Comparison of Double-Stranded DNA at the 5′ and 3′ Ends of the G-Triplex and Its Application in the Detection of Hg(II)
Abstract
:1. Introduction
2. Results and Discussion
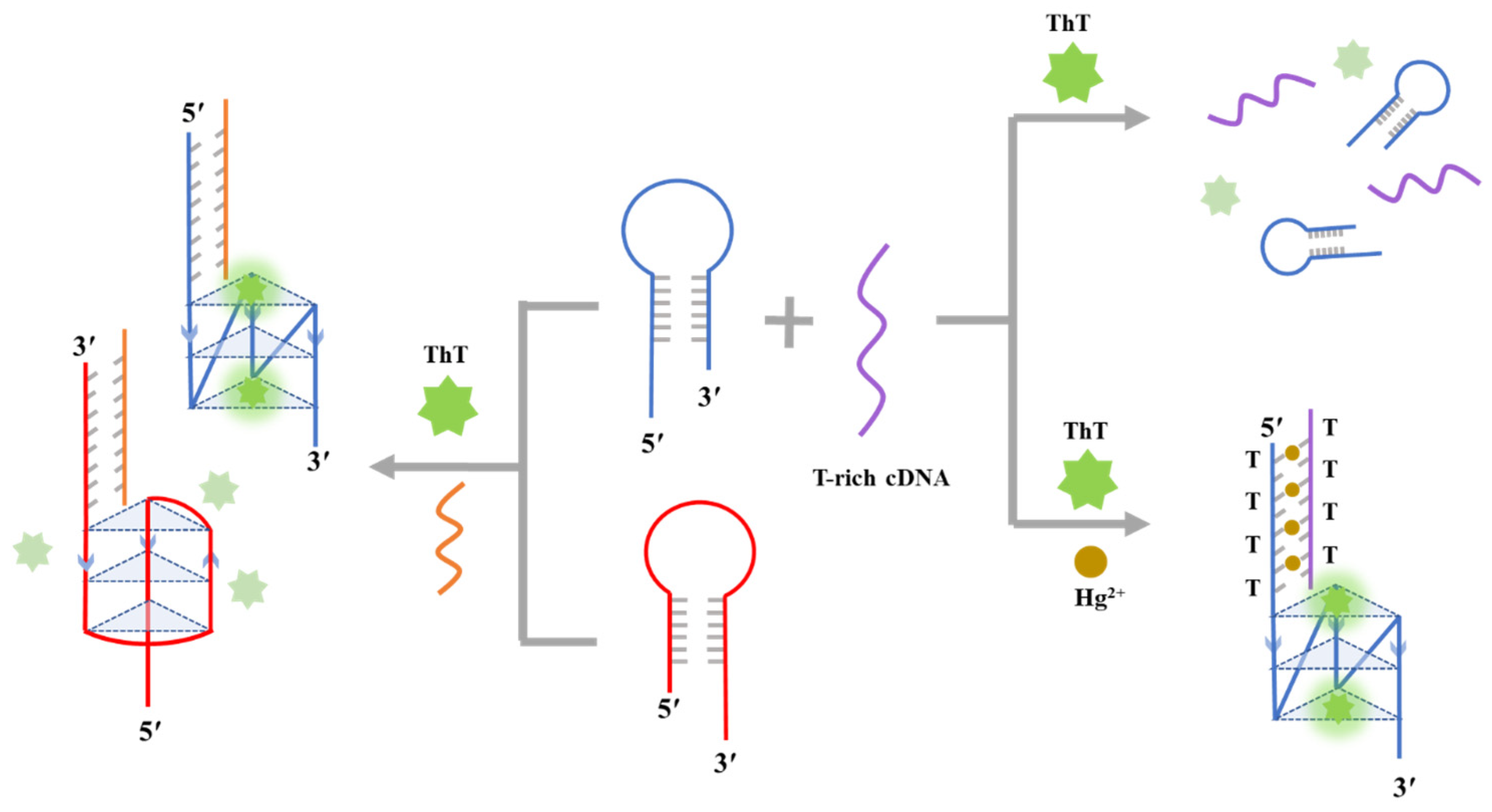
2.1. Principles of Detecting Hg(II)
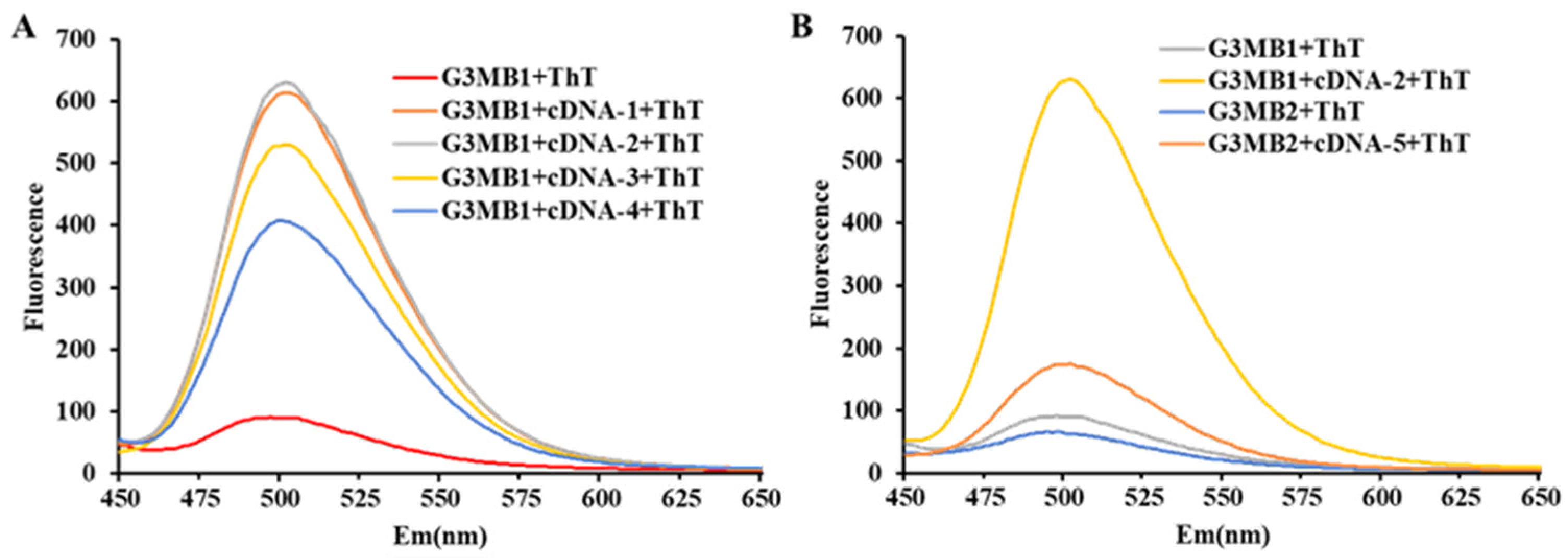
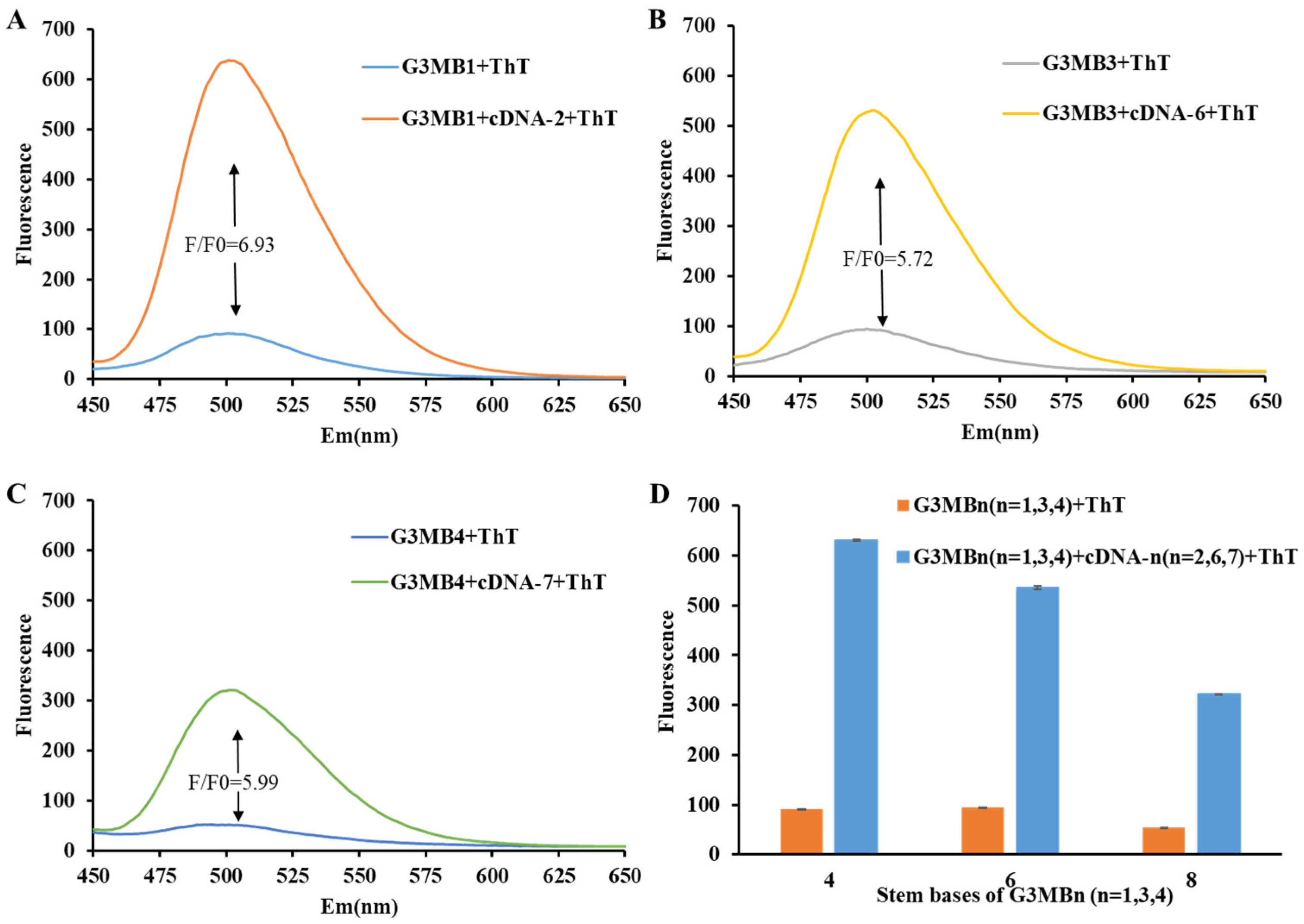
2.2. Effect of 5′/3′-dsDNA on the Fluorescence Signal of G3/ThT
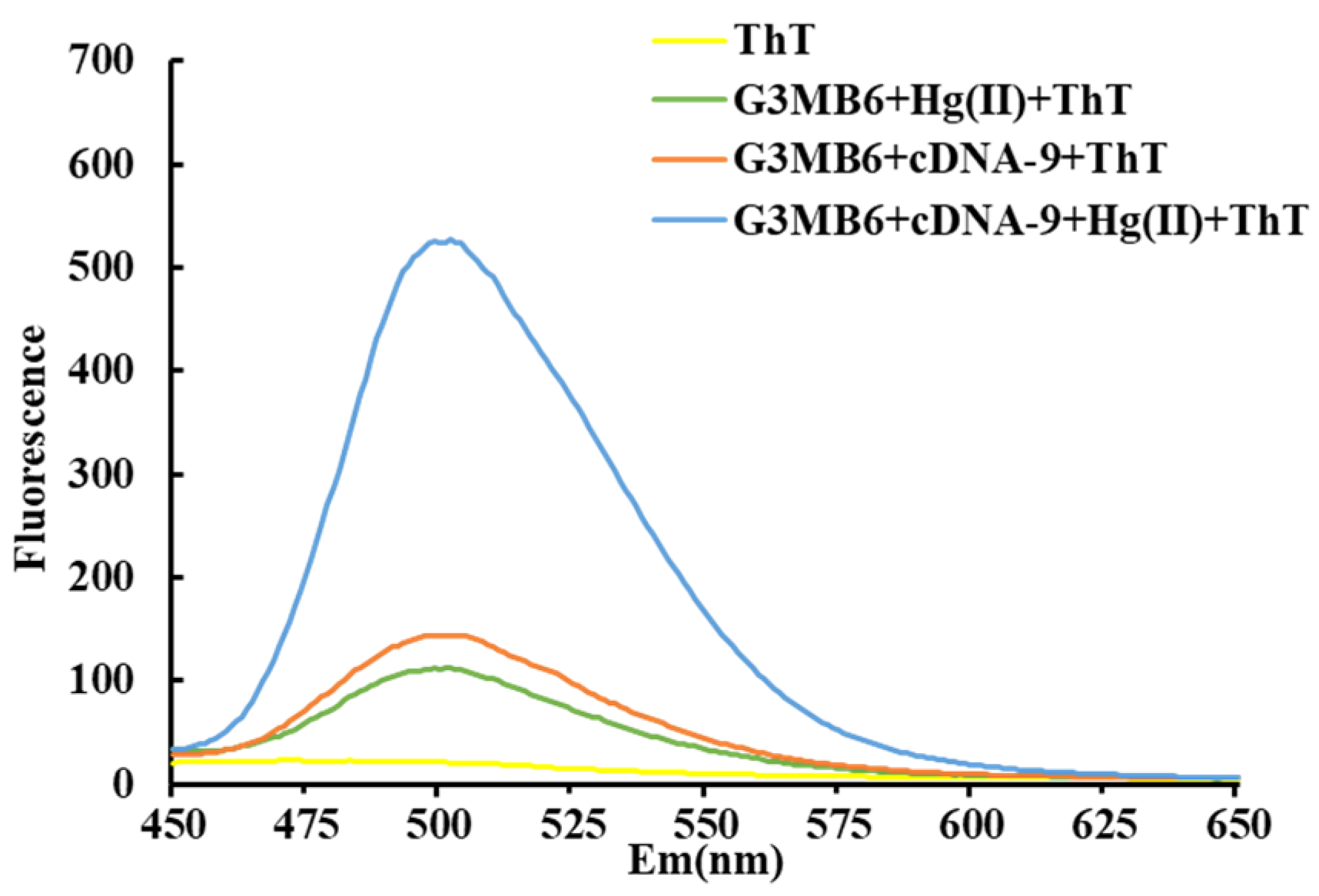
2.3. Feasibility of G3MB6/ThT for Monitoring Hg(II)
2.4. Sensitivity and Selectivity of G3MB6/ThT for Detecting Hg(II)
2.5. Analysis of Hg(II) in Tap Water and Milk
3. Materials and Methods
3.1. Chemicals and Apparatus
3.2. Fluorescence of the G-Triplex with the Adjacent dsDNA
3.3. Fluorescence of Hg(II)
3.4. Detection of Hg(II) in Natural Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Zheng, G.; Kang, X.; Zhang, X.; Guo, J.; Wang, S.; Chen, Y.; Xue, L. Aquatic Bacteria Rheinheimera tangshanensis New Ability for Mercury Pollution Removal. Int. J. Mol. Sci. 2023, 24, 5009. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Ren, X.; Shi, Y.; Zheng, H.; Zhang, Y.; Zuo, Q. A novel covalent organic polymer with hierarchical pore structure for rapid and selective trace Hg(II) removal from drinking water. Sep. Purif. Technol. 2022, 285, 120306. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, G.; Li, J.; Zhang, Y.; Yu, Y.; Wei, Q. Photoelectrochemical determination of Hg(II) via dual signal amplification involving SPR enhancement and a folding-based DNA probe. Microchim. Acta 2017, 184, 1379–1387. [Google Scholar] [CrossRef]
- Garkani-Nejad, Z.; Javar, H.A.; Mahmoudi-Moghaddam, H. An efficient sensor for simultaneous determination of Hg(II) and As(III) using a carbon paste electrode modified with needle-shaped Pt-doped NiCo2O4 nanograss. Sens. Actuators B Chem. 2022, 358, 131445. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, A.; Fang, Y.; Fan, C.; Guo, Y.; Tan, Z.; Yin, Y.; Cai, Y.; Jiang, G. Dithizone-functionalized C18 online solid-phase extraction-HPLC-ICP-MS for speciation of ultra-trace organic and inorganic mercury in cereals and environmental samples. J. Environ. Sci. 2022, 115, 403–410. [Google Scholar] [CrossRef]
- Suárez-Criado, L.; Rodríguez-González, P.; Marrugo-Negrete, J.; Alonso, J.I.G.; Díez, S. Determination of methylmercury and inorganic mercury in human hair samples of individuals from Colombian gold mining regions by double spiking isotope dilution and GC-ICP-MS. Environ. Res. 2023, 231, 115970. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A. DNA-functionalized dye-loaded carbon dots: Ultrabright FRET platform for ratiometric detection of Hg(II) in serum samples and cell microenvironment. Ionics 2019, 25, 4469–4479. [Google Scholar] [CrossRef]
- Muhammad, S.P.; Shah, M.R.; Ullah, R.; Ahmad, I.; Ali, K. Synthesis and Characterization of Functionalized Silver Nanoparticles for Selective Screening of Mercury (II) Ions. Arab. J. Sci. Eng. 2022, 47, 7135–7145. [Google Scholar] [CrossRef]
- Pourbadiei, B.; Eftekhari-Sis, B.; Kordzadeh, A.; Pourjavadi, A. Simultaneous detection of mercury and cadmium ions: A colorimetric method in aqueous media. Heliyon 2023, 9, e21674. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Sui, N.; Liu, M.; Yu, W.W. A colorimetric assay for Hg(II) based on the use of a magnetic aptamer and a hybridization chain reaction. Microchim. Acta 2016, 183, 2855–2860. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Ju, X.; Zhang, J.; Wang, M.; Xing, C.; Yuan, J. Development and optimization of an immunoassay for the detection of Hg(II) in lake water. Food Sci. Nutrition 2019, 7, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, H.; Yang, G.; Liu, S.; Liu, R.; Lv, C. Detection of radon with biosensors based on the lead(II)-induced conformational change of aptamer HTG and malachite green fluorescence probe. J. Environ. Radioact. 2018, 195, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Oda, S.; Yamaguchi, H.; Kondo, Y.; Kojima, C.; Ono, A. 15N-15N J-coupling across Hg(II): Direct observation of Hg(II)-mediated T-T base pairs in a DNA duplex. J. Am. Chem. Soc. 2007, 129, 244–245. [Google Scholar] [CrossRef]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. Mercury(II)-mediated formation of thymine-Hg-II-thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, T.; Wu, Z.; Wang, D.; Hu, F.; Xu, J.; Li, X.; Qiu, J. Label-free hairpin probe for the rapid detection of Hg(II) based on T-Hg(II)-T. Anal. Chim. Acta 2022, 1221, 340113. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, N.; Zhang, Y.; Wei, M.; Li, H.; Yao, S. Label-free DNA sensor for Pb2+ based on a duplex–quadruplex exchange. Anal. Methods 2013, 5, 6100–6150. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Li, X.; Cai, Y.; Gao, Z.; Qiu, J. Development of a split G-quadruplex and DAPI-based fluorescent probe for Hg(III) and Pb(II) ions detection. Anal. Methods 2024, 16, 83–90. [Google Scholar] [CrossRef]
- Ge, J.; Li, X.P.; Jiang, J.H.; Yu, R.Q. A highly sensitive label-free sensor for Mercury ion (Hg2+) by inhibiting thioflavin T as DNA G-quadruplexes fluorescent inducer. Talanta 2014, 122, 85–90. [Google Scholar] [CrossRef]
- Yan, X.; Huang, J.; Xiao, X.; Ma, C.; Zhang, J.; Zhur, O.; Zhou, M.; He, H.; Wu, C. A new method for determination of polysaccharides in adsorption of Hg2+. Microchem. J. 2022, 183, 107962. [Google Scholar] [CrossRef]
- Liu, B. Highly sensitive oligonucleotide-based fluorometric detection of mercury(II) in aqueous media. Biosens. Bioelectron. 2008, 24, 756–760. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, Z.F.; Han, Q.J.; Zhong, H.M.; Peng, J.B.; Li, X.; Fan, X.L. Stable and Label-Free Fluorescent Probe Based on G-triplex DNA and Thioflavin T. Anal. Chem. 2018, 90, 3220–3226. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Wang, P. The effect of adjacent double-strand DNA on the G-triplex-ThT complex fluorescence intensity enhancement and its application in TNOS and Hg2+ detection. Talanta 2023, 252, 123884. [Google Scholar] [CrossRef]
- Lu, X.M.; Li, H.; You, J.; Li, W.; Wang, P.Y.; Li, M.; Dou, S.X.; Xi, X.G. Folding Dynamics of Parallel and Antiparallel G-Triplexes under the Influence of Proximal DNA. J. Phys. Chem. B 2018, 122, 9499–9506. [Google Scholar] [CrossRef]
- Liu, S.; Peng, P.; Wang, H.; Shi, L.; Li, T. Thioflavin T binds dimeric parallel-stranded GA-containing non-G-quadruplex DNAs: A general approach to lighting up double-stranded scaffolds. Nucleic Acids Res. 2017, 45, 12080–12089. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jiang, X.Q.; Lu, L.F.; Zhang, M.; Shi, G. “Molecular beacon”-hosted thioflavin T: Applications for label-free fluorescent detection of iodide and logic operations. Talanta 2016, 150, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yue, Y.; Deng, S.; He, G.; Gao, H.; Zhou, M.; Zhong, K.; Deng, R. Ratiometric-enhanced G-Quadruplex Probes for Amplified and Mix-to-Read Detection of Mercury Pollution in Aquatic Products. J. Agric. Food Chem. 2020, 68, 12124–12131. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Xing, Y.; Zhou, X. Duplex functional G-quadruplex/NMM fluorescent probe for label-free detection of lead(II) and mercury(II) ions. J. Hazard. Mater. 2018, 355, 50–55. [Google Scholar] [CrossRef]
- Song, X.; Fu, B.; Lan, Y.; Chen, Y.; Wei, Y.; Dong, C. Label-free fluorescent aptasensor berberine-based strategy for ultrasensitive detection of Hg2+; ion. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2018, 204, 301–307. [Google Scholar] [CrossRef]
- Yuan, T.; Hu, L.; Liu, Z.; Qi, W.; Zhu, S.; Xu, G. A label-free and signal-on supersandwich fluorescent platform for Hg2+ sensing. Anal. Chim. Acta 2013, 793, 86–89. [Google Scholar] [CrossRef]
- Kong, R.M.; Ma, L.; Han, X.; Ma, C.; Qu, F.; Xia, L. Hg2+-mediated stabilization of G-triplex based molecular beacon for label-free fluorescence detection of Hg2+, reduced glutathione, and glutathione reductase activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117855. [Google Scholar] [CrossRef] [PubMed]


| Intercalator | Linear Range (μM) | LOD (nM) | References |
|---|---|---|---|
| DAPI + NMM | 0–20 | 300 | [27] |
| DAPI | 0–1.4 | 2.87 | [16] |
| NMM | 0–1 | 18.6 | [28] |
| Berberine | 0–10 | 7.7 | [29] |
| Genefinder | 0–0.3 | 2.5 | [30] |
| ThT | 0–4 | 20 | [31] |
| ThT | 0–0.3 | 5.32 | This work |
| Sample | Group | Added (nM) | Repeats | Found (nM) | Recovery (%) | Avg. (nM) |
|---|---|---|---|---|---|---|
| Milk | 1 | 19.24 | ||||
| No.1 | 20 | 2 | 19.24 | 100.30 | 20.061 | |
| 3 | 21.70 | |||||
| 1 | 102.11 | |||||
| No.2 | 100 | 2 | 102.93 | 103.20 | 103.203 | |
| 3 | 104.57 | |||||
| 1 | 253.08 | |||||
| No.3 | 250 | 2 | 255.54 | 101.12 | 252.803 | |
| 3 | 249.79 | |||||
| Water | 1 | 19.24 | ||||
| No.1 | 20 | 2 | 20.06 | 101.67 | 20.334 | |
| 3 | 21.70 | |||||
| 1 | 102.93 | |||||
| No.2 | 100 | 2 | 103.75 | 103.75 | 103.750 | |
| 3 | 104.57 | |||||
| 1 | 254.72 | |||||
| No.3 | 250 | 2 | 256.36 | 102.54 | 256.359 | |
| 3 | 258.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wu, Z.; Li, X.; Hu, X.; Wu, J.; You, Z.; Qiu, J. Comparison of Double-Stranded DNA at the 5′ and 3′ Ends of the G-Triplex and Its Application in the Detection of Hg(II). Int. J. Mol. Sci. 2024, 25, 8159. https://doi.org/10.3390/ijms25158159
Cai Y, Wu Z, Li X, Hu X, Wu J, You Z, Qiu J. Comparison of Double-Stranded DNA at the 5′ and 3′ Ends of the G-Triplex and Its Application in the Detection of Hg(II). International Journal of Molecular Sciences. 2024; 25(15):8159. https://doi.org/10.3390/ijms25158159
Chicago/Turabian StyleCai, Yule, Ziyi Wu, Xiangxiang Li, Xingting Hu, Jiamin Wu, Zhengying You, and Jieqiong Qiu. 2024. "Comparison of Double-Stranded DNA at the 5′ and 3′ Ends of the G-Triplex and Its Application in the Detection of Hg(II)" International Journal of Molecular Sciences 25, no. 15: 8159. https://doi.org/10.3390/ijms25158159





