Comparative Transcriptome Analysis of Hepatopancreas Reveals Sexual Dimorphic Response to Methyl Farnesoate Injection in Litopenaeus vannamei
Abstract
1. Introduction
2. Results
2.1. Overview of Hepatopancreases Transcriptome Data after Injection
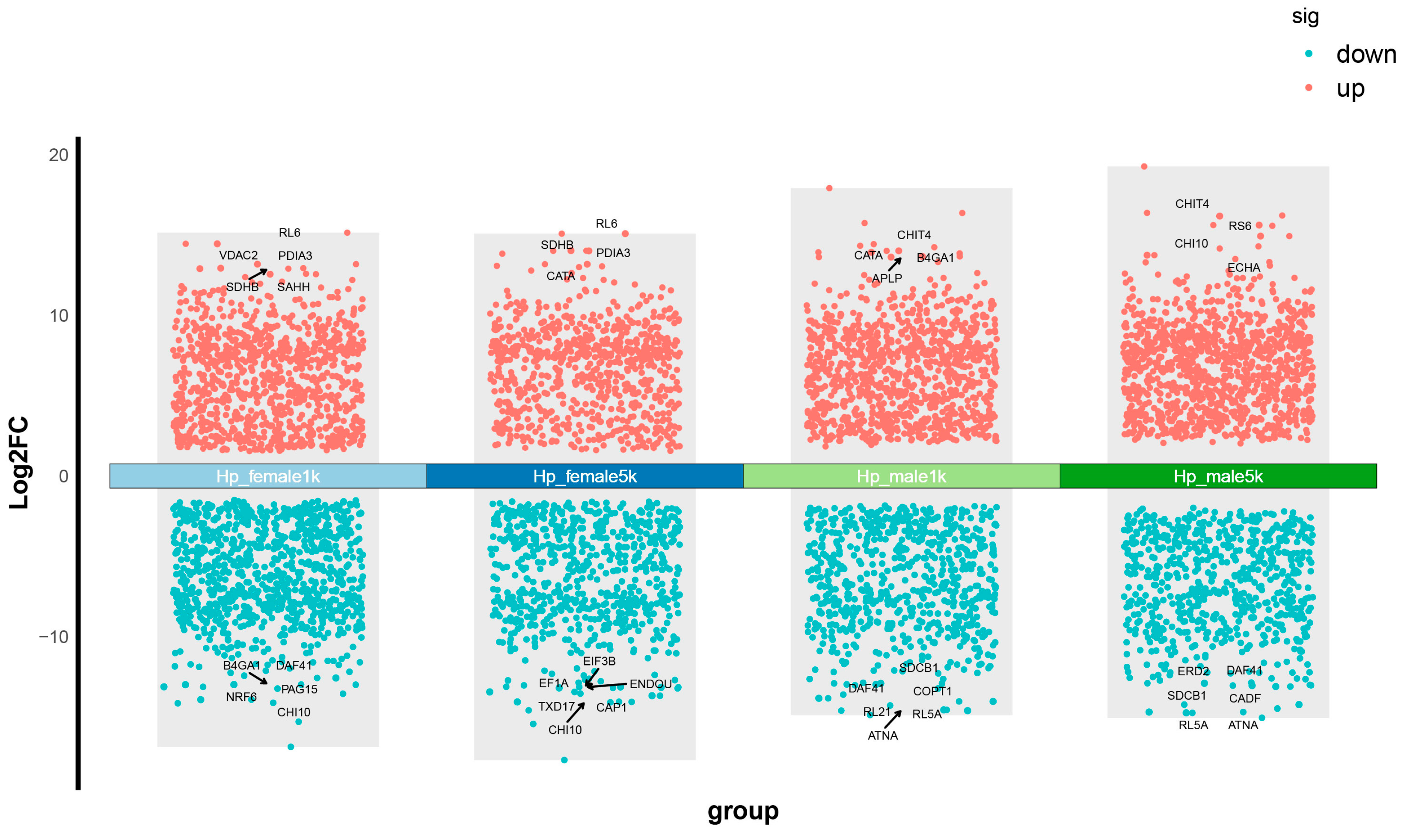
2.2. Transcriptomic Responses of Hepatopancreases to MF Injection in L. vannamei
2.3. Sexually Dimorphic Responses of Key Components in Hormone-Related Pathways
2.4. Clusters of the Gender-Specific Responding DETs
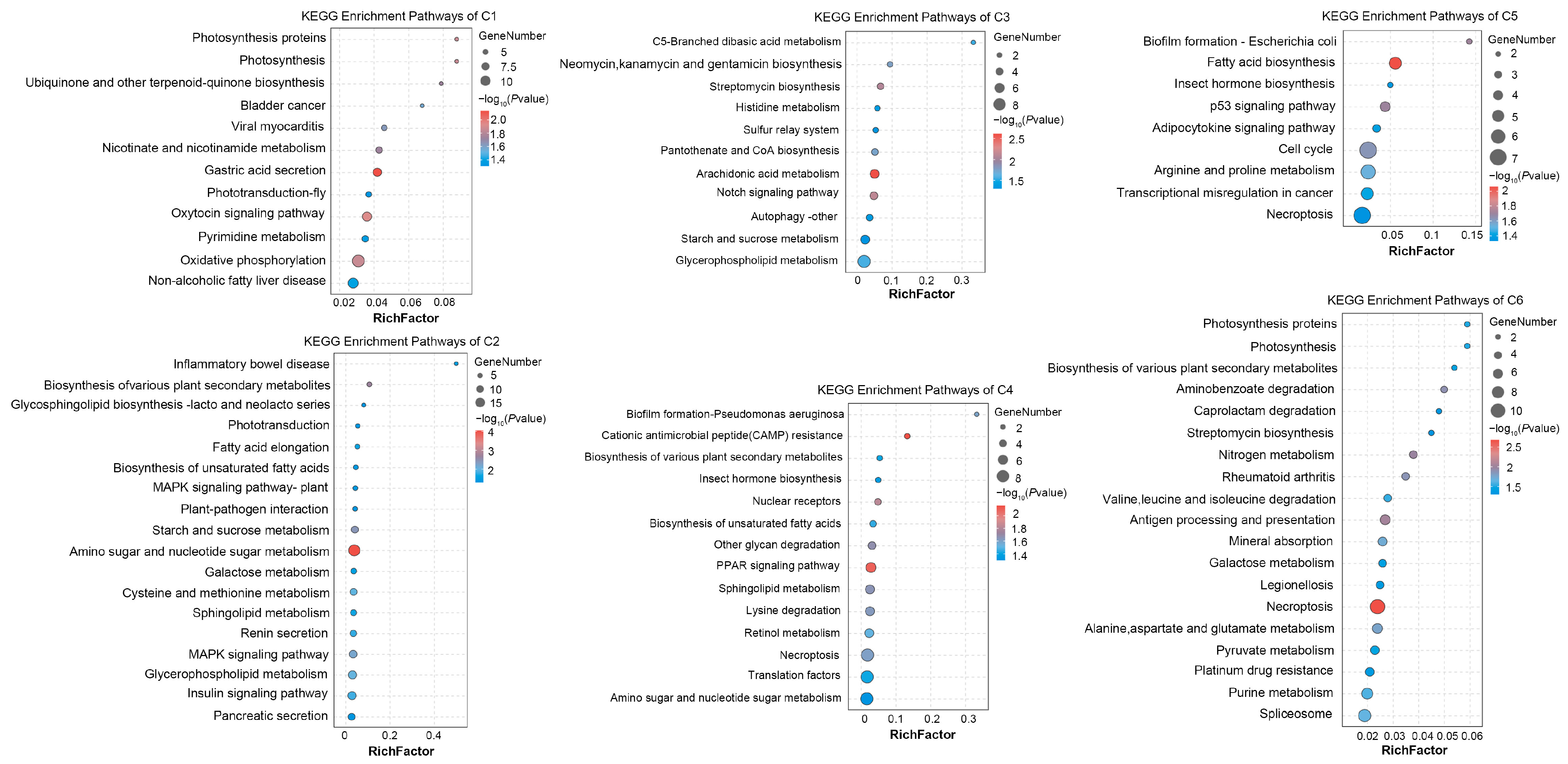
2.5. GO and KEGG Enrichment Analysis of the Gender-Specific Responding DETs Clusters
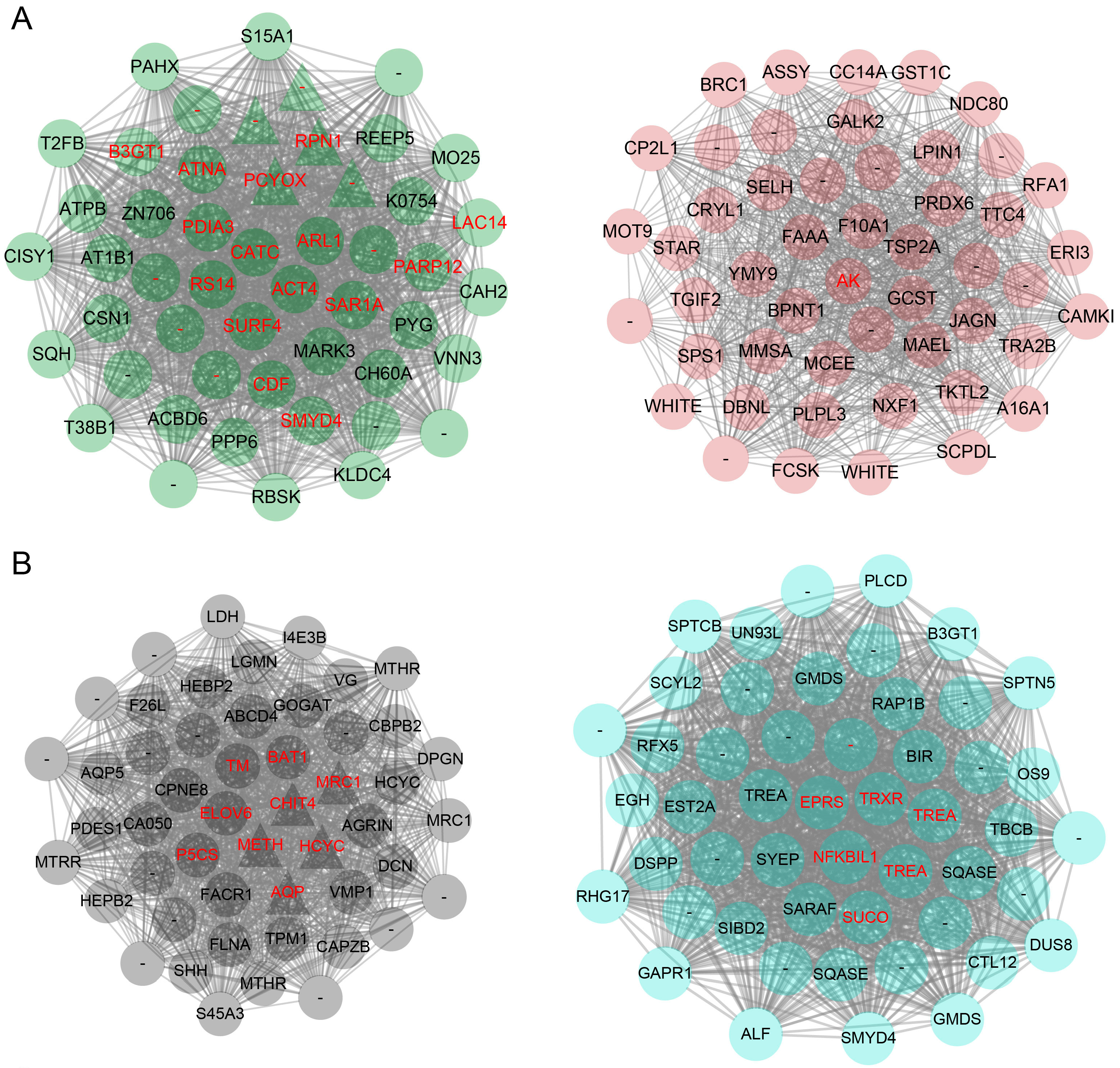
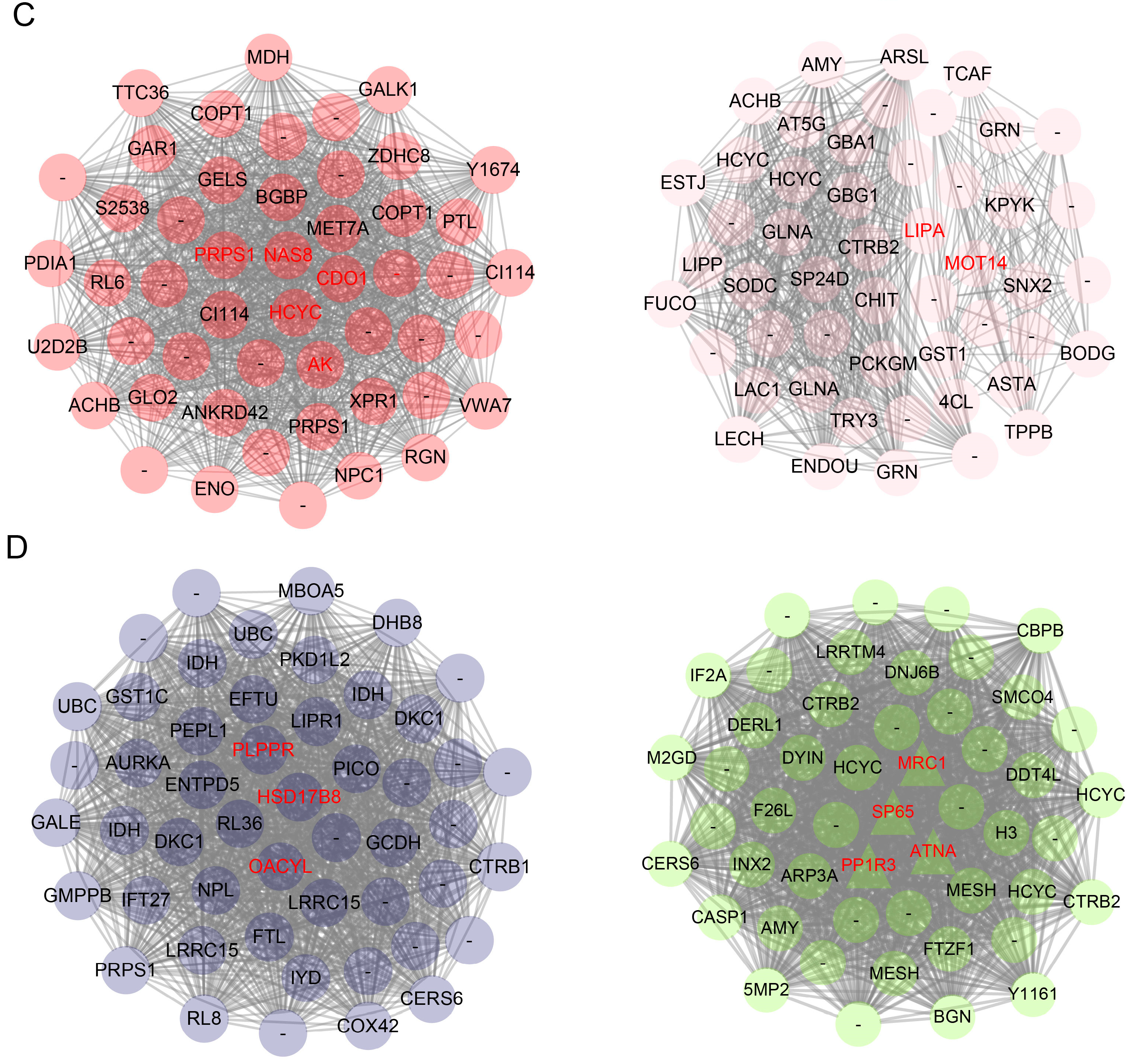
2.6. Sexual Dimorphic Modules Responding to Sesquiterpenoid Hormone
3. Discussion
4. Materials and Methods
4.1. Animal Culture
4.2. Hormone Treatment and Tissue Sample Collection
4.3. RNA Sequencing and Data Processing
4.4. Differential Expression and Functional Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | methyl farnesoate |
| JH | juvenile hormone |
| Hp | hepatopancreas |
| TPM | transcripts per million |
| DEGs | differentially expressed genes |
| DETs | differentially expressed transcripts |
| WGCNA | weighted gene co-expression network analysis |
| FC | FoldChange |
| JHE-L | juvenile hormone esterase-like carboxylesterase |
| Vg | vitellogenin |
| VgR | vitellogenin receptor |
| DDX17 | DEAD-box helicase 17 |
| SMAD3 | mothers against decapentaplegic homolog 3 |
| AK | arginine kinase |
| ELOV6 | very long chain fatty acids protein 6 |
| TM | tropomyosin |
| TRXR | thioredoxin reductase |
| CDO | cysteine dioxygenase |
| LIPA | lysosomal acid lipase |
| HSD17B8 | estradiol 17-beta-dehydrogenase 8 |
| ATP1A | sodium/potassium-transporting ATPase subunit alpha |
References
- Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Juvenile hormone and sesquiterpenoids in arthropods: Biosynthesis, signaling, and role of MicroRNA. J. Steroid. Biochem. Mol. Biol. 2018, 184, 69–76. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Borst, D.W. Methyl farnesoate couples environmental changes to testicular development in the crab Carcinus maenas. J. Exp. Biol. 2008, 211 Pt 17, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.H.; Hayward, A.; Bendena, W.G.; Takahashi, T.; Tobe, S.S. Evolution and functional divergence of enzymes involved in sesquiterpenoid hormone biosynthesis in crustaceans and insects. Peptides 2010, 31, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Tamone, S.L.; Prestwich, G.D.; Chang, E.S. Identification and characterization of methyl farnesoate binding proteins from the crab, Cancer magister. Gen. Comp. Endocrinol. 1997, 105, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, J.; Zhang, X.; Xiang, J.; Li, F. Genomic Characterization and Expression of Juvenile Hormone Esterase-Like Carboxylesterase Genes in Pacific White Shrimp, Litopenaeus vannamei. Int. J. Mol. Sci. 2020, 21, 5444. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Toyota, K.; Ishibashi, H.; Tominaga, N.; Sato, T.; Tatarazako, N.; Iguchi, T. Molecular Insights into Structural and Ligand Binding Features of Methoprene-Tolerant in Daphnids. Chem. Res. Toxicol. 2020, 33, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, M.; Jiang, Q.; Zheng, H.; Zheng, L.; Zhu, D. Role of Kruppel homolog 1 (Kr-h1) in methyl farnesoate-mediated vitellogenesis in the swimming crab Portunus trituberculatus. Gene 2018, 679, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, X.; Tao, T.; Jiang, Q.; Shao, J.; Zhu, D. Molecular characterization of methoprene-tolerant gene (Met) in the swimming crab Portunus trituberculatus: Its putative role in methyl farnesoate-mediated vitellogenin transcriptional activation. Anim Reprod. Sci. 2016, 174, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Han, Y.; Huang, M.; Jiang, H.; Huang, J.; Tao, M.; Xu, R.; Xie, Q.; Su, S. Potential role of Methoprene-tolerant (Met) in methyl farnesoate-mediated vitellogenesis in the Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 252, 110524. [Google Scholar] [CrossRef] [PubMed]
- Kakaley, E.K.; Wang, H.Y.; LeBlanc, G.A. Agonist-mediated assembly of the crustacean methyl farnesoate receptor. Sci. Rep. 2017, 7, 45071. [Google Scholar] [CrossRef]
- Yudin, A.; Diener, R.; Clark, W.; Chang, E. Mandibular Gland of the Blue Crab. Biological. Bull. 1980, 159, 760. [Google Scholar] [CrossRef]
- Taketomi, T.; Motono, M.; Miyawaki, M. On the function of the mandibular gland of decapod Crustacea. Cell Biol. Int. Rep. 1989, 13, 463–469. [Google Scholar] [CrossRef]
- Tamone, S.L.; Chang, E.S. Methyl farnesoate stimulates ecdysteroid secretion from crab Y-organs in vitro. Gen. Comp. Endocrinol. 1993, 89, 425–432. [Google Scholar] [CrossRef]
- Hyde, C.J.; Elizur, A.; Ventura, T. The crustacean ecdysone cassette: A gatekeeper for molt and metamorphosis. J. Steroid. Biochem. Mol. Biol. 2019, 185, 172–183. [Google Scholar] [CrossRef]
- Yuan, H.; Cai, P.; Zhang, W.; Jin, S.; Jiang, S.; Xiong, Y.; Gong, Y.; Qiao, H.; Fu, H. Identification of genes regulated by 20-Hydroxyecdysone in Macrobrachium nipponense using comparative transcriptomic analysis. BMC Genom. 2024, 25, 35. [Google Scholar] [CrossRef]
- Tiu, S.H.; Hui, J.H.; He, J.G.; Tobe, S.S.; Chan, S.M. Characterization of vitellogenin in the shrimp Metapenaeus ensis: Expression studies and hormonal regulation of MeVg1 transcription in vitro. Mol. Reprod. Dev. 2006, 73, 424–436. [Google Scholar] [CrossRef]
- Chan, S.M.; Mak, A.S.; Choi, C.L.; Ma, T.H.; Hui, J.H.; Tiu, S.H. Vitellogenesis in the red crab, Charybdis feriatus: Contributions from small vitellogenin transcripts (CfVg) and farnesoic acid stimulation of CfVg expression. Ann. N. Y. Acad. Sci. 2005, 1040, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Medesani, D.; Ferré, L.; Canosa, I.; Silveyra, G.; Rodríguez, E. Induction of vitellogenesis by 17-hydroxyprogesterone and methyl farnesoate during post-reproductive period, in the estuarine crab Neohelice granulata. Invertebr. Reprod. Dev. 2015, 59, 104–110. [Google Scholar] [CrossRef]
- Olmstead, A.W.; Leblanc, G.A. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J. Exp. Zool 2002, 293, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Tatarazako, N.; Oda, S.; Watanabe, H.; Morita, M.; Iguchi, T. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere 2003, 53, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, H.; Miyakawa, H.; Ishikawa, A.; Ishikawa, Y.; Sugime, Y.; Emlen, D.J.; Lavine, L.C.; Miura, T. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via juvenile hormone signaling in stag beetles. PLoS Genet. 2014, 10, e1004098. [Google Scholar] [CrossRef]
- Gotoh, H.; Hust, J.A.; Miura, T.; Niimi, T.; Emlen, D.J.; Lavine, L.C. The Fat/Hippo signaling pathway links within-disc morphogen patterning to whole-animal signals during phenotypically plastic growth in insects. Dev. Dyn. 2015, 244, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Sagi, A.; Homola, E.; Laufer, H. Methyl farnesoate in the prawn Macrobrachium rosenbergii: Synthesis by the mandibular organ in vitro, and titers in the hemolymph. Comp. Biochem. Physiol. B 1991, 99, 879–882. [Google Scholar] [CrossRef] [PubMed]
- The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [CrossRef]
- Pérez-Rostro, C.; Ibarra, A.M. Heritabilities and genetic correlations of size traits at harvest in sexually dimorphic Pacific white shrimp (Litopenaeus vannamei) grown in two environments. Aquac. Res. 2003, 34, 1079–1085. [Google Scholar] [CrossRef]
- Gitterle, T.; Rye, M.; Salte, R.; Cock, J.; Johansen, H.; Lozano, C.; Suárez, J.; Gjerde, B. Genetic (co)variation in harvest body weight and survival in Penaeus (Litopenaeus) vannamei under standard commercial conditions. Aquaculture 2005, 243, 83–92. [Google Scholar] [CrossRef]
- Zhang, J. ClusterGVis: One-Step to Cluster and Visualize Gene Expression Matrix. 2022. Available online: https://github.com/junjunlab/ClusterGVis (accessed on 19 November 2022).
- Aiken, D.E. Photoperiod, endocrinology and the crustacean molt cycle. Science 1969, 164, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Nguyen, T.; Nguyen, L.; Quang Pham, H.; Nguyen, T.; Pham, H.; Hoa, N.; Hoa, T.; Dau, T.; Vu, T.H.; et al. De novo assembly and transcriptome characterization of major growth-related genes in various tissues of Penaeus monodon. Aquaculture 2016, 464, 545–553. [Google Scholar] [CrossRef]
- Rodríguez, E.; López Greco, L.; Medesani, D.; Laufer, H.; Fingerman, M. Effect of Methyl Farnesoate, Alone and in Combination with Other Hormones, on Ovarian Growth of the Red Swamp Crayfish, Procambarus clarkii, during Vitellogenesis. Gen. Comp. Endocrinol. 2002, 125, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Xie, X.; Liu, M.; Jiang, Q.; Zhu, D. Cloning of two carboxylesterase cDNAs from the swimming crab Portunus trituberculatus: Molecular evidences for their putative roles in methyl farnesotae degradation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 203, 100–107. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, M.; Deng, Y.; Yang, Y.; Li, X.; Lu, Q.; Ge, J.; Pan, J.; Xu, Z. Molecular cloning, characterization and expression analysis of two juvenile hormone esterase-like carboxylesterase cDNAs in Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 205, 46–53. [Google Scholar] [CrossRef]
- Gong, J.; Huang, C.; Shu, L.; Bao, C.; Huang, H.; Ye, H.; Zeng, C.; Li, S. The retinoid X receptor from mud crab: New insights into its roles in ovarian development and related signaling pathway. Sci. Rep. 2016, 6, 23654. [Google Scholar] [CrossRef]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef]
- Ngo, T.D.; Partin, A.C.; Nam, Y. RNA Specificity and Autoregulation of DDX17, a Modulator of MicroRNA Biogenesis. Cell Rep. 2019, 29, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J. The role of miRNA biogenesis and DDX17 in tumorigenesis and cancer stemness. Biomed. J. 2020, 43, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Lin, C.Y.; Xu, W.B.; Zhang, Y.M.; Shao, Q.J.; Dong, W.R.; Shu, M.A. The first identification and functional analysis of two drosophila mothers against decapentaplegic protein genes (SpSmad1 and SpSmad2/3) and their involvement in the innate immune response in Scylla paramamosain. Fish Shellfish Immunol. 2023, 143, 109183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Lin, C.Y.; Li, B.Z.; Dong, W.R.; Shu, M.A. Identification and functional analysis of two drosophila mothers against decapentaplegic protein(Smad)genes and their involvement in immune responses in the red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2022, 131, 1255–1263. [Google Scholar] [CrossRef]
- Yin, X.; Wei, W.; Zhuang, X.; Li, Z.; Liu, C.; Ou, M.; Dong, W.; Wang, F.; Huang, L.; Liao, M.; et al. Determining the function of LvSmad3 on Litopenaeus vannamei in response to acute low temperature stress. Dev. Comp. Immunol. 2021, 125, 104209. [Google Scholar] [CrossRef]
- Healy, T.M.; Burton, R.S. Strong selective effects of mitochondrial DNA on the nuclear genome. Proc. Natl. Acad. Sci. USA 2020, 117, 6616–6621. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Zhang, S.; Rong, L.; Yang, X.; Wu, Z.; Sun, W. Metabolic changes and stress damage induced by ammonia exposure in juvenile Eriocheir sinensis. Ecotoxicol. Environ. Saf. 2021, 223, 112608. [Google Scholar] [CrossRef]
- Salma, U.; Uddowla, M.H.; Kim, M.; Kim, J.M.; Kim, B.K.; Baek, H.J.; Park, H.; Mykles, D.L.; Kim, H.W. Five hepatopancreatic and one epidermal chitinases from a pandalid shrimp (Pandalopsis japonica): Cloning and effects of eyestalk ablation on gene expression. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 197–207. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, S.; Xiong, Y.; Fu, H.; Sun, S.; Qiao, H.; Zhang, W.; Jiang, F.; Jin, S.; Gong, Y. Six chitinases from oriental river prawn Macrobrachium nipponense: cDNA characterization, classification and mRNA expression during post-embryonic development and moulting cycle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 167, 30–40. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Kim, W.S.; Park, K.; Kim, J.; Lee, M.O.; Kwak, I.S. Chitinase gene responses and tissue sensitivity in an intertidal mud crab (Macrophthalmus japonicus) following low or high salinity stress. Cell Stress Chaperones 2015, 20, 517–526. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Sun, Y.; Li, S.; Gao, Y.; Yu, Y.; Liu, C.; Wang, Q.; Lv, X.; Zhang, X.; et al. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019, 10, 356. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Cheng, X.; Ladd, T.; Lingohr, E.J.; Krell, P.J.; Arif, B.M.; Retnakaran, A.; Feng, Q. A molt-associated chitinase cDNA from the spruce budworm, Choristoneura fumiferana. Insect. Biochem. Mol. Biol. 2002, 32, 1813–1823. [Google Scholar] [CrossRef]
- Reddy, P.; Arifullah, M. Dietary methyl farnesoate, a potential growth inducer in male crab Oziothelphusa senex senex. IOP Conf. Ser. Earth Environ. Sci. 2021, 756, 012062. [Google Scholar] [CrossRef]
- Laufer, H.; Ahl, J.; Rotllant, G.; Baclaski, B. Evidence that ecdysteroids and methyl farnesoate control allometric growth and differentiation in a crustacean. Insect. Biochem. Mol. Biol. 2002, 32, 205–210. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.; Huang, G.; Shi, W.; Yang, X.; Jiang, Q.; Jia, Y.; Yang, X.; Jiang, H. Hepatopancreatic metabolomics shedding light on the mechanism underlying unsynchronized growth in giant freshwater prawn, Macrobrachium rosenbergii. PLoS ONE 2020, 15, e0243778. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Z.; Aweya, J.J.; Wang, F.; Li, S.; Tuan, T.N.; Yao, D.; Zhang, Y. Litopenaeus vannamei Notch interacts with COP9 signalosome complex subunit 1 (CNS1) to negatively regulate the NF-κB pathway. J. Proteom. 2021, 232, 104074. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Shi, B.; Jiao, L.F.; Jin, M.; Sun, P.; Ding, L.Y.; Yuan, Y. Hepatopancreas and ovarian transcriptome response to different dietary soybean lecithin levels in Portunus trituberculatus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100600. [Google Scholar] [CrossRef]
- Zhou, M.; Abbas, M.N.; Kausar, S.; Jiang, C.X.; Dai, L.S. Transcriptome profiling of red swamp crayfish (Procambarus clarkii) hepatopancreas in response to lipopolysaccharide (LPS) infection. Fish Shellfish Immunol. 2017, 71, 423–433. [Google Scholar] [CrossRef]
- Lu, J.; Tao, X.; Luo, J.; Zhu, T.; Jiao, L.; Jin, M.; Zhou, Q. Dietary choline promotes growth, antioxidant capacity and immune response by modulating p38MAPK/p53 signaling pathways of juvenile Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2022, 131, 827–837. [Google Scholar] [CrossRef]
- Strong, S.J.; Ellington, W.R. Isolation and sequence analysis of the gene for arginine kinase from the chelicerate arthropod, Limulus polyphemus: Insights into catalytically important residues. Biochim. Biophys. Acta 1995, 1246, 197–200. [Google Scholar] [CrossRef]
- Ellington, W.R. Evolution and physiological roles of phosphagen systems. Annu. Rev. Physiol. 2001, 63, 289–325. [Google Scholar] [CrossRef]
- Yao, C.L.; Ji, P.F.; Kong, P.; Wang, Z.Y.; Xiang, J.H. Arginine kinase from Litopenaeus vannamei: Cloning, expression and catalytic properties. Fish Shellfish Immunol. 2009, 26, 553–558. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Huang, C.; Lin, H.; Zhang, M.; Chen, M.; Han, K.; Huang, W.; Ruan, S. Cloning and expression characterization of elongation of very long-chain fatty acids protein 6 (elovl6) with dietary fatty acids, ambient salinity and starvation stress in Scylla paramamosain. Front. Physiol. 2023, 14, 1221205. [Google Scholar] [CrossRef]
- Zhou, Y.; Aweya, J.J.; Huang, Z.; Chen, Y.; Tang, Z.; Shi, Z.; Zheng, Z.; Zhang, Y. The ELOVL6 homolog in Penaeus vannamei plays a dual role in fatty acid metabolism and immune response. Mol. Immunol. 2023, 164, 7–16. [Google Scholar] [CrossRef]
- West, J. Ultrastructural and Contractile Activation Properties of Crustacean Muscle Fibres Over the Moult Cycle. Comp. Biochem. Physiol. Part B Comp. Biochem. 1997, 117, 333–345. [Google Scholar] [CrossRef]
- Tan, S.H.; Degnan, B.M.; Lehnert, S.A. The Penaeus monodon Chitinase 1 Gene Is Differentially Expressed in the Hepatopancreas During the Molt Cycle. Mar. Biotechnol. 2000, 2, 126–135. [Google Scholar] [CrossRef]
- Hu, J.H.; Zhang, F.Y.; Jiang, K.J.; Fang, Y.B.; Wang, J.; Zhao, M.; Qiao, Z.G.; Ma, L.B. Molecular characterization of thioredoxin-1 and thioredoxin reductase activity in mud crab Scylla paramamosain. Genet. Mol. Res. 2014, 13, 10241–10255. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Huang, Y.; Zhu, Z.; Li, T.; Cai, Z.; Li, M.; Gong, H.; Yan, M. Combined effects of microplastics and antibiotic-resistant bacteria on Daphnia magna growth and expression of functional genes. Sci. Total Environ. 2023, 905, 166880. [Google Scholar] [CrossRef] [PubMed]
- Bagley, P.J.; Hirschberger, L.L.; Stipanuk, M.H. Evaluation and modification of an assay procedure for cysteine dioxygenase activity: High-performance liquid chromatography method for measurement of cysteine sulfinate and demonstration of physiological relevance of cysteine dioxygenase activity in cysteine catabolism. Anal. Biochem. 1995, 227, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Charmantier, G.; Voss-Foucart, M.F.; Trilles, J.P.; Jeuniaux, C. Free amino acids of hemolymph during pubertal molting and senescence in Spaeroma serratum (Isopoda, Flabellifera. Arch. Int. Physiol. Biochim. 1975, 83, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, V.Z.; Duax, W.L. Rational proteomics IV: Modeling the primary function of the mammalian 17beta-hydroxysteroid dehydrogenase type 8. J. Steroid. Biochem. Mol. Biol. 2005, 94, 327–335. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Kastaniotis, A.J.; Autio, K.J.; Jiang, G.; Chen, Z.; Glumoff, T. 17B-hydroxysteroid dehydrogenases as acyl thioester metabolizing enzymes. Mol. Cell Endocrinol. 2019, 489, 107–118. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H. Lysosomal Acid Lipase in Lipid Metabolism and Beyond. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, C.; García-Carreño, F. Effect of fasting on digestive gland lipase transcripts expression in Penaeus vannamei. Mar. Genom. 2011, 4, 273–278. [Google Scholar] [CrossRef]
- Olmstead, A.W.; LeBlanc, G.A. Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean Daphnia magna. Environ. Health Perspect. 2003, 111, 919–924. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yang, X.; Du, J.; Wei, C.; Liu, P.; Hu, J.; Bao, Z.; Qu, Z. Comparative Transcriptome Analysis of Hepatopancreas Reveals Sexual Dimorphic Response to Methyl Farnesoate Injection in Litopenaeus vannamei. Int. J. Mol. Sci. 2024, 25, 8152. https://doi.org/10.3390/ijms25158152
Yang Z, Yang X, Du J, Wei C, Liu P, Hu J, Bao Z, Qu Z. Comparative Transcriptome Analysis of Hepatopancreas Reveals Sexual Dimorphic Response to Methyl Farnesoate Injection in Litopenaeus vannamei. International Journal of Molecular Sciences. 2024; 25(15):8152. https://doi.org/10.3390/ijms25158152
Chicago/Turabian StyleYang, Zhihui, Xiaoliu Yang, Jiahao Du, Cun Wei, Pingping Liu, Jingjie Hu, Zhenmin Bao, and Zhe Qu. 2024. "Comparative Transcriptome Analysis of Hepatopancreas Reveals Sexual Dimorphic Response to Methyl Farnesoate Injection in Litopenaeus vannamei" International Journal of Molecular Sciences 25, no. 15: 8152. https://doi.org/10.3390/ijms25158152
APA StyleYang, Z., Yang, X., Du, J., Wei, C., Liu, P., Hu, J., Bao, Z., & Qu, Z. (2024). Comparative Transcriptome Analysis of Hepatopancreas Reveals Sexual Dimorphic Response to Methyl Farnesoate Injection in Litopenaeus vannamei. International Journal of Molecular Sciences, 25(15), 8152. https://doi.org/10.3390/ijms25158152




