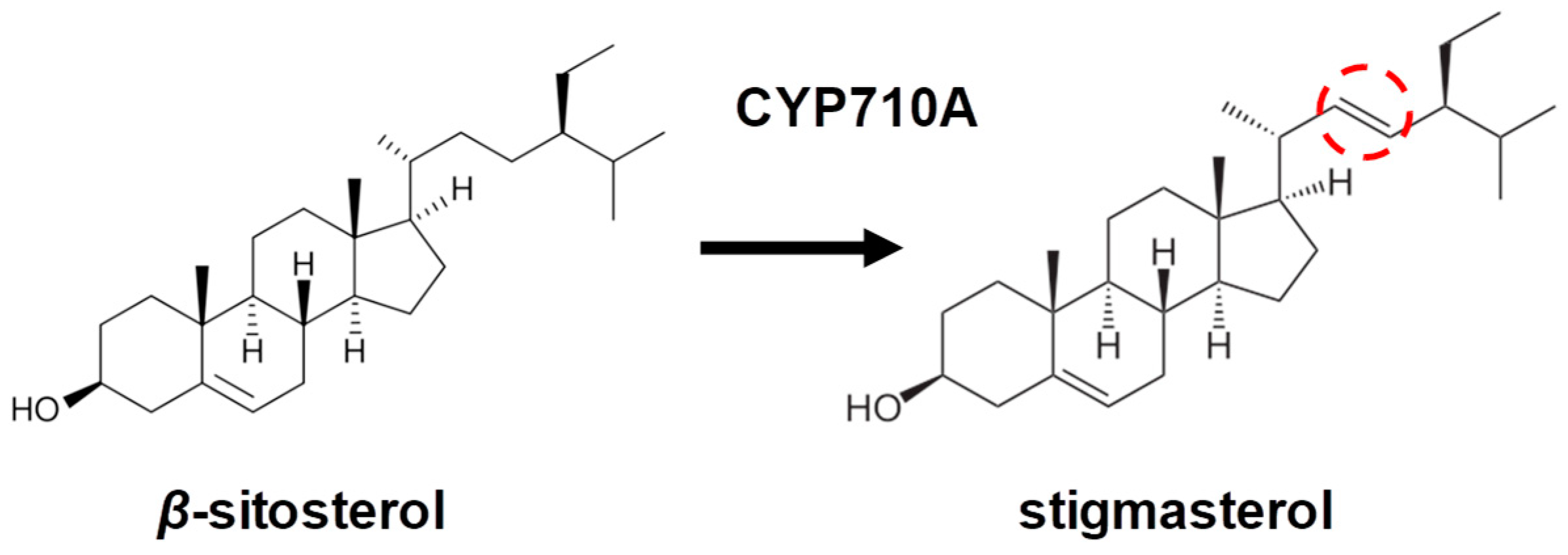
4. C22-Sterol Desaturase (CYP710A) as a Key Enzyme of Stigmasterol Biosynthesis
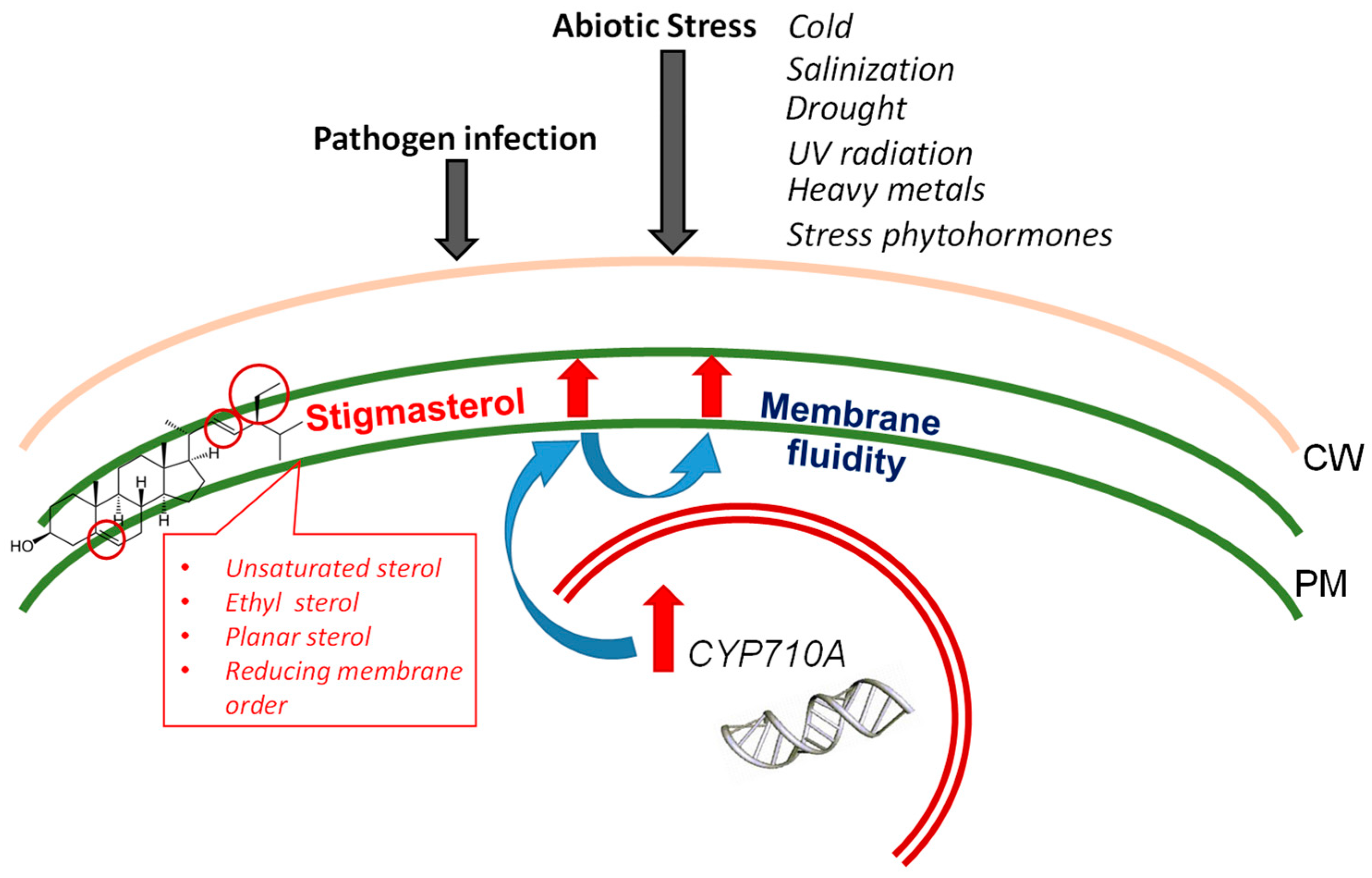
5. Stigmasterol Levels during Biotic and Abiotic Stresses
5.1. Pathogen Infection
5.2. Salinization
5.3. Unfavorable Temperatures
5.4. Drought
5.5. UV Radiation
5.6. Heavy Metals
5.7. Stress Phytohormones
5.8. Summary of the Role of Stigmasterol in the Response of Plants to Stresses
6. Application of Stigmasterol in Medicine
7. Conclusion: Stigmasterol as a Stress Sterol of Plants
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benveniste, P. Biosynthesis and Accumulation of Sterols. Annu. Rev. Plant Biol. 2004, 55, 429–457. [Google Scholar] [CrossRef]
- Darnet, S.; Schaller, H. Metabolism and Biological Activities of 4-Methyl-sterols. Molecules 2019, 24, 451. [Google Scholar] [CrossRef]
- Hartmann, M.A. Plant Sterols and the Membrane Environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging Roles for Conjugated Sterols in Plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Szakiel, A. The Role of Sterols in Plant Response to Abiotic Stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Aboobucker, S.I.; Suza, W.P. Why Do Plants Converts β-Sitosterol to Stigmasterol? Front. Plant Sci. 2019, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Griebel, T.; Zeier, J. A Role for Beta-Sitosterol to Stigmasterol Conversion in Plant-Pathogen Interactions. Plant J. 2010, 63, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Zhou, J.; Yuan, Y.-J. Analysis of Phospholipids, Sterols, and Fatty Acids in Taxus chinensis var. mairei Cells in Response to Shear Stress. Biotechnol. Appl. Biochem. 2009, 54, 105–112. [Google Scholar] [CrossRef]
- Dalal, J.; Lewis, D.R.; Tietz, O.; Brown, E.M.; Brown, C.S.; Palme, K.; Muday, G.K.; Sederoff, H.W. ROSY1, A Novel Regulator of Gravitropic Response is a Stigmasterol Binding Protein. J. Plant Physiol. 2016, 196–197, 28–40. [Google Scholar] [CrossRef]
- Schuller, I.; Milon, A.; Nakatani, Y.; Ourisson, G.; Albrecht, A.-M.; Benveniste, P.; Hartmann, M.-A. Differential Effects of Plant Sterols on Water Permeability and On Acyl Chain Ordering of Soybean Phosphatidylcholine Bilayers. Proc. Nat. Acad. Sci. USA 1991, 88, 6926–6930. [Google Scholar] [CrossRef]
- Bernsdorff, C.; Winter, R. Differential Properties of The Sterols: Cholesterol, Ergosterol, β-Sitosterol, Trans-7-Dehydrocholesterol, Stigmasterol and Lanosterol on DPPC Bilayer Order. J. Phys. Chem. B 2003, 107, 10658–10664. [Google Scholar] [CrossRef]
- Xu, X.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of The Structure of Natural Sterols and Sphingolipids on The Formation of Ordered Sphingolipid/Sterol Domains (Rafts) Comparison of Cholesterol to Plant, Fungal, and Disease-Associated Sterols and Comparison of Sphingomyelin, Cerebrosides, and Ceramide. J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [PubMed]
- Fakih, O.; Sanver, D.; Kane, D.; Thorne, J.L. Exploring the Biophysical Properties of Phytosterols in the Plasma Membrane for Novel Cancer Prevention Strategies. Biochimie 2018, 153, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ohvo-Rekila, H.; Ramstedt, B.; Leppim, P.; Slotte, J.P. Cholesterol Interactions with Phospholipids in Membranes. Prog. Lipid Res. 2002, 66, 9789–9792. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, A.; Rappolt, M.; Amenitsch, H.; Laggner, P.; Pabst, G. Differential Modulation of Membrane Structure and Fluctuations by Plant Sterols and Cholesterol. Biophys. J. 2008, 94, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. The Role of Phytosterols in Plant Adaptation to Temperature. Plant Signal Behav. 2008, 3, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, K.; Mongrand, S.; Beney, L.; Simon-Plas, F.; Gerbeau-Pissot, P. Differential Effect of Plant Lipids on Membrane Organization: Specificities of Phytosphingolipids and Phytosterols. J. Biol. Chem. 2015, 290, 5810–5825. [Google Scholar] [CrossRef]
- De Almeida, R.F.; Joly, E. Crystallization Around Solid-Like Nanosized Docks Can Explain the Specificity, Diversity, and Stability of Membrane Microdomains. Front. Plant Sci. 2014, 5, 72. [Google Scholar] [CrossRef]
- Wagatsuma, T. The Membrane Lipid Bilayer as A Regulated Barrier to Cope with Detrimental Ionic Conditions: Making New Tolerant Plant Lines with Altered Membrane Lipid Bilayer. Soil Sci. Plant Nutr. 2017, 63, 507–516. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Effect of Different Culture Medias on Shoot Multiplication and Stigmasterol Content in Accessions of Centella asiatica. Int. J. Ayurvedic Herb. Med. 2017, 7, 2643–2650. [Google Scholar] [CrossRef]
- Chowdhary, A.; Chaturvedi, P.; Memon, R. Stigmasterol Variation in a Medhya Rasayan Plant (Centella asiatica L.: Apiaceae) Collected from Different Regions. Indian Drugs 2014, 51, 44–49. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Mishra, S. Simultaneous HPTLC Analysis of Ursolic Acid, Betulinic Acid, Stigmasterol and Lupeol for the Identification of Four Medicinal Plants Commonly Available in the Indian Market as Shankhpushpi. J. Chromatogr. Sci. 2015, 53, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Blair, J.E.; Venturi, M.L.; Shoe, J.L. A Molecular Timescale of Eukaryote Evolution and the Rise of Complex Multicellular Life. BMC Evol Biol. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Saga, H.; Hashizume, H.; Ohta, D. CYP710A Genes Encoding Sterol C22-desaturase in Physcomitrella patens as Molecular Evidence for The Evolutionary Conservation of a Sterol Biosynthetic Pathway in Plants. Planta 2009, 229, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Tolstikov, G.A. Organic Metabolites of Lichens; Publishing House of SB RAS, “Geo” Branch: Novosibirsk, Russia, 2005; 135p. [Google Scholar]
- Ling, Q.; Bogang, L.; Jiafa, G.; Guolin, Z. Chemical Study on Aspergillus sp 136. Chin. J. Appl. Environ. Biol. 2007, 13, 66–68. [Google Scholar]
- Yan, H.; Gao, S.; Li, C.; Li, X.; Wang, B. Chemical Constituents of a Marine-Derived Endophytic Fungus Penicillium commune G2M. Molecules 2010, 15, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, A.H.; León, F.; Radwan, M.M.; Rosa, L.H.; Cutler, S.J. Secondary Metabolites from the Fungus Emericella nidulans. Nat. Prod. Commun. 2013, 8, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.M.; Olimpio da Paixão, L.K.; Dolabela, M.F.; Marinho, P.S.B.; Marinho, A.M.d.R. Phytosterols Isolated from Endophytic Fungus Colletotrichum gloeosporioides (Melanconiaceae). Acta Amaz. 2016, 46, 69–72. [Google Scholar] [CrossRef]
- Patterson, G.W. The Distribution of Sterols in Algae. Lipids 1971, 6, 120–127. [Google Scholar] [CrossRef]
- Geng, H.-X.; Yua, R.-C.; Chena, Z.-F.; Peng, Q.-C.; Yan, T.; Zhou, M.-J. Analysis of Sterols in Selected Bloom-Forming Algae in China. Harmful Algae 2017, 66, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Voshall, A.; Christie, N.T.M.; Rose, S.L.; Khasin, M.; Van Etten, J.L.; Riekhof, W.R.; Nickerson, K.W. Sterol Biosynthesis in Four Green Algae: A Bioinformatic Analysis of The Ergosterol Versus Phytosterol Decision Point. J. Phycol. 2021, 57, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, P. Sterol metabolism. Arab. Book 2002, 1, e0004. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Mizutani, M.; Aoki, N.; Watanabe, B.; Saga, H.; Saito, S.; Oikawa, A.; Suzuki, H.; Sakurai, N.; Shibata, D.; et al. Cytochrome P450 CYP710A Encodes the Sterol C-22 Desaturase in Arabidopsis and Tomato. Plant Cell 2006, 18, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Raksha, B.R.; Siva, R.; Vino, S.; Babu, S. Spatio-varietal Differences in Stigmasterol Biosynthesis in Tomato and Overexpression of a Sterol Desaturase Gene for Enhanced Stigmasterol Production. In Vitro Cell Dev. Biol Plant 2016, 52, 571–579. [Google Scholar] [CrossRef]
- Sewelam, N.; Jaspert, N.; Van Der Kelen, K.; Tognetti, V.B.; Schmitz, J.; Frerigmann, H.; Stahl, E.; Zeier, J.; Van Breusegem, F.; Maurino, V.G. Spatial H2O2 Signaling Specificity: H2O2 from Chloroplasts and Peroxisomes Modulates the Plant Transcriptome Differentially. Mol Plant. 2014, 7, 1191–1210. [Google Scholar] [CrossRef]
- Schrick, K.; Mayer, U.; Horrichs, A.; Kuhnt, C.; Bellini, C.; Dangl, J.; Schmidt, J.; Jürgens, G. FACKEL is a Sterol C-14 Reductase Required for Organized Cell Division and Expansion in Arabidopsis Embryogenesis. Genes Dev. 2000, 14, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Souter, M.; Topping, J.; Pullen, M.; Friml, J.; Palme, K.; Hackett, R.; Grierson, D.; Lindsey, K. Hydra Mutants of Arabidopsis are Defective in Sterol Profiles and Auxin and Ethylene Signaling. Plant Cell 2002, 14, 1017–1031. [Google Scholar] [CrossRef]
- Lindsey, K.; Pullen, M.L.; Topping, J.F. Importance of Plant Sterols in Pattern Formation and Hormone Signalling. Trends Plant Sci. 2003, 8, 521–525. [Google Scholar] [CrossRef]
- Masucci, J.D.; Rerie, W.G.; Foreman, D.R.; Zhang, M.; Galway, M.E.; Marks, M.D.; Schiefelbein, J.W. The Homeobox Gene GLABRA2 Is Required for Position-Dependent Cell Differentiation in The Root Epidermis of Arabidopsis thaliana. Development 1996, 122, 1253–1260. [Google Scholar] [CrossRef]
- Lung, S.C.; Liao, P.; Yeung, E.C.; Hsiao, A.S.; Xue, Y.; Chye, M.L. Acyl-CoA-binding Protein ACBP1 Modulates Sterol Synthesis During Embryogenesis. Plant Physiol. 2017, 174, 1420–1435. [Google Scholar] [CrossRef]
- Aboobucker, S.I.; Showman, L.J.; Lübberstedt, T.; Suza, W.P. Maize Zmcyp710a8 Mutant as a Tool to Decipher the Function of Stigmasterol in Plant Metabolism. Front. Plant Sci. 2021, 12, 732216. [Google Scholar] [CrossRef]
- García, L.G. Unveiling the Biological Role of Stigmasterol Biosynthesis in Tomato Plants. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Senthil-Kumar, M.; Wang, K.; Mysore, K.S. AtCYP710A1 Gene-Mediated Stigmasterol Production Plays a Role in Imparting Temperature Stress Tolerance in Arabidopsis thaliana. Plant Signal Behav. 2013, 8, e23142. [Google Scholar] [CrossRef]
- Nelson, D.R. Plant cytochrome P450s from moss to poplar. Phytochem. Rev. 2006, 5, 193–204. [Google Scholar] [CrossRef]
- Wang, K.; Senthil-Kumar, M.; Ryu, C.M.; Kang, L.; Mysore, K.S. Phytosterols Play a Key Role in Plant Innate Immunity Against Bacterial Pathogens by Regulating Nutrient Efflux into the Apoplast. Plant Physiol. 2012, 158, 1789–1802. [Google Scholar] [CrossRef]
- Arnqvist, L.; Persson, M.; Jonsson, L.; Dutta, P.C.; Sitbon, F. Overexpression of CYP710A1 and CYP710A4 in Transgenic Arabidopsis Plants Increases the Level of Stigmasterol at The Expense of Sitosterol. Planta. 2008, 227, 309–317. [Google Scholar] [CrossRef]
- Renkova, A.; Valitova, J.; Schaller, H.; Minibayeva, F. The Homoeologous Genes Encoding C24-Sterol Methyltransferase 1 in Triticum aestivum: Structural Characteristics and Effects of Cold Stress. Biol. Plantarum. 2019, 63, 59–69. [Google Scholar] [CrossRef]
- Renkova, A.G.; Khabibrakhmanova, V.R.; Valitova, J.N.; Mukhitova, F.K.; Minibayeva, F.V. Effects of Stress Phytohormones on Sterol Metabolism of Triticum aestivum L. Russ. J. Plant Physiol. 2021, 68, 474–482. [Google Scholar] [CrossRef]
- Chen, W.; Lee, M.-K.; Jefcoate, C.; Kim, S.-C.; Chen, F.; Yu, J.-H. Fungal Cytochrome P450 Monooxygenases: Their Distribution, Structure, Functions, Family Expansion, and Evolutionary Origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef]
- Kelly, S.L.; Kelly, D.E. Microbial cytochromes P450: Biodiversity and biotechnology. Where Do Cytochromes P450 Come From, What Do They Do and What Can They Do for Us? Philos. Trans. R. Soc. B 2013, 368, 20120476. [Google Scholar] [CrossRef]
- Nelson, D.; Werck-Reichhart, D. The Plant Genome: An Evolutionary View on Structure and Function A P450-Centric View of Plant Evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Hatada, M.; Akiyama, R.; Yamagishi, M.; Ishizaki, K.; Mizutani, M. MpDWF5A-Encoded Sterol Δ7-Reductase Is Essential for the Normal Growth and Development of Marchantia polymorpha. Plant Cell Physiol. 2023, 64, 826–838. [Google Scholar] [CrossRef]
- Gotoh, O. Substrate Recognition Sites in Cytochrome P450 Family 2 (CYP2) Proteins Inferred from Comparative Analyzes of Amino Acids and Coding Nucleotide Sequences. J. Biol. Chem. 1992, 267, 83–90. [Google Scholar] [CrossRef]
- Tang, J.; Ohyama, K.; Kawaura, K.; Hashinokuchi, H.; Kamiya, Y.; Suzuki, M.; Muranaka, T.; Ogihara, Y. A New Insight into Application for Barley Chromosome Addition Lines of Common Wheat: Achievement of Stigmasterol Accumulation. Plant Physiol. 2011, 157, 1555–1567. [Google Scholar] [CrossRef]
- Schaller, H. The Role of Sterols in Plant Growth and Development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Huang, L.; Li, G.; Wang, Q.; Meng, Q.; Xu, F.; Chen, Q.; Liu, F.; Hu, Y.; Luo, M. GhCYP710A1 Participates in Cotton Resistance to Verticillium Wilt by Regulating Stigmasterol Synthesis and Plasma Membrane Stability. Int. J. Mol. Sci. 2022, 23, 8437. [Google Scholar] [CrossRef]
- Hedin, P.A.; Callahan, F.E.; Dollar, D.A.; Greech, R.G. Total Sterols in Root-Knot Nematode Meloidogyne incognita infected cotton Gossypium hirsutum (L.). Plant Roots. Comp. Biochem. Physiol. 1995, 111, 447–452. [Google Scholar] [CrossRef]
- Cabianca, A.; Ruthes, A.C.; Pawlowski, K.; Dahlin, P. Tomato Sterol 22-desaturase Gene CYP710A11: Its Roles in Meloidogyne incognita Infection and Plant Stigmasterol Alteration. Int. J. Mol. Sci. 2022, 23, 15111. [Google Scholar] [CrossRef]
- Beck, J.G.; Mathieu, D.; Loudet, C.; Buchoux, S.; Dufourc, E.J. Plant Sterols in “Rafts”: A Better Way to Regulate Membrane Thermal Shocks. FASEB J. 2007, 21, 1714–1723. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Kerkeb, L.; Donaire, J.P.; Venema, K.; Rodríguez-Rosales, M.P. Tolerance to NaCl induces changes in plasma membrane lipid composition, fluidity and H+-ATPase activity of tomato calli. Physiol. Plantarum. 2001, 113, 217–224. [Google Scholar] [CrossRef]
- Morales-Cedillo, F.; González-Solís, A.; Gutiérrez-Angoa, L.; Cano-Ramírez, D.L.; Gavilanes-Ruiz, M. Plant Lipid Environment and Membrane Enzymes: The Case of the Plasma Membrane H+-ATPase. Plant Cell Rep. 2015, 34, 617–629. [Google Scholar] [CrossRef]
- Guo, Q.; Barkla, L.B. Membrane Lipid Remodeling in Response to Salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef]
- Salama, K.H.A.; Mansour, M.M.F. Choline Priming-Induced Plasma Membrane Lipid Alterations Contributed to Improved Wheat Salt Tolerance. Acta Physiol. Plant. 2015, 37, 1–7. [Google Scholar] [CrossRef]
- Salama, K.H.A.; Mansour, M.M.F.; Ali, F.Z.M.; Abou-Hadid, A.F. NaCl-Induced Changes in Plasma Membrane Lipids and Proteins of Zea mays L. Cultivars Differing in Their Response to Salinity. Acta Physiol. Plant. 2007, 29, 351–359. [Google Scholar] [CrossRef]
- Blits, K.; Gallagher, J. Effect of NaCl on Lipid Content of Plasma Membranes Isolated from Roots and Cell Suspension Cultures of The Dicot Halophyte Kosteletzkya virginica (L.) Presl. Plant Cell Rep. 1990, 9, 156–159. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.A.; Al-Mutawa, M.M.; Abou Hadid, A.F. Effect of NaCl and Polyamines on Plasma Membrane Lipids of Wheat Roots. Biol. Plantarum. 2002, 45, 235–239. [Google Scholar] [CrossRef]
- Douglas, T.J.; Walker, R.R. 4-Desmethylsterol Composition of Citrus Rootstocks of Different Salt Exclusion Capacity. Physiol. Plant. 1983, 58, 69–74. [Google Scholar] [CrossRef]
- Douglas, T.J.; Csiro, S.R.S. Phospholipid, Galactolipid and Free Sterol Composition of Fibrous Roots from Citrus Genotypes Differing in Chloride Exclusion Ability. Plant Cell Environ. 1985, 8, 693–699. [Google Scholar] [CrossRef]
- López-Pérez, L.; Martínez-Ballesta, M.D.C.; Maurel, C.; Carvajal, M. Changes in Plasma Membrane Lipids, Aquaporins and Proton Pump of Broccoli Roots, as an Adaptation Mechanism to Salinity. Phytochemistry 2009, 70, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.A.; Kotlova, E.R.; Bogdanova, E.S.; Nesterov, V.N.; Senik, S.V.; Shavarda, A.L. Balance of Δ5 -and Δ7 -Sterols and Stanols in Halophytes in Connection with Salinity Tolerance. Phytochemistry 2022, 198, 113156. [Google Scholar] [CrossRef]
- Hashem, H.A.; Bassuony, F.M.; Hassanein, R.A.; Baraka, D.M.; Khalil, R.R. Stigmasterol Seed Treatment Alleviates the Drastic Effect of NaCl and Improves Quality and Yield in Flax Plants. Aust. J. Crop Sci. 2011, 5, 1858–1867. [Google Scholar]
- Hassanein, R.A.; Hashem, H.A.; Khalil, R.R. Stigmasterol Treatment Increases Salt Stress Tolerance of Faba Bean Plants by Enhancing Antioxidant Systems. Plant Omics 2012, 5, 476–485. [Google Scholar]
- Shabala, S.; Shabala, L.; Volkenburgh, E.V. Effect of Calcium on Root Development and Root Ion Fluxes in Salinised Barley Seedlings. Funct. Plant Biol. 2003, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Pilar, C.; Antonio, O.; Antonio, C. Effects of Saline Stress and Calcium on Lipid Composition in Bean Roots. Phytochemistry 1993, 32, 1131–1136. [Google Scholar] [CrossRef]
- Grandmougin-Ferjani, A.; Schuler-Muller, I.; Hartmann, M.A. Sterol Modulation of The Plasma Membrane H+-ATPase Activity from Corn Roots Reconstituted into Soybean Lipids. Plant Physiol. 1997, 113, 163–174. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Barkla, B.J.; Vera-Estrella, R.; Zhu, J.K.; Schumaker, K.S. Na+/H+ Exchange Activity in the Plasma Membrane of Arabidopsis. Plant Physiol. 2003, 132, 1041–1052. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.; Miettinen, T.; Toivo, J.; Lampi, A.-M. Plant Sterols: Biosynthesis, Biological Function and Their Importance to Human Nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Vlahakis, C.; Hazebroek, J. Phytosterol Accumulation in Canola, Sunflower, and Soybean Oils: Effects of Genetics, Planting Location, and Temperature. J. Am. Oil Chem. Soc. 2000, 77, 49. [Google Scholar] [CrossRef]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Vear, F.; Merah, O. Sterol Content in Sunflower Seeds (Helianthus annuus L.) as Affected by Genotypes and Environmental Conditions. J. Fed. Am. Soc. Exp. Biol. 2010, 22, 3980–3991. [Google Scholar] [CrossRef]
- Pose, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis dry2/sqe1-5 Mutant Reveals a Central Role for Sterols in Drought Tolerance and Regulation of Reactive Oxygen Species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Sena, F.; Sotelo-Silveira, M.; Astrada, S.; Botella, M.A.; Malacrida, L.; Borsani, O. Spectral Phasor Analysis Reveals Altered Membrane Order and Function of Root Hair Cells in Arabidopsis dry2/sqe1-5 Drought Hypersensitive Mutant. Plant Physiol. Biochem. 2017, 119, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Ali, K.; Dahuja, A.; Tyagi, A. Role of Phytosterols in Drought Stress Tolerance in Rice. Plant Physiol. Biochem. 2015, 96, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Mawlong, I.; Ali, K.; Tyagi, A. Regulation of Phytosterol Biosynthetic Pathway During Drought Stress in Rice. Plant Physiol. Biochem. 2018, 129, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Aphalo, P.J.; Banaś, A.K.; Barnes, P.W.; Brelsford, C.C.; Jenkins, G.I.; Kotilainen, T.K.; Łabuz, J.; Martínez-Abaigar, J.; Morales, L.O.; et al. A Perspective on Ecologically Relevant Plant-UV Research and Its Practical Application. Atthew. Photochem. Photobiol. Sci. 2019, 18, 970. [Google Scholar] [CrossRef]
- Lake, J.A.; Field, K.J.; Davey, M.P.; Beerling, D.J.; Lomax, B.H. Metabolomic and Physiological Responses Reveal Multi-Phasic Acclimation of Arabidopsis thaliana to Chronic UV Radiation. Plant Cell Environ. 2009, 32, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Matus, J.T. Transcriptomic and Metabolomic Networks in The Grape Berry Illustrate That It Takes More Than Flavonoids to Fight Against Ultraviolet Radiation. Front Plant Sci. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Pontin, M.; Berli, F.; Bottini, R.; Piccoli, P. Metabolism of Terpenes in The Response of Grape (Vitis vinifera L.) Leaf Tissues to UV-B Radiation. Phytochemistry 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. β-Sitosterol Modulates Antioxidant Enzyme Response in RAW 264.7 Macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Ahmed, F.; Schenk, P.M. UV–C Radiation Increases Sterol Production in the Microalga Pavlova lutheri. Phytochemistry 2017, 139, 25–32. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methyl-Glyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Barbafieri, M.; Tassi, E. Brassinosteroids for Phytoremediation Application. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 403–437. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Wagatsuma, T.; Maejima, E.; Watanabe, T.; Khan, M.S.H.; Ishikawa, S. Significant role of the plasma membrane lipid bilayers in aluminum tolerance of plants. In Aluminum Stress Adaptation in Plants. Signaling and Communication in Plants; Panda, S.K., Baluška, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 99–124. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, 15. [Google Scholar] [CrossRef]
- Stillwell, W.; Cheng, Y.F.; Wassall, S.R. Plant Sterol Inhibition of Abscisic Acid-Induced Perturbations in Phospholipid Bilayers. Biochim. Biophys. Acta Biomembr. 1990, 1024, 345. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Chemical Elicitor-Induced Modulation and Antioxidant Metabolism and Enhancement of Secondary Metabolite Accumulation in Cell Suspension Cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci. 2016, 17, 399. [Google Scholar] [CrossRef]
- He, J.-X.; Fujioka, S.; Li, T.-C.; Kang, S.G.; Seto, H.; Takatsuto, S.; Yoshida, S.; Jang, J.-C. Sterols Regulate Development and Gene Expression in Arabidopsis. Plant Physiol. 2003, 131, 1258–1269. [Google Scholar] [CrossRef]
- Ashraf, R.; Bhatti, H.N. Stigmasterol. In A Centum of Valuable Plant Bioactives; Elsevier: Lahore, Pakistan, 2021; pp. 213–232. [Google Scholar]
- Awad, A.B.; Fink, C.S. Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Su, P.; Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A Comprehensive Review. Int. J. Pharm. Sci. Res. 2011, 2, 2259. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on Its Anti-Tumor Effect and Mechanism of Action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Yu, C.; Hu, P.F.; Bao, J.P.; Tang, J.L.; Wu, L.D. Stigmasterol Blocks Cartilage Degradation in Rabbit Model of Osteoarthritis. Acta Biochim. Pol. 2012, 59, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Jie, F.; Yang, X.; Yang, B.; Liu, Y.; Wu, L.; Lu, B. Stigmasterol Attenuates Inflammatory Response of Microglia via NF-kB and NLRP3 Signaling by AMPK Activation. Biomed Pharmacother. 2022, 153, 113317. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-Diabetic Activity of Stigmasterol from Soybean Oil by Targeting the GLUT4 Glucose Transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid Inhibitory, Antiperoxidative and Hypoglycemic Effects of Stigmasterol Isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.I.B.; Oliveira, S.M.; Tonello, R.; Rossato, M.F.; da Silva Brum, E.; Ferreira, J.; Trevisan, G. Anti-Nociceptive Effect of Stigmasterol in Mouse Models of Acute and Chronic Pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Parle, M.; Jindal, D.K.; Dhingra, S. Protective Effects of Stigmasterol Against Ketamine-Induced Psychotic Symptoms: Possible Behavioral, Biochemical and Histopathological Changes in Mice. Pharmacol. Rep. 2018, 70, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of Stigmasterol and β-Sitosterol Alters Lipid Metabolism and Alleviates NAFLD in Mice Fed a High-Fat Western-Style Diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The Ameliorating Effects of Stigmasterol on Scopolamine-Induced Memory Impairments in Mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef]
- Bansal, R.; Sen, S.S.; Muthuswami, R.; Madhubala, R. Stigmasterol as a Potential Biomarker for Amphotericin B Resistance in Leishmania donovani. J. Antimicrob. Chemother. 2020, 75, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Husche, C.; Schött, H.; Pettersson, H.; Lütjohann, D. Plant Sterol Oxidation Products—Analogs to Cholesterol Oxidation Products from Plant Origin? Biochimie 2013, 95, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingärtner, O.; Laufs, U.; et al. Plant Sterols The Better Cholesterol in Alzheimer’s Disease? A Mechanistical Study. J. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.J.; Lütjohann, D.; Abildayeva, K.; Vanmierlo, T.; Plösch, T.; Plat, J.; von Bergmann, K.; Groen, A.K.; Ramaekers, F.C.; Kuipers, F. Dietary Plant Sterols Accumulate in the Brain. Biochim. Biophys. Acta 2006, 1761, 445–453. [Google Scholar] [CrossRef]
- Khabazian, I.; Bains, J.; Williams, D.; Cheung, J.; Wilson, J.; Pasqualotto, B.; Pelech, S.; Andersen, R.; Wang, Y.T.; Liu, L. Isolation of Various Forms of Sterol β-d-Glucoside from The Seed of Cycas circinalis: Neurotoxicity and Implications for ALS-parkinsonism Dementia Complex. J. Neurochem. 2002, 82, 516–528. [Google Scholar] [CrossRef]
- Sultana, N.; Khalid, A. Phytochemical and Enzyme Inhibitory Studies on Indigenous Medicinal Plant Rhazya stricta. Nat. Prod. Res. 2010, 24, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wang, J.; Lai, Z.; Liang, S.; Zou, W.; Wang, J.; Zheng, D.; Li, Y.; He, Y.; Cheng, J.; et al. Arisaema Heterophyllum Blume Monomer Stigmasterol Targets PPARg and Inhibits the Viability and Tumorigenicity of Lung Adenocarcinoma Cells NCI-H1975. Evid.-Based Complement. Altern. Med. 2022, 2022, 5377690. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V.K. Elucidation of the Chemopreventive Role of Stigmasterol Against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Nayakavadi, S.R.V.; Zameer, F.; Dhananjaya, B.L.; Dhananjayaf, B.L.; Prasad, N.M.N. The Effect of a Plant Extract Enriched in Stigmasterol and β-Sitosterol on Glycaemic Status and Glucose Metabolism in Alloxan-Induced Diabetic Rats. Food Funct. 2016, 7, 3999–4011. [Google Scholar] [CrossRef] [PubMed]
- Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and Neuroprotective Activities of Stigmasterol against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Hannan, M.A.; Dash, R.; Choi, S.M.; Moon, I.S. The Potential LXRβ Agonist Stigmasterol Protects Against Hypoxia/Reoxygenation Injury by Modulating Mitophagy in Primary Hippocampal Neurons. Phytomedicine 2021, 81, 153415. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, V.; Scott, B.A.; Crowder, C.M. Autophagy Protects Against Hypoxic Injury in C. elegans. Autophagy 2008, 4, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Bhuiyan, M.M.H.; Moon, I.S. Stigmasterol Activates Cdc42-Arp2 and Erk1/2-Creb Pathways to Enrich Glutamatergic Synapses in Cultures of Brain Neurons. Nutr. Res. 2018, 56, 71–78. [Google Scholar] [CrossRef]
- Spencer, T.A.; Li, D.; Russel, J.S.; Collins, J.L.; Bledsoe, R.K.; Consler, T.G.; Moore, L.B.; Galardi, C.M.; McKee, D.D.; Moore, J.T. Pharmacophore Analysis of The Nuclear Oxysterol Receptor LXRα. J. Med. Chem. 2001, 44, 886–897. [Google Scholar] [CrossRef]
| Parameter | Marchantia polymorpha (Mapoly0103s0038) | Physcomitrium patens (Pp3c2_27810V3) | Physcomitrium patens (Pp3c1_11690V3) | Sphagnum fallax (Sphfalx0121s0046) |
|---|---|---|---|---|
| Genomic seq. (bp) | 4049 | 3340 | 3177 | 3517 |
| Transcript seq. (bp) | 3791 | 2853 | 2524 | 2619 |
| CDS seq. (bp) | 1518 | 1512 | 1509 | 1578 |
| Peptide seq. (aa) | 505 | 503 | 502 | 525 |
| Exon number | 1 | 2 | 2 | 3 |
| Stress | Stigmasterol Stress Response | References |
|---|---|---|
| Pathogen infection | Inoculation of Arabidopsis with Pseudomonas syringae induces stigmasterol biosynthesis. | [8] |
| The level of stigmasterol increases after H2O2 production by chloroplasts following contact with a pathogen. | [37] | |
| Infection with the fungal pathogen Verticillium dahliae upregulates the expression of GhCYP710A1, increasing the ratio of stigmasterol to β-sitosterol. | [58] | |
| Infection with the nematode Meloidogyne incognita reduces the ratio of stigmasterol to β-sitosterol. | [59,60] | |
| Salinization | Salt treatment of citrus leads to both the exclusion of Cl− and an increase in stigmasterol. | [67,72] |
| Halophytes growing under different levels of salinity and soil moisture actively synthesize stigmasterol. | [74] | |
| Exogenous applications of stigmasterol to germinating seeds of Vicia faba and Linum usitatissimum increase their tolerance to NaCl. | [75,76] | |
| Unfavorable temperatures | In sunflower seeds, heat stress increases the content of β-sitosterol and campesterol, while the level of stigmasterol remains unchanged. | [83] |
| Drought | In the seedlings of two varieties of Oryza sativa during drought stress, the tolerant cultivar accumulated more stigmasterol than the sensitive cultivar. | [86,87] |
| UV radiation | Treating leaves of the medicinal plant Withania somnifera with UV increases the level of stigmasterol. | [5] |
| Heavy metals | A decrease in stigmasterol can lower plasma membrane permeability, thereby increasing resistance to aluminum. | [20,97] |
| Stress phytohormones | The content of stigmasterol in the roots and leaves of Triticum aestivum increases following treatment with ABA and SA. | [50] |
| Medicinal Effect of Stigmasterol | Animal Model System(s) | Possible Mechanisms | Reference(s) |
|---|---|---|---|
| Anti-osteoarthritis | Rabbit | Stigmasterol could be related to six targets, namely, NCOA2, PGR, PTGS1, PTGS2, RXRA, and NR3C2. These primary target genes are linked to signaling pathways involved in cartilage degeneration in knee OA, including the PI3K–Akt signaling pathway and the TNF-α signaling pathway. | [108] |
| Anti-inflammatory | Mice and the BV2 cells | Stigmasterol therapy markedly inhibited the expression of pro-inflammatory mediators, including iNOS, IL-6, IL-1β, COX-2, and TNF-α, and upregulated the expression of anti-inflammatory cytokines such as IL-10 via negative regulation of p38MAPK expression and NF-kBp65 (suppression of p-IKB-α activation) in the joints. | [109] |
| Anti-diabetic | L6 cells | Stigmasterol binds to sirtuin 4, an NAD-dependent deacylase enzyme that down-regulates leucine and glutamate-dehydrogenase-induced insulin secretion. | [107,110] |
| Neuroprotective | Hippocampal neuronal cells, hippocampal neurons, cultures of brain neurons | The neuroprotective effects of stigmasterol are caused by its ability to reduce oxidative stress by decreasing the levels of ROS and lipid peroxidation and its ability to inhibit apoptosis. A study by Haque et al. showed that stigmasterol induces mitophagy in H/R (hypoxia/reoxygenation)-exposed hippocampal cells. It is known that the activation of autophagy plays an important neuroprotective role after hypoxic injury. Transcriptome studies have demonstrated that stigmasterol can function as a liver X receptor (LXR) agonist and protect neurons from pathological excitation by modulating N-methyl-D-aspartate (NMDA) signaling and promoting mitophagy. In silico studies of the interaction between stigmasterol and LXR β have shown that stigmasterol forms multiple hydrogen bonds with the GLU281 and ARG319 LXR β residues in an orientation similar to that of other endogenous steroid–nuclear receptor complexes. | [106,127,128,129,130,131,132] |
| Anti-tumor potential | |||
| Lung cancer | NCI-H1975 cells | Proliferation | [107,122] |
| Gall bladder cancer | Human gall bladder cancer cells | Apoptosis | [107,123] |
| Gastric cancer | Blood cells | Proliferation, apoptosis, autophagy | [107,124] |
| Ovarian cancer | ES2 and OV90 cells | Apoptosis, migration (ROS, calcium) | [107,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. https://doi.org/10.3390/ijms25158122
Valitova J, Renkova A, Beckett R, Minibayeva F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. International Journal of Molecular Sciences. 2024; 25(15):8122. https://doi.org/10.3390/ijms25158122
Chicago/Turabian StyleValitova, Julia, Albina Renkova, Richard Beckett, and Farida Minibayeva. 2024. "Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions" International Journal of Molecular Sciences 25, no. 15: 8122. https://doi.org/10.3390/ijms25158122
APA StyleValitova, J., Renkova, A., Beckett, R., & Minibayeva, F. (2024). Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. International Journal of Molecular Sciences, 25(15), 8122. https://doi.org/10.3390/ijms25158122






