Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis
Abstract
1. Introduction
2. Results
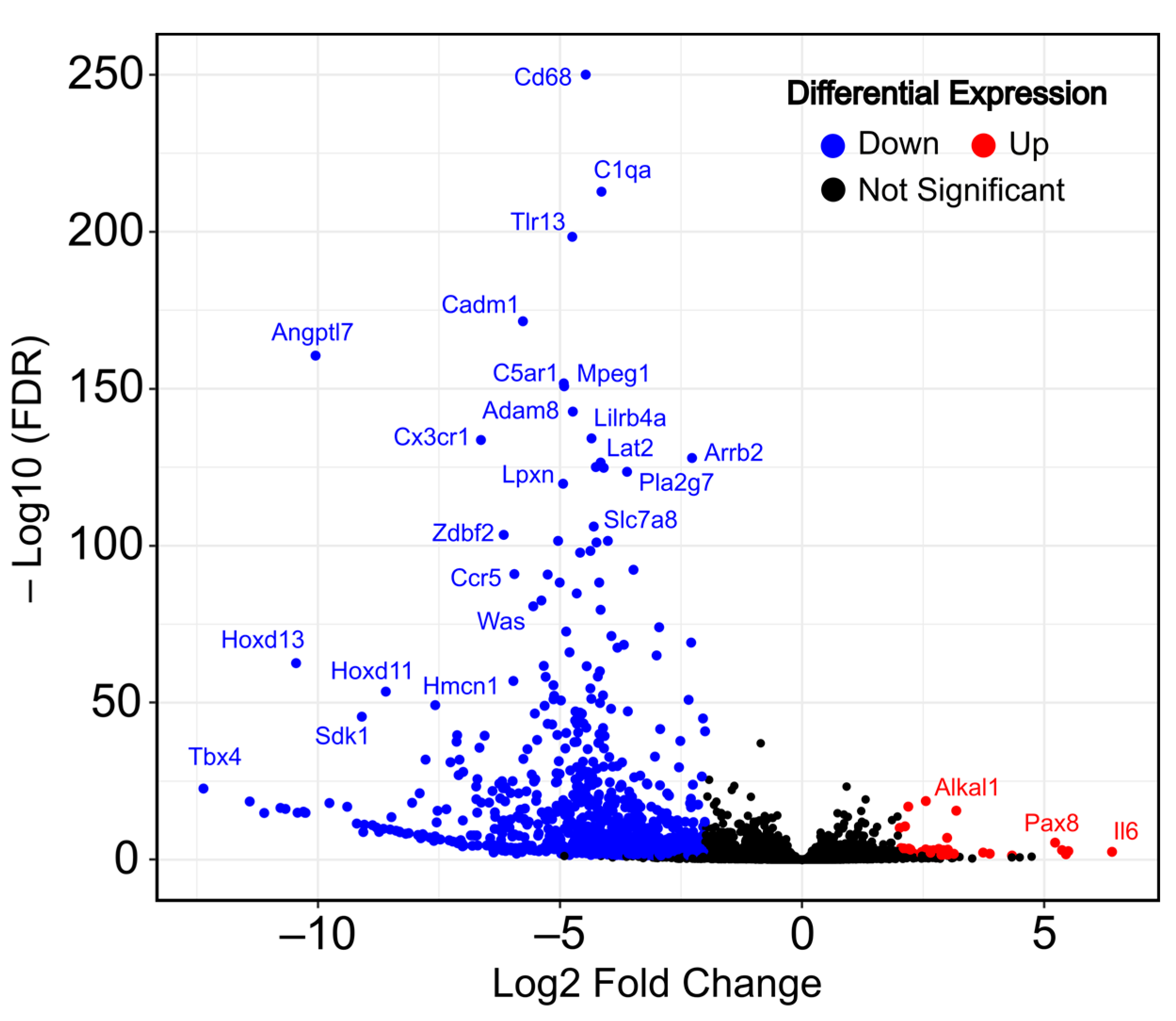
2.1. Transcriptome Changes during MEF Spontaneous Immortalization Show a Predominant Gene Downregulation Pattern
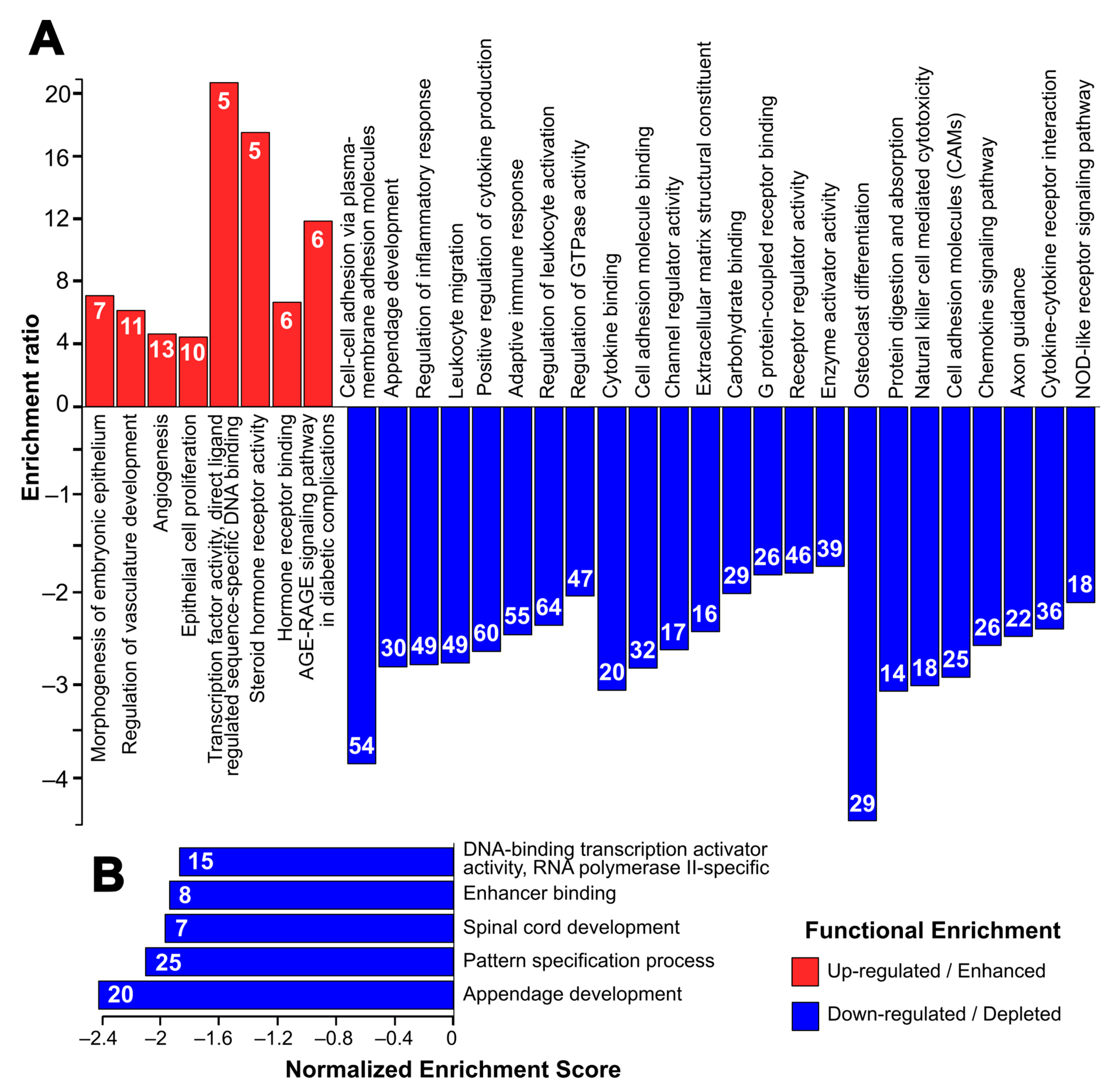
2.2. Spontaneous MEF Immortalization is Associated with the Upregulation of Genes Involved in Epithelial Cell Proliferation and the Downregulation of Cell Adhesion and Immune Response
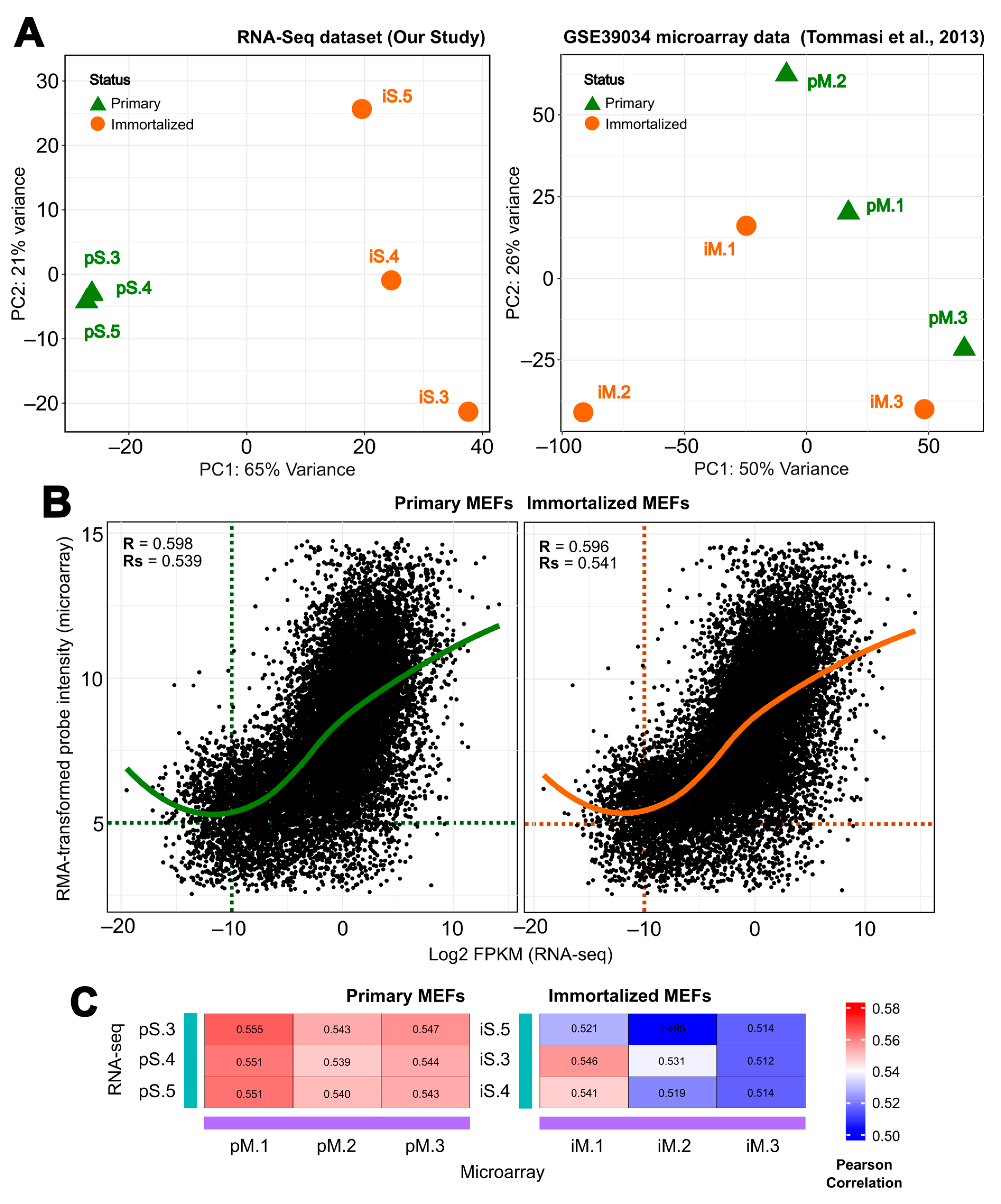
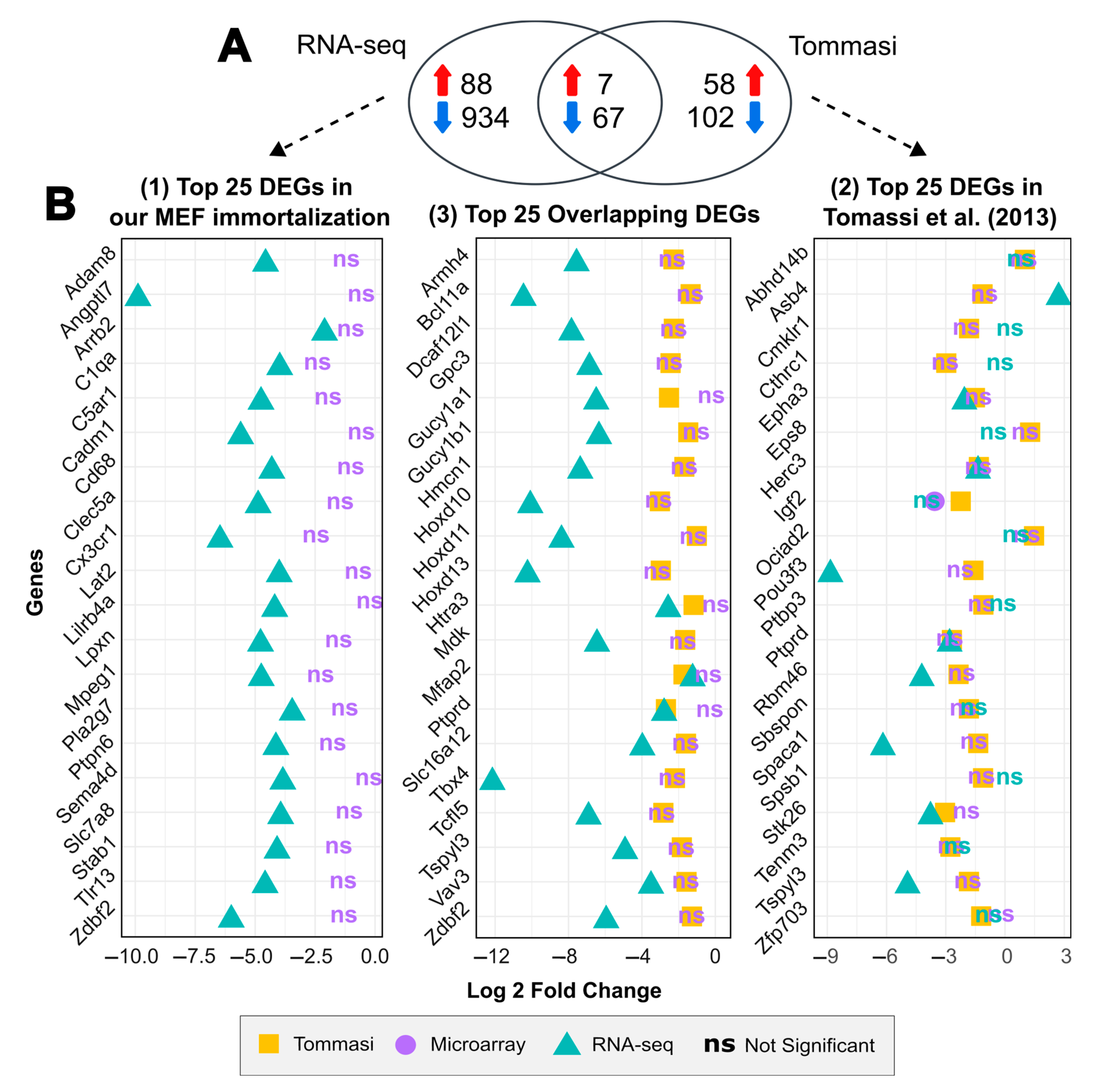
2.3. Comparative Analysis Shows a Positive Correlation between Gene Expression Levels for Both Immortalization Statuses between Different Spontaneous MEF Immortalization Studies, Revealing Novel Genes Differentially Expressed during this Process
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Immortalization
4.2. RNA Sequencing and Differential Expression Analysis
4.3. Protein–Protein Interaction (PPI) Analysis and Hub Gene Identification
4.4. Gene Ontology and Pathway Enrichment Analysis
4.5. Microarray Data
4.6. Microarray and RNA-seq Expression Data Correlation
4.7. Transcription Factor (TF)—Target Gene Regulatory Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The Essence of Senescence. Genes. Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. The Biology of Replicative Senescence. Eur. J. Cancer Part A 1997, 33, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Purcell, M.; Kruger, A.; Tainsky, M.A. Gene Expression Profiling of Replicative and Induced Senescence. Cell Cycle 2014, 13, 3927–3937. [Google Scholar] [CrossRef]
- Zheng, H.; Seit-Nebi, A.; Han, X.; Aslanian, A.; Tat, J.; Liao, R.; Yates, J.R.; Sun, P. A Posttranslational Modification Cascade Involving P38, Tip60, and PRAK Mediates Oncogene-Induced Senescence. Mol. Cell 2013, 50, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, N.R.; Shalgi, R.; Frankel, L.B.; Leucci, E.; Lees, M.; Klausen, M.; Pilpel, Y.; Nielsen, F.C.; Oren, M.; Lund, A.H. P53-Independent Upregulation of MiR-34a during Oncogene-Induced Senescence Represses MYC. Cell Death Differ. 2010, 17, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Villot, R.; Poirier, A.; Bakan, I.; Boulay, K.; Fernández, E.; Devillers, R.; Gama-Braga, L.; Tribouillard, L.; Gagné, A.; Duchesne, É.; et al. ZNF768 Links Oncogenic RAS to Cellular Senescence. Nat. Commun. 2021, 12, 4841. [Google Scholar] [CrossRef]
- Sedic, M.; Skibinski, A.; Brown, N.; Gallardo, M.; Mulligan, P.; Martinez, P.; Keller, P.J.; Glover, E.; Richardson, A.L.; Cowan, J.; et al. Haploinsufficiency for BRCA1 Leads to Cell-Type-Specific Genomic Instability and Premature Senescence. Nat. Commun. 2015, 6, 7505. [Google Scholar] [CrossRef] [PubMed]
- Wangsa, D.; Quintanilla, I.; Torabi, K.; Vila-Casadesús, M.; Ercilla, A.; Klus, G.; Yuce, Z.; Galofré, C.; Cuatrecasas, M.; Lozano, J.J.; et al. Near-Tetraploid Cancer Cells Show Chromosome Instability Triggered by Replication Stress and Exhibit Enhanced Invasiveness. FASEB J. 2018, 32, 3502–3517. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- de Bardet, J.C.; Cardentey, C.R.; González, B.L.; Patrone, D.; Mulet, I.L.; Siniscalco, D.; Robinson-Agramonte, M.D.L.A. Cell Immortalization: In Vivo Molecular Bases and In Vitro Techniques for Obtention. BioTech 2023, 12, 14. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, J.Y.; Yun, J.Y.; Ahn, D.K.; Lee, J.Y. Immortalization of Human Embryonic Fibroblasts by Overexpression of C-Myc and Simian Virus 40 Large T Antigen. Exp. Mol. Med. 2001, 33, 293–298. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.; Li, J.; Li, J.; Cui, L.; Dong, J.; Meng, X.; Qian, C.; Wang, H. Immortalization Effect of SV40T Lentiviral Vectors on Canine Corneal Epithelial Cells. BMC Vet. Res. 2022, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Pestana, A.; Vinagre, J.; Sobrinho-Simões, M.; Soares, P. TERT Biology and Function in Cancer: Beyond Immortalisation. J. Mol. Endocrinol. 2017, 58, R129–R146. [Google Scholar] [CrossRef]
- Pino-Barrio, M.J.; García-García, E.; Menéndez, P.; Martínez-Serrano, A. V-Myc Immortalizes Human Neural Stem Cells in the Absence of Pluripotency-Associated Traits. PLoS ONE 2015, 10, e118499. [Google Scholar] [CrossRef] [PubMed]
- Foudah, D.; Redaelli, S.; Donzelli, E.; Bentivegna, A.; Miloso, M.; Dalprà, L.; Tredici, G. Monitoring the Genomic Stability of in Vitro Cultured Rat Bone-Marrow-Derived Mesenchymal Stem Cells. Chromosome Res. 2009, 17, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Méndez-López, L.F.; Davila-Velderrain, J.; Domínguez-Hüttinger, E.; Enríquez-Olguín, C.; Martínez-García, J.C.; Alvarez-Buylla, E.R. Gene Regulatory Network Underlying the Immortalization of Epithelial Cells. BMC Syst. Biol. 2017, 11, 24. [Google Scholar] [CrossRef]
- Xu, J. Preparation, Culture, and Immortalization of Mouse Embryonic Fibroblasts. Curr. Protoc. Mol. Biol. 2005, 70, 28.1.1–28.1.8. [Google Scholar] [CrossRef]
- Christman, S.A.; Kong, B.W.; Landry, M.M.; Kim, H.; Foster, D.N. Contributions of Differential P53 Expression in the Spontaneous Immortalization of a Chicken Embryo Fibroblast Cell Line. BMC Cell Biol. 2006, 7, 12–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amand, M.M.; Hanover, J.A.; Shiloach, J. A Comparison of Strategies for Immortalizing Mouse Embryonic Fibroblasts. J. Biol. Methods 2016, 3, e41. [Google Scholar] [CrossRef]
- Rittling, S.R. Clonal Nature of Spontaneously Immortalized 3T3 Cells. Exp. Cell Res. 1996, 229, 7–13. [Google Scholar] [CrossRef]
- Sharpless, N.E. Preparation and Immortalization of Primary Murine Cells. In Cell Biology: A Laboratory Handbook; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Zindy, F.; Eischen, C.M.; Randle, D.H.; Kamijo, T.; Cleveland, J.L.; Sherr, C.J.; Roussel, M.F. Myc Signaling via the ARF Tumor Suppressor Regulates P53-Dependent Apoptosis and Immortalization. Genes. Dev. 1998, 12, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Zindy, F.; Quelle, D.E.; Roussel, M.F.; Sherr, C.J. Expression of the P16(INK4a) Tumor Suppressor versus Other INK4 Family Members during Mouse Development and Aging. Oncogene 1997, 15, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Evangelista, M.; Simili, M.; Mariani, L.; Pitto, L.; Rainaldi, G. Immortalization of MEF Is Characterized by the Deregulation of Specific MiRNAs with Potential Tumor Suppressor Activity. Aging 2011, 3, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Polo, J.M.; Stadtfeld, M.; Maherali, N.; Kulalert, W.; Walsh, R.M.; Khalil, A.; Rheinwald, J.G.; Hochedlinger, K. Immortalization Eliminates a Roadblock during Cellular Reprogramming into IPS Cells. Nature 2009, 460, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Ouelle, D.E.; Zindy, F.; Ashmun, R.A.; Sherr, C.J. Alternative Reading Frames of the INK4a Tumor Suppressor Gene Encode Two Unrelated Proteins Capable of Inducing Cell Cycle Arrest. Cell 1995, 83, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, Y.; Fujimori, H.; Fukuda, H.; Inase, A.; Shinohe, K.; Yoshioka, Y.; Shikanai, M.; Ichijima, Y.; Unno, J.; Mizutani, S.; et al. Onset of Quiescence Following P53 Mediated Down-Regulation of H2AX in Normal Cells. PLoS ONE 2011, 6, e23432. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Cicalese, A.; Fumagalli, M.; Dobreva, M.; Verrecchia, A.; Pelicci, P.G.; Di Fagagna, F.D.A. DNA Damage Response Activation in Mouse Embryonic Fibroblasts Undergoing Replicative Senescence and Following Spontaneous Immortalization. Cell Cycle 2008, 7, 3601–3606. [Google Scholar] [CrossRef]
- Bailey, S.M. Editorial: Hallmark of Cancer: Replicative Immortality. Front. Oncol. 2023, 13, 1204094. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Cragg, M.S. Three Steps to the Immortality of Cancer Cells: Senescence, Polyploidy and Self-Renewal. Cancer Cell Int. 2013, 13, 92. [Google Scholar] [CrossRef]
- Duesberg, P.; McCormack, A. Immortality of Cancers. Cell Cycle 2013, 12, 783–802. [Google Scholar] [CrossRef][Green Version]
- Schulz, W.A. Molecular Biology of Human Cancers; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Todaro, G.J.; Green, H. Quantitative Studies of the Growth of Mouse Embryo Cells in Culture and Their Development into Established Lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- vom Brocke, J.; Schmeiser, H.H.; Reinbold, M.; Hollstein, M. MEF Immortalization to Investigate the Ins and Outs of Mutagenesis. Carcinogenesis 2006, 27, 2141–2147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olivier, M.; Weninger, A.; Ardin, M.; Huskova, H.; Castells, X.; Vallée, M.P.; McKay, J.; Nedelko, T.; Muehlbauer, K.R.; Marusawa, H.; et al. Modelling Mutational Landscapes of Human Cancers in Vitro. Sci. Rep. 2014, 4, 4482. [Google Scholar] [CrossRef] [PubMed]
- Rogan, E.M.; Bryan, T.M.; Hukku, B.; Maclean, K.; Chang, A.C.-M.; Moy, E.L.; Englezou, A.; Warneford, S.G.; Dalla-Pozza, L.; Reddel, R.R. Alterations in p53 and p16INK4 Expression and Telomere Length during Spontaneous Immortalization of Li-Fraumeni Syndrome Fibroblasts. Mol. Cell Biol. 1995, 15, 4745–4753. [Google Scholar] [CrossRef] [PubMed]
- Fridman, A.L.; Tainsky, M.A. Critical Pathways in Cellular Senescence and Immortalization Revealed by Gene Expression Profiling. Oncogene 2008, 27, 5975–5987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Meng, L.; Hu, H.; Wang, X.; Shi, F.; Wang, Y.; Li, Q.; Lin, A. Spontaneously Immortalised Bovine Mammary Epithelial Cells Exhibit a Distinct Gene Expression Pattern from the Breast Cancer Cells. BMC Cell Biol. 2010, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, L.; Heidari, M.; Sun, S.; Chang, S.; Xie, Q.; Ai, Y.; Dong, K.; Zhang, H. Small RNA Deep Sequencing Revealed MicroRNAs’ Involvement in Modulating Cellular Senescence and Immortalization State. Poult. Sci. 2023, 102, 102474. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Margolin, A.A.; Stolovitzky, G.; Klein, U.; Dalla-Favera, R.; Califano, A. Reverse Engineering of Regulatory Networks in Human B Cells. Nat. Genet. 2005, 37, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, Y.; Hao, H.; Wang, Y.; Zhou, Z.; Wang, Z.; Chu, X. Screening Hub Genes as Prognostic Biomarkers of Hepatocellular Carcinoma by Bioinformatics Analysis. Cell Transplant. 2019, 28, 76S–86S. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lim, J.; Wang, X.; Liang, F.; Xiao, G. Enhanced Construction of Gene Regulatory Networks Using Hub Gene Information. BMC Bioinform. 2017, 18, 186. [Google Scholar] [CrossRef]
- Tommasi, S.; Zheng, A.; Weninger, A.; Bates, S.E.; Li, X.A.; Wu, X.; Hollstein, M.; Besaratinia, A. Mammalian Cells Acquire Epigenetic Hallmarks of Human Cancer during Immortalization. Nucleic Acids Res. 2013, 41, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Klaus, B.; Reisenauer, S. An End to End Workflow for Differential Gene Expression Using Affymetrix Microarrays. F1000Res 2018, 5, 1384. [Google Scholar] [CrossRef]
- Hammelman, J.; Patel, T.; Closser, M.; Wichterle, H.; Gifford, D. Ranking Reprogramming Factors for Cell Differentiation. Nat. Methods 2022, 19, 812–822. [Google Scholar] [CrossRef]
- Hu, H.; Miao, Y.R.; Jia, L.H.; Yu, Q.Y.; Zhang, Q.; Guo, A.Y. AnimalTFDB 3.0: A Comprehensive Resource for Annotation and Prediction of Animal Transcription Factors. Nucleic Acids Res. 2019, 47, D33–D38. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Bates, S.E.; Besaratinia, A. Spontaneous and Photosensitization-Induced Mutations in Primary Mouse Cells Transitioning through Senescence and Immortalization. J. Biol. Chem. 2020, 295, 9974–9985. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Jiang, X.; Li, W.; Zhai, C.; Shang, F.; Chen, S.; Zhao, Z.; Yu, W. TRIM67 Inhibits Tumor Proliferation and Metastasis by Mediating MAPK11 in Colorectal Cancer. J. Cancer 2020, 11, 6025–6037. [Google Scholar] [CrossRef]
- Song, X.; Dong, C.; Man, X. Phosphorylated MAPK11 Promotes the Progression of Clear Cell Renal Cell Carcinoma by Maintaining RUNX2 Protein Abundance. J. Cell Mol. Med. 2023, 27, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, R.; Yuan, L.; Xu, M.; Zhuang, H.; Li, Y.; Zhang, Y.; Lin, N. Circ_0001955 Facilitates Hepatocellular Carcinoma (HCC) Tumorigenesis by Sponging MiR-516a-5p to Release TRAF6 and MAPK11. Cell Death Dis. 2019, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, M.; Ostalé, C.M.; van der Burg, L.R.; Galán-Martínez, J.; Hardwick, J.C.H.; López-Pérez, R.; Hawinkels, L.J.A.C.; Stamatakis, K.; Fresno, M. DUSP10 Is a Regulator of YAP1 Activity Promoting Cell Proliferation and Colorectal Cancer Progression. Cancers 2019, 11, 1767. [Google Scholar] [CrossRef]
- Nomura, M.; Shiiba, K.I.; Katagiri, C.; Kasugai, I.; Masuda, K.; Sato, I.; Sato, M.; Kakugawa, Y.; Nomura, E.; Hayashi, K.; et al. Novel Function of MKP-5/DUSP10, a Phosphatase of Stress-Activated Kinases, on ERK-Dependent Gene Expression, and Upregulation of Its Gene Expression in Colon Carcinomas. Oncol. Rep. 2012, 28, 931–936. [Google Scholar]
- Jiménez-Martínez, M.; Stamatakis, K.; Fresno, M. The Dual-Specificity Phosphatase 10 (DUSP10): Its Role in Cancer, Inflammation, and Immunity. Int. J. Mol. Sci. 2019, 20, 1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Campbell, H.G.; Wiles, A.K.; Eccles, M.R.; Reddel, R.R.; Braithwaite, A.W.; Royds, J.A. PAX8 Regulates Telomerase Reverse Transcriptase and Telomerase RNA Component in Glioma. Cancer Res. 2008, 68, 5724–5732. [Google Scholar] [CrossRef]
- Li, C.G.; Nyman, J.E.; Braithwaite, A.W.; Eccles, M.R. PAX8 Promotes Tumor Cell Growth by Transcriptionally Regulating E2F1 and Stabilizing RB Protein. Oncogene 2011, 30, 4824–4834. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Kaul, S.C.; Mitsui, Y.; Wadhwa, R. Enhanced Expression of Multiple Forms of VEGF Is Associated with Spontaneous Immortalization of Murine Fibroblasts. BBA—Mol. Cell Res. 1994, 1224, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Xu, F.; Xu, F.; Wei, M.; Ge, Y.; Chenge, S. CADM1 Inhibits Ovarian Cancer Cell Proliferation and Migration by Potentially Regulating the PI3K/Akt/MTOR Pathway. Biomed. Pharmacother. 2020, 123, 109717. [Google Scholar] [CrossRef]
- Bostanabad, S.Y.; Noyan, S.; Dedeoglu, B.G.; Gurdal, H. Overexpression of β-Arrestins Inhibits Proliferation and Motility in Triple Negative Breast Cancer Cells. Sci. Rep. 2021, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shin, J.H.; Kim, M.J.; Kang, Y.; Lee, J.S.; Son, J.; Jeong, S.K.; Kim, D.; Kim, D.H.; Chun, E.; et al. Β-arrestin 2 Negatively Regulates Lung Cancer Progression by Inhibiting the TRAF6 Signaling Axis for NF-ΚB Activation and Autophagy Induced by TLR3 and TLR4. Cell Death Dis. 2023, 14, 422. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Irie-Sasaki, J.; Horie, Y.; Bachmaler, K.; Fata, J.E.; Li, M.; Suzuki, A.; Bouchard, D.; Ho, A.; Redston, M.; et al. Colorectal Carcinomas in Mice Lacking the Catalytic Subunit of PI(3)K. Nature 2000, 406, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Semba, S.; Itoh, N.; Ito, M.; Youssef, E.M.; Harada, M.; Moriya, T.; Kimura, W.; Yamakawa, M. Down-Regulation of PIK3CG, a Catalytic Subunit of Phosphatidylinositol 3-OH Kinase, by CpG Hypermethylation in Human Colorectal Carcinoma. Clin. Cancer Res. 2002, 8, 3824–3831. [Google Scholar]
- Singhal, P.K.; Sassi, S.; Lan, L.; Au, P.; Halvorsen, S.C.; Fukumura, D.; Jain, R.K.; Seed, B. Mouse Embryonic Fibroblasts Exhibit Extensive Developmental and Phenotypic Diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 122–127. [Google Scholar] [CrossRef]
- Zhao, S.; Fung-Leung, W.P.; Bittner, A.; Ngo, K.; Liu, X. Comparison of RNA-Seq and Microarray in Transcriptome Profiling of Activated T Cells. PLoS ONE 2014, 9, e78644. [Google Scholar] [CrossRef]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2019, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell Adhesion Molecules and Their Relation to (Cancer) Cell Stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Mei, C.M.; Shen, S. The Roles of Cell Adhesion Molecules in Tumor Suppression and Cell Migration: A New Paradox. Cell Adh Migr. 2009, 3, 334–336. [Google Scholar]
- Nair, K.S.; Naidoo, R.; Chetty, R. Expression of Cell Adhesion Molecules in Oesophageal Carcinoma and Its Prognostic Value. J. Clin. Pathol. 2005, 58, 343–351. [Google Scholar] [CrossRef]
- Okegawa, T.; Li, Y.; Pong, R.C.; Hsieh, J.T. Cell Adhesion Proteins as Tumor Suppressors. J. Urol. 2002, 167, 1836–1843. [Google Scholar] [CrossRef]
- Li, Z.; Xu, T.; Li, X.; Wang, T.; Tang, G.; Zhao, H.; Zhao, Y.; Ye, K.; Gao, P. Viral Integration Promotes SV40T-Induced Immortalization by Disturbing the Expression of DNA/Chromosome- and ECM-Associated Functional Genes. Gene 2024, 896, 148060. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.M.; Xie, Y.M.; Liao, L.D.; Li, L.Y.; Chen, B.; Xie, J.J.; Xu, L.Y.; Li, E.M. Biological Characterization of Three Immortalized Esophageal Epithelial Cell Lines. Mol. Med. Rep. 2016, 14, 4802–4810. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shen, M.; Wang, X.; Zhang, S.; Yu, S.; Chen, G.; Gu, X.; Ding, F. Comparative Proteomic Analysis of Primary Schwann Cells and a Spontaneously Immortalized Schwann Cell Line RSC 96: A Comprehensive Overview with a Focus on Cell Adhesion and Migration Related Proteins. J. Proteome Res. 2012, 11, 3186–3198. [Google Scholar] [CrossRef]
- Zhou, L.N.; Hua, X.; Deng, W.Q.; Wu, Q.N.; Mei, H.; Chen, B. PCDH10 Interacts with HTERT and Negatively Regulates Telomerase Activity. Medicine 2015, 94, e2230. [Google Scholar] [CrossRef]
- Ying, J.; Li, H.; Seng, T.J.; Langford, C.; Srivastava, G.; Tsao, S.W.; Putti, T.; Murray, P.; Chan, A.T.C.; Tao, Q. Functional Epigenetics Identifies a Protocadherin PCDH10 as a Candidate Tumor Suppressor for Nasopharyngeal, Esophageal and Multiple Other Carcinomas with Frequent Methylation. Oncogene 2006, 25, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, W.; Xie, J.; Wang, Y.; Tang, A.; Li, X.; Ye, J.; Gui, Y.; Cai, Z. Epigenetic Inactivation of PCDH10 in Human Prostate Cancer Cell Lines. Cell Biol. Int. 2011, 35, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, H.; Zhang, C.; Wu, Q.; Shao, Y.; Zhang, J.; Guan, M.; Wan, J.; Zhang, W. High-Resolution Melting Analysis of PCDH10 Methylation Levels in Gastric, Colorectal and Pancreatic Cancers. Neoplasma 2010, 57, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.; Peng, X.; Li, Y.; Liu, Q.; Chen, J. Nuclear Factor-ΚB Is Involved in the Protocadherin-10-Mediated pro-Apoptotic Effect in Multiple Myeloma. Mol. Med. Rep. 2014, 10, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Gao, X.; Luo, W.; Ou, K.; Lu, H.; Liu, H.; Zhuang, Q. Expression of CHL1 in Clear Cell Renal Cell Carcinoma and Its Association with Prognosis. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, M.; Pagnan, G.; Marimpietri, D.; Cangelosi, D.; Cilli, M.; Benedetti, M.C.; Boldrini, R.; Garaventa, A.; Frassoni, F.; Eva, A.; et al. CHL1 Gene Acts as a Tumor Suppressor in Human Neuroblastoma. Oncotarget 2018, 9, 25903–25921. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, C.; Fu, L.; Zhu, C.L.; Xiang, Y.Q.; Jiang, L.X.; Chen, Q.; Liu, W.M.; Chen, J.N.; Zhang, L.Y.; et al. CHL1 Suppresses Tumor Growth and Metastasis in Nasopharyngeal Carcinoma by Repressing PI3K/AKT Signaling Pathway via Interaction with Integrin Β1 and Merlin. Int. J. Biol. Sci. 2019, 15, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Li, S.; Zhu, Y.; Dong, X.; Wang, R.; Jing, F. CHL1 Inhibits Cell Proliferation, Migration and Invasion by Regulating the NF-κB Signaling Pathway in Colorectal Cancer. Exp. Ther. Med. 2024, 27, 165. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N. Tumor Suppressor Gene E-Cadherin and Its Role in Normal and Malignant Cells. Cancer Cell Int. 2003, 3, 17. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-Cadherin-Integrin Crosstalk in Cancer Invasion and Metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef]
- Zohn, I.E.; Li, Y.; Skolnik, E.Y.; Anderson, K.V.; Han, J.; Niswander, L. P38 and a P38-Interacting Protein Are Critical for Downregulation of E-Cadherin during Mouse Gastrulation. Cell 2006, 125, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, X.; Zhu, Y.; Yao, Z.; Zhao, W.; Zhu, Y.; Sun, F.; Mu, X.; Wang, Y.; He, W.; et al. IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer. Biomed. Res. Int. 2021, 2021, 5535578. [Google Scholar] [CrossRef]
- Shen, T.N.Y.; Kanazawa, S.; Kado, M.; Okada, K.; Luo, L.; Hayashi, A.; Mizuno, H.; Tanaka, R. Interleukin-6 Stimulates Akt and P38 MAPK Phosphorylation and Fibroblast Migration in Non-Diabetic but Not Diabetic Mice. PLoS ONE 2017, 12, e178232. [Google Scholar] [CrossRef]
- Zu, L.; He, J.; Zhou, N.; Zeng, J.; Zhu, Y.; Tang, Q.; Jin, X.; Zhang, L.; Xu, S. The Profile and Clinical Significance of ITGB2 Expression in Non-Small-Cell Lung Cancer. J. Clin. Med. 2022, 11, 6421. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Collins, S.; Cuddington, B.; Mossman, K. The Importance of Physiologically Relevant Cell Lines for Studying Virus-Host Interactions. Viruses 2016, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Barrett, J.W.; Ma, Y.; Dekaban, G.A.; McFadden, G. Induction of Alpha/Beta Interferon by Myxoma Virus Is Selectively Abrogated When Primary Mouse Embryo Fibroblasts Become Immortalized. J. Virol. 2009, 83, 5928–5932. [Google Scholar] [CrossRef]
- Fu, J.Q.; Chen, Z.; Hu, Y.J.; Fan, Z.H.; Guo, Z.X.; Liang, J.Y.; Ryu, B.M.; Ren, J.L.; Shi, X.J.; Li, J.; et al. A Single Factor Induces Neuronal Differentiation to Suppress Glioma Cell Growth. CNS Neurosci. Ther. 2019, 25, 486–495. [Google Scholar] [CrossRef]
- Milet, C.; MacZkowiak, F.; Roche, D.D.; Monsoro-Burq, A.H. Pax3 and Zic1 Drive Induction and Differentiation of Multipotent, Migratory, and Functional Neural Crest in Xenopus Embryos. Proc. Natl. Acad. Sci. USA 2013, 110, 5528–5533. [Google Scholar] [CrossRef]
- Thottappillil, N.; Gomez-Salazar, M.A.; Xu, M.; Qin, Q.; Xing, X.; Xu, J.; Broderick, K.; Yea, J.H.; Archer, M.; Ching-Yun Hsu, G.; et al. ZIC1 Dictates Osteogenesis Versus Adipogenesis in Human Mesenchymal Progenitor Cells Via a Hedgehog Dependent Mechanism. Stem Cells 2023, 41, 862–876. [Google Scholar] [CrossRef]
- Rodriǵuez-Rodero, S.; Ferńandez, A.F.; Fernández-Morera, J.L.; Castro-Santos, P.; Bayon, G.F.; Ferrero, C.; Urdinguio, R.G.; Gonzalez-Marquez, R.; Suarez, C.; Fernández-Vega, I.; et al. DNA Methylation Signatures Identify Biologically Distinct Thyroid Cancer Subtypes. J. Clin. Endocrinol. Metab. 2013, 98, 2811–2821. [Google Scholar] [CrossRef]
- Wang, L.J.; Jin, H.C.; Wang, X.; Lam, E.K.Y.; Zhang, J.B.; Liu, X.; Chan, F.K.L.; Si, J.M.; Sung, J.J.Y. ZIC1 Is Downregulated through Promoter Hypermethylation in Gastric Cancer. Biochem. Biophys. Res. Commun. 2009, 379, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Chen, S.; Zhong, J.; Wang, X.; Lam, E.K.Y.; Liu, X.; Zhang, J.; Zhou, T.; Yu, J.; Si, J.; et al. ZIC1 Is Downregulated through Promoter Hypermethylation, and Functions as a Tumor Suppressor Gene in Colorectal Cancer. PLoS ONE 2011, 6, e16916. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, S.; Xue, M.; Du, Q.; Cai, J.; Jin, H.; Si, J.; Wang, L. ZIC1 Modulates Cell-Cycle Distributions and Cell Migration through Regulation of Sonic Hedgehog, PI3K and MAPK Signaling Pathways in Gastric Cancer. BMC Cancer 2012, 12, 290. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Yang, J.; Wang, J.; Zhang, R.; Li, J.; Zhang, R. CircMTO1 Suppresses Hepatocellular Carcinoma Progression via the MiR-541-5p/ZIC1 Axis by Regulating Wnt/β-Catenin Signaling Pathway and Epithelial-to-Mesenchymal Transition. Cell Death Dis. 2022, 13, 12. [Google Scholar] [CrossRef]
- Ge, Q.; Hu, Y.; He, J.; Chen, F.; Wu, L.; Tu, X.; Qi, Y.; Zhang, Z.; Xue, M.; Chen, S.; et al. Zic1 Suppresses Gastric Cancer Metastasis by Regulating Wnt/β-Catenin Signaling and Epithelial-Mesenchymal Transition. FASEB J. 2020, 34, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Zhao, Y.; Yang, Q.; Liu, W.; Guan, H.; Lv, S.; Ji, M.; Shi, B.; Hou, P. ZIC1 Is a Putative Tumor Suppressor in Thyroid Cancer by Modulating Major Signaling Pathways and Transcription Factor FOXO3a. J. Clin. Endocrinol. Metab. 2014, 99, E1163–E1172. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Cheng, Y.; Yan, L.L.; An, R.; Wang, X.Y.; Wang, H.Y. Exploring Dna Methylation Profiles Altered in Cryptogenic Hepatocellular Carcinomas by High-Throughput Targeted Dna Methylation Sequencing: A Preliminary Study for Cryptogenic Hepatocellular Carcinoma. Onco Targets Ther. 2020, 13, 9901–9916. [Google Scholar] [CrossRef]
- Han, Z.; Jia, J.; Lv, Y.; Wang, R.; Cao, K. Transcriptional Expression of ZICs as an Independent Indicator of Survival in Gliomas. Sci. Rep. 2021, 11, 17532. [Google Scholar] [CrossRef] [PubMed]
- Diamand, K.E.M.; Barratt, K.S.; Arkell, R.M. Overview of Rodent Zic Genes. Adv. Exp. Med. Biol. 2018, 1046, 179–207. [Google Scholar]
- Houtmeyers, R.; Souopgui, J.; Tejpar, S.; Arkell, R. The ZIC Gene Family Encodes Multi-Functional Proteins Essential for Patterning and Morphogenesis. Cell. Mol. Life Sci. 2013, 70, 3791–3811. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Baba, Y.; Nakauchi, H.; Mizota, A.; Watanabe, S. The Role of Zic Family Zinc Finger Transcription Factors in the Proliferation and Differentiation of Retinal Progenitor Cells. Biochem. Biophys. Res. Commun. 2011, 415, 42–47. [Google Scholar] [CrossRef]
- Zha, Y.; Ding, E.; Yang, L.; Mao, L.; Wang, X.; McCarthy, B.A.; Huang, S.; Ding, H.F. Functional Dissection of HOXD Cluster Genes in Regulation of Neuroblastoma Cell Proliferation and Differentiation. PLoS ONE 2012, 7, e40728. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.H.; Wang, H.Y.; Lai, Y.; Zhong, W.; Liang, W.L.; Yan, F.D.; Yu, Z.; Chen, J.K.; Lin, Y. Epigenetic Inactivation of HOXD10 Is Associated with Human Colon Cancer via Inhibiting the RHOC/AKT/MAPK Signaling Pathway. Cell Commun. Signal. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Niu, Y.; Fan, B.; Zhang, A. Upregulation of Homeobox D10 Expression Suppresses Invasion and Migration of Clear Cell Renal Cell Carcinoma through Targeting of E-Cadherin. Mol. Biol. Rep. 2022, 49, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Malyankar, U.M.; Rittling, S.R.; Connor, A.; Denhard, D.T. The Mitogen-Regulated Protein/Proliferin Transcript Is Degraded in Primary Mouse Embryo Fibroblast but Not 3T3 Nuclei: Altered RNA Processing Correlates with Immortalization. Proc. Natl. Acad. Sci. USA 1994, 91, 335–339. [Google Scholar] [CrossRef]
- Kondoh, H.; Lleonart, M.E.; Gil, J.; Wang, J.; Degan, P.; Peters, G.; Martinez, D.; Carnero, A.; Beach, D. Glycolytic Enzymes Can Modulate Cellular Life Span. Cancer Res. 2005, 65, 177–185. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Zhang, T.; Braun, U.; Leitges, M. PKD3 Deficiency Causes Alterations in Microtubule Dynamics during the Cell Cycle. Cell Cycle 2016, 15, 1844–1854. [Google Scholar] [CrossRef]
- Zhang, T.; Sell, P.; Braun, U.; Leitges, M. PKD1 Protein Is Involved in Reactive Oxygen Species-Mediated Mitochondrial Depolarization in Cooperation with Protein Kinase Cδ (PKCδ). J. Biol. Chem. 2015, 290, 10472–10485. [Google Scholar] [CrossRef]
- Hirst, M. Gene Targeting: A Practical Approach. J. Med. Genet. 1994, 31, 821. [Google Scholar] [CrossRef][Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2–27. [Google Scholar] [CrossRef]
- Ma, H.; He, Z.; Chen, J.; Zhang, X.; Song, P. Identifying of Biomarkers Associated with Gastric Cancer Based on 11 Topological Analysis Methods of CytoHubba. Sci. Rep. 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Henze, H.; Hüttner, S.S.; Koch, P.; Schüler, S.C.; Groth, M.; von Eyss, B.; von Maltzahn, J. Denervation Alters the Secretome of Myofibers and Thereby Affects Muscle Stem Cell Lineage Progression and Functionality. NPJ Regen. Med. 2024, 9, 10. [Google Scholar] [CrossRef]
- Han, H.; Cho, J.W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: An Expanded Reference Database of Human and Mouse Transcriptional Regulatory Interactions. Nucleic Acids Res. 2018, 46, D380–D386. [Google Scholar] [CrossRef]
- Liska, O.; Bohár, B.; Hidas, A.; Korcsmáros, T.; Papp, B.; Fazekas, D.; Ari, E. TFLink: An Integrated Gateway to Access Transcription Factor-Target Gene Interactions for Multiple Species. Database 2022, 2022, baac083. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loaiza-Moss, J.; Braun, U.; Leitges, M. Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis. Int. J. Mol. Sci. 2024, 25, 8116. https://doi.org/10.3390/ijms25158116
Loaiza-Moss J, Braun U, Leitges M. Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis. International Journal of Molecular Sciences. 2024; 25(15):8116. https://doi.org/10.3390/ijms25158116
Chicago/Turabian StyleLoaiza-Moss, Jocshan, Ursula Braun, and Michael Leitges. 2024. "Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis" International Journal of Molecular Sciences 25, no. 15: 8116. https://doi.org/10.3390/ijms25158116
APA StyleLoaiza-Moss, J., Braun, U., & Leitges, M. (2024). Transcriptome Profiling of Mouse Embryonic Fibroblast Spontaneous Immortalization: A Comparative Analysis. International Journal of Molecular Sciences, 25(15), 8116. https://doi.org/10.3390/ijms25158116







