Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats
Abstract
:1. Introduction
2. Results
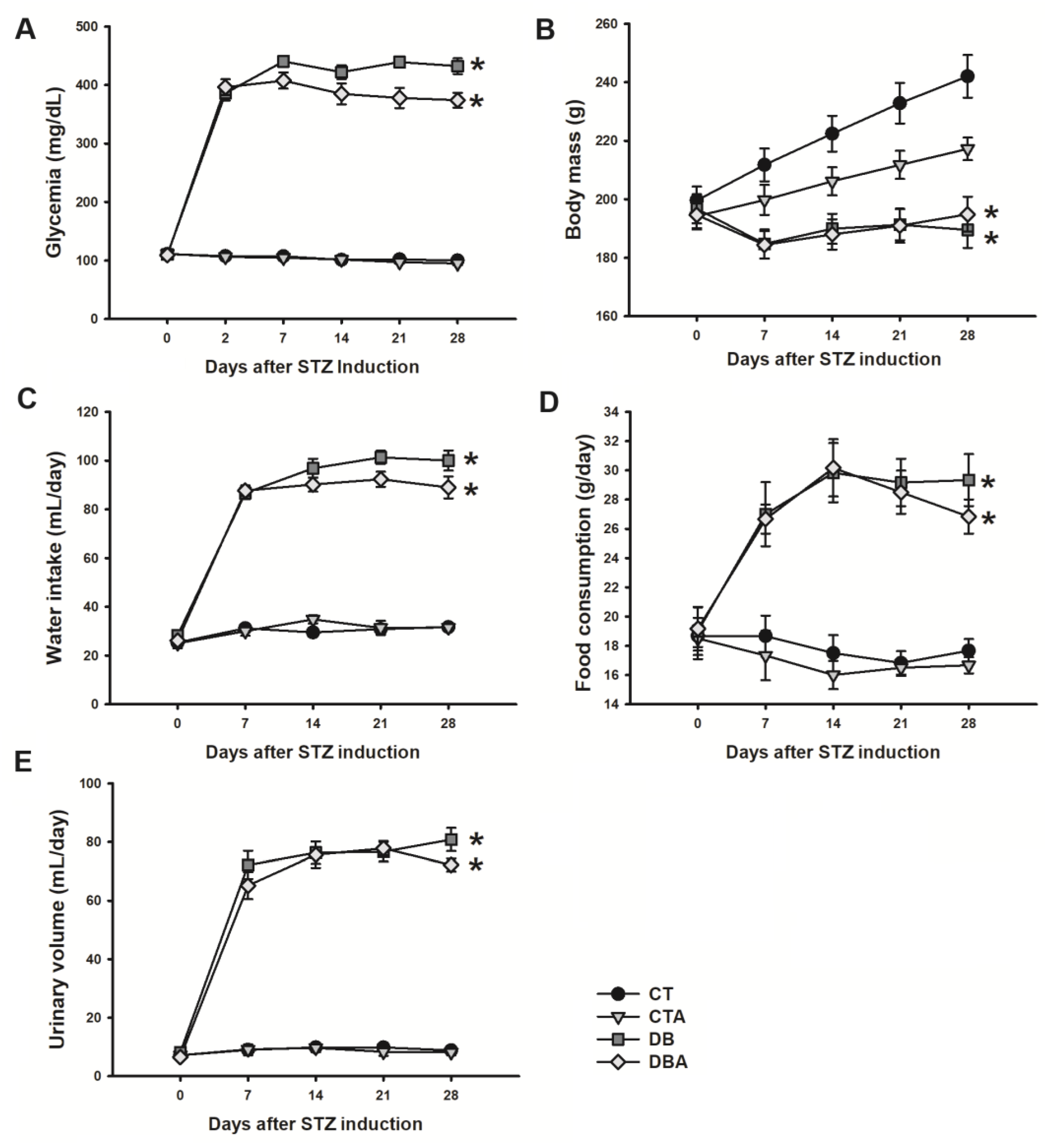
2.1. Diabetic Model Characterization and Anethole Effects
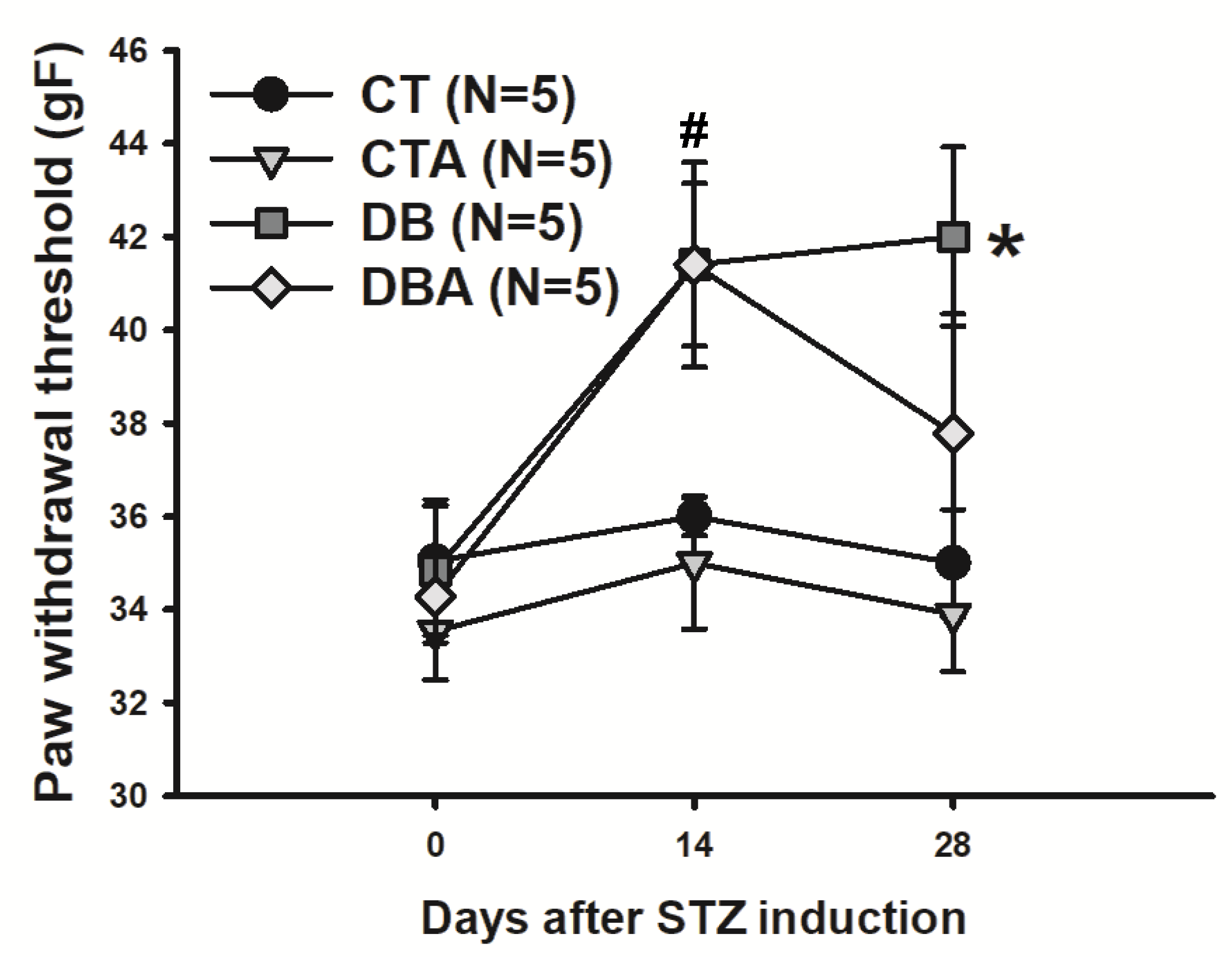
2.2. Mechanical Sensitivity Test
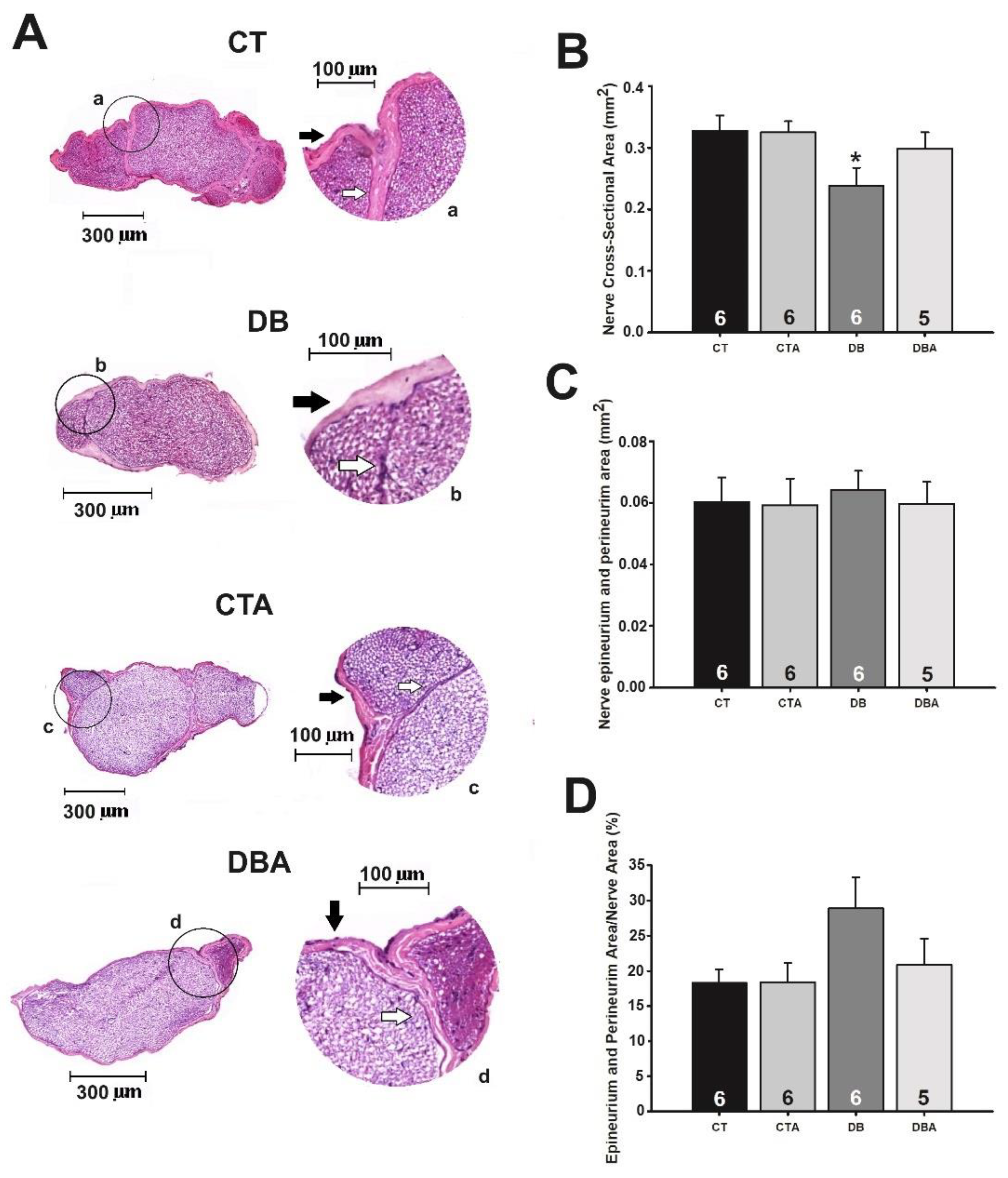
2.3. Morphology and Morphometry of SN Medial Portion
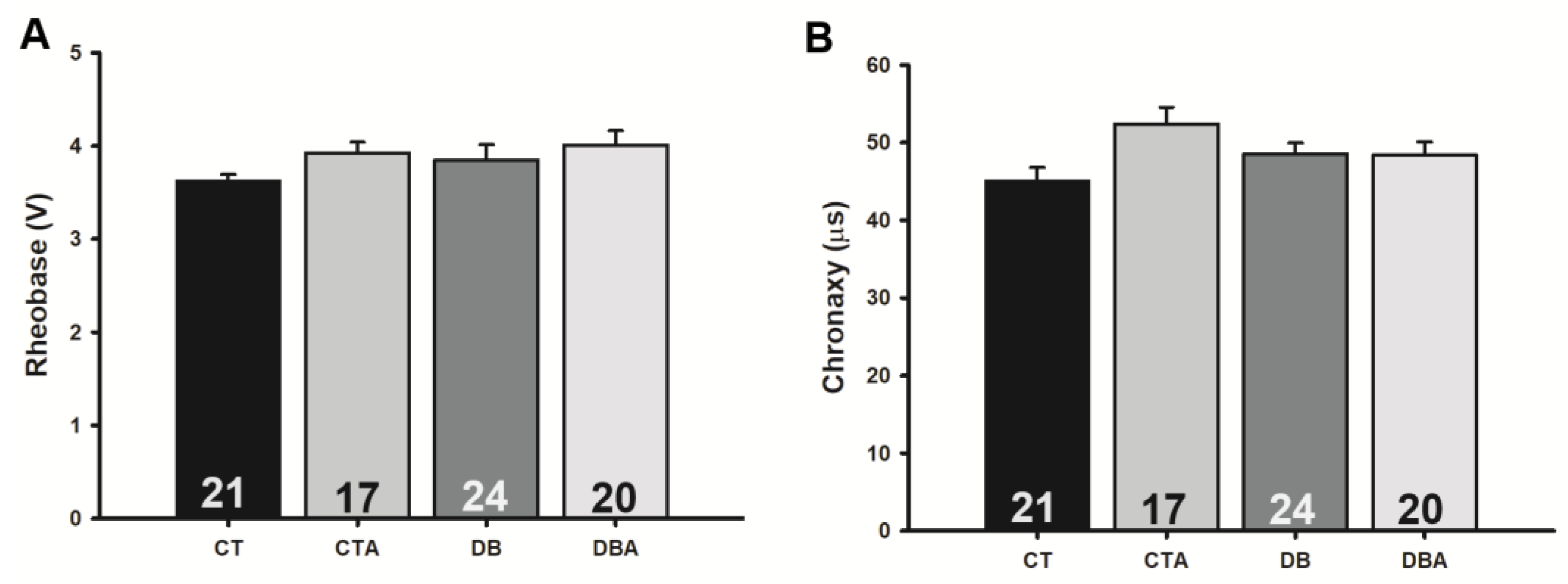
2.4. Anethole Effects on Electrophysiological Changes Promoted by DM on Myelinated SN Fibers
2.5. Anethole Effects on Oxidative Stress Markers Promoted by DM on SN
3. Discussion
4. Materials and Methods
4.1. Animals, Diabetes Mellitus Induction, and Anethole Treatment
4.2. Evaluation of Mechanical Sensitivity
4.3. Tissue Histological Preparation
4.4. Morphological and Morphometric Analysis
4.5. Electrophysiology
4.6. Sciatic Nerve Oxidative Stress Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aprotosoaie, A.C.; Costache, I.I.; Miron, A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [PubMed]
- Calvancanti, J.M.; Leal-Cardoso, J.H.; Diniz, L.R.; Portella, V.G.; Costa, C.O.; Linard, C.F.; Alves, K.; Rocha, M.V.; Lima, C.C.; Ceccato, V.M.; et al. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J. Ethnopharmacol. 2012, 144, 240–247. [Google Scholar]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.; Pinheiro, D.C. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Albuquerque, A.A.C.; Do Vale, O.C.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri and its major constituent anethole block excitability of rat peripheral nerve. Planta Medica 2015, 81, 292–297. [Google Scholar] [PubMed]
- Moreira-Junior, L.; Leal-Cardoso, J.H.; Cassola, A.C.; Carvalho-de-Souza, J.L. State-dependent blockade of dorsal root ganglion voltage-gated na+ channels by anethole. Int. J. Mol. Sci. 2024, 25, 1034. [Google Scholar] [CrossRef]
- Ritter, A.M.; Ames, F.Q.; Otani, F.; De Oliveira, R.M.; Cuman, R.K.; Bersani-Amado, C.A. Effects of anethole in nociception experimental models. Evid. Based Complement. Alternat. Med. 2014, 2014, 345829. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, G.; Yang, M.; Liu, N.; Li, Y.X.; Ma, H.; Ma, L.; Sun, T.; Tan, H.; Yu, J. Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochem. Res. 2018, 43, 2404–2422. [Google Scholar] [CrossRef] [PubMed]
- Ponte, E.L.; Sousa, P.L.; Rocha, M.V.; Soares, P.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; Asseury, A.M. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012, 64, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri prevents conduction alterations produced by diabetes mellitus on vagus nerve. Plants 2021, 10, 893. [Google Scholar] [CrossRef]
- Ferreira-da-Silva, F.W.; Silva-Alves, K.S.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri prevents electrophysiological alterations in dorsal root ganglia of streptozotocin-induced diabetes mellitus in rats. Phytomedicine Plus 2023, 3, 100443. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Boulton, A.J.M.; Feldman, E.L.; Marcus, R.L.; Mizokami-Stout, K.; Singleton, J.R.; Ziegler, D. Diagnosis and treatment of painful diabetic peripheral neuropathy. Arlingt. Am. Diab. Assoc. 2022, 2022, 1–31. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.; Di Salvo, J.; Epifani, R.; Piavocele, A.; Tagliaferri, G.; et al. Peripheral neuropathy in diabetes mellitus: Pathogenetic mechanisms and diagnostic options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef]
- Elafros, M.A.; Andersen, H.; Bennett, D.L.; Savelieff, M.G.; Viswanathan, V.; Callaghan, B.C.; Feldman, E.L. Towards prevention of diabetic peripheral neuropathy: Clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022, 21, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-da-Silva, F.W.; Silva-Alves, K.S.; Lemos-dos-Santos, M.; Oliveira-Abreu, K.; Joca, H.C.; Do Vale, O.C.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. n5-stz diabetic model develops alterations in sciatic nerve and dorsal root ganglia neurons of Wistar rats. ISRN Endocrinol. 2013, 2013, 13. [Google Scholar] [CrossRef]
- Silva-dos-Santos, N.M.; Oliveira-Abreu, K.; Moreira-Junior, L.; Santos-Nascimento, T.D.; Silva-Alves, K.S.; Coelho-de-Souza, A.N.; Ferreira-da-Silva, F.W.; Leal-Cardoso, J.H. Diabetes mellitus alters electrophysiological properties in neurons of superior cervical ganglion of rats. Brain 2019, 1729, 146599. [Google Scholar] [CrossRef] [PubMed]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Weinreich, D.; Leal-Cardoso, J.H. Diabetes mellitus differently affects electrical membrane properties of vagal afferent neurons of rats. Physiol. Rep. 2023, 11, e15605. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cardoso, J.H.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Silva-Alves, K.S. Diabetes-induced electrophysiological alterations on neurosomes in ganglia of peripheral nervous system. Biophys. Rev. 2023, 11, e15605. [Google Scholar] [CrossRef]
- Chang, M.C.; Yang, S. Diabetic peripheral neuropathy essentials: A narrative review. Ann. Palliat. Med. 2023, 12, 390–398. [Google Scholar] [CrossRef]
- Varejão, A.S.; Melo-Pinto, P.; Meek, M.F.; Filipe, V.M.; Bulas-Cruz, J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol. Res. 2004, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; De Cruz, J.; Navarro, X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef]
- Leal-Julià, M.; Vilches, J.J.; Onieva, A.; Verdés, S.; Sánchez, Á.; Chillón, M.; Navarro, X.; Bosch, A. Proteomic quantitative study of dorsal root ganglia and sciatic nerve in type 2 diabetic mice. Mol. Metab. 2022, 55, 101408. [Google Scholar] [CrossRef]
- Bayir, M.H.; Yıldızhan, K.; Altındağ, F. Effect of hesperidin on sciatic nerve damage in STZ-induced diabetic neuropathy: Modulation of TRPM2 channel. Neurotox. Res. 2023, 41, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.; Monem, M.A. A review of diabetic peripheral neuropathy management given recent guidelines updates. Arch. Gen. Intern. Med. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Frizzi, K.E.; Guernsey, L.; Marquez, A.; Ochoa, J.; Rodriguez, M.; Calcutt, N.A. Peripheral neuropathy in mouse models of diabetes. Curr. Protoc. Mouse Biol. 2016, 6, 223–255. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Cloete, L. Diabetes mellitus: An overview of the types, symptoms, complications, and management. Nurs. Stand. 2022, 1, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.A.; Pari, L.; Rathinam, A.; Chandramohan, R. Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochimie 2015, 112, 57–65. [Google Scholar] [CrossRef]
- Samadi-Noshahr, Z.; Ebrahimzadeh-Bideskan, A.; Hadjzadeh, M.A.; Shafei, M.N.; Salmani, H.; Hosseinian, S.; Khajavi-Rad, A. Trans-Anethole attenuated renal injury and reduced expressions of angiotensin II receptor (AT1R) and TGF-β in streptozotocin-induced diabetic rats. Biochimie 2021, 185, 117–127. [Google Scholar] [CrossRef]
- Liao, C.; Yang, M.; Liu, P.; Zhong, W.; Zhang, W. Stable rat model of mechanical allodynia in diabetic peripheral neuropathy: The role of nerve compression. J. Reconstr. Microsurg. 2018, 34, 264–269. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Yin, Y.; Zhu, L.; Zhang, F.; Yang, J. Investigation of the role of miR-221 in diabetic peripheral neuropathy and related molecular mechanisms. Adv. Clin. Exp. Med. 2021, 30, 623–632. [Google Scholar] [CrossRef]
- Cifuentes-Mendiola, S.E.; Solís-Suarez, D.L.; Martínez-Davalos, A.; García-Hernández, A.L. Macrovascular and microvascular type 2 diabetes complications are interrelated in a mouse model. J. Diabetes Complicat. 2023, 37, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Vastegani, S.; Khoshnam, S.E.; Mansouri, E.; Ghafouri, S.; Bakhtiari, N.; Farbood, Y.; Sarkaki, A. Anti-inflammatory, anti-apoptotic, and neuroprotective potentials of anethole in Parkinson’s disease-like motor and non-motor symptoms induced by rotenone in rats. Metab. Brain Dis. 2023, 38, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S.S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990, 13, 771–784. [Google Scholar] [PubMed]
- Fazan, V.P.; Salgado, H.C.; Barreira, A.A. Aortic depressor nerve myelinated fibers in acute and chronic experimental diabetes. Am. J. Hypertens. 2006, 19, 153–160. [Google Scholar] [CrossRef]
- Fazan, V.P.; Rodrigues Filho, O.A.; Jordão, C.E.; Moore, K.C. Phrenic nerve diabetic neuropathy in rats: Unmyelinated fibers morphometry. J. Peripher. Nerv. Syst. 2009, 19, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Srilatha, K.; Reddy, K.P. Sciatic nerve structural and functional recovery with extract of phyllanthus amarus and esculetin in STZ-induced hyperglycemic rats. Ann. Neurosci. 2019, 26, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sanada, L.S.; Tavares, M.R.; Sato, K.L.; Ferreira, R.d.S.; Neubern, M.C.; Castania, J.A.; Salgado, H.C.; Fazan, V.P. Association of chronic diabetes and hypertension in sural nerve morphometry: An experimental study. Diabetol. Metab. Syndr. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cardoso, J.H.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Santos-Nascimento, T.; Joca, H.C.; Macedo, F.H.P.; Albuquerque-Neto, P.M.; Magalhães, P.J.C.; Lahlou, S.; Cruz, J.S.; et al. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur. J. Pharmacol. 2010, 645, 86–93. [Google Scholar] [CrossRef]
- Junge, D. Nervous and Muscular Excitement, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 1992. [Google Scholar]
- Aidley, D.J. The Physiology of Excitable Cells, 4th ed.; Cambridge University Press: Cambrige, UK, 1998. [Google Scholar]
- Langlois, V.; Bedat-Millet, A.L.; Lebesnerais, M.; Miranda, S.; Marguet, F.; Benhamou, Y.; Marcorelles, P.; Lévesque, H. Small fiber neuropathy. Rev. Med. Interne 2018, 39, 99–106. [Google Scholar] [CrossRef]
- Wisniewski-Rebecca, E.S.; Rocha, B.A.; Wiirzler, L.A.; Cuman, R.K.; Velazquez-Martinez, C.A.; Bersani-Amado, C.A. Synergistic effects of anethole and ibuprofen in acute inflammatory response. Chem. Biol. Interact. 2015, 242, 247–252. [Google Scholar] [CrossRef]
- Raman, S.; Asle-Rousta, M.; Rahnema, M. Protective effect of fennel, and its major component trans-anethole against social isolation induced behavioral deficits in rats. Physiol. Int. 2020, 107, 30–39. [Google Scholar] [CrossRef]
- Pasnoor, M.; Dimachkie, M.M.; Kluding, P.; Barohn, R.J. Diabetic neuropathy part 1: Overview and symmetric phenotypes. Neurol Clin. 2013, 31, 425–445. [Google Scholar] [CrossRef]
- Farias, V.X.; Macêdo, F.H.; Oquendo, M.B.; Tomé, A.R.; Báo, S.N.; Cintra dos Santos, C.F.; Albuquerque, A.A.; Heimark, D.B.; Larner, J.; Fonteles, M.C.; et al. Chronic treatment with D-chiro-inositol prevents autonomic and somatic neuropathy in STZ-induced diabetic mice. Diabetes Obes. Metab. 2011, 13, 243–250. [Google Scholar] [CrossRef]
- Julu, P.O.O. The correlation between sensory nerve conduction velocities and three metabolic indices in rats treated with streptozotocin. Diabetology 1988, 31, 247–253. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dickenson, A.H. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef]
- Farias, V.X.; Uchoa, P.N.; Aquino, C.P.; Britto, L.R.G.; Fonteles, M.C.; Leal-Cardoso, J.H.; Silva-Alves, K.S.; Havt, A.; Prata, M.M.G.; Heimark, D.B.; et al. Expression of myo-inositol cotransporters in the sciatic nerve and dorsal root ganglia in experimental diabetes. Braz. J. Med. Biol. Res. 2019, 52, e8589. [Google Scholar] [CrossRef]
- Ayaz, M.; Tuncer, S.; Okudan, N.; Gokbel, H. Coenzyme Q(10) and alpha-lipoic acid supplementation in diabetic rats: Conduction velocity distributions. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 367–374. [Google Scholar]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Chainy, G.B.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a Medicinal Plant Compound, Decreases the Production of Pro-Inflammatory TNF-α and IL-1β in a Rat Model of LPS-Induced Periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding Diabetic Neuropathy: Focus on Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 9524635. [Google Scholar] [CrossRef]
- European Food Safety Authority. Safety of ‘γ-methyl-α-(p-methylphenyl)benzyl alcohol’ [ANETHOLE] as a flavouring substance—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 6, 721. [Google Scholar]
- Newberne, P.; Smith, R.L.; Doull, J.; Goodman, J.I.; Munro, I.C.; Portoghese, P.S.; Wagner, B.M.; Weil, C.S.; Woods, L.A.; Adams, T.B.; et al. The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Flavour and Extract Manufacturer’s Association. Food Chem. Toxicol. 1999, 37, 789–811. [Google Scholar] [CrossRef] [PubMed]
- Coelho-de-Souza, A.N.; Rocha, M.V.A.P.; Oliveira, K.A.; Vasconcelos, Y.A.G.; Santos, E.C.; Silva-Alves, K.S.; Diniz, L.R.L.; Ferreira-da-Silva, F.W.; Oliveira, A.C.; Ponte, E.L.; et al. Volatile oil of Croton zehntneri per oral sub-acute treatment offers small toxicity: Perspective of therapeutic use. Braz. J. Pharmacogn. 2019, 29, 228–233. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bannister, J.V.; Calabrese, L. Assays for superoxide dismutase. Methods Biochem. Anal. 1987, 32, 279–312. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa-Ferreira, B.d.S.; Silva, F.E.R.d.; Gomes-Vasconcelos, Y.d.A.; Joca, H.C.; Coelho-de-Souza, A.N.; Ferreira-da-Silva, F.W.; Leal-Cardoso, J.H.; Silva-Alves, K.S.d. Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats. Int. J. Mol. Sci. 2024, 25, 8133. https://doi.org/10.3390/ijms25158133
Barbosa-Ferreira BdS, Silva FERd, Gomes-Vasconcelos YdA, Joca HC, Coelho-de-Souza AN, Ferreira-da-Silva FW, Leal-Cardoso JH, Silva-Alves KSd. Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats. International Journal of Molecular Sciences. 2024; 25(15):8133. https://doi.org/10.3390/ijms25158133
Chicago/Turabian StyleBarbosa-Ferreira, Bianca de Sousa, Francisca Edilziane Rodrigues da Silva, Yuri de Abreu Gomes-Vasconcelos, Humberto Cavalcante Joca, Andrelina Noronha Coelho-de-Souza, Francisco Walber Ferreira-da-Silva, José Henrique Leal-Cardoso, and Kerly Shamyra da Silva-Alves. 2024. "Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats" International Journal of Molecular Sciences 25, no. 15: 8133. https://doi.org/10.3390/ijms25158133





