Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments
Abstract
1. Introduction
1.1. Background on Bone Metastases
1.2. Pathophysiology of Bone Metastases
1.3. Introduction to Interleukins
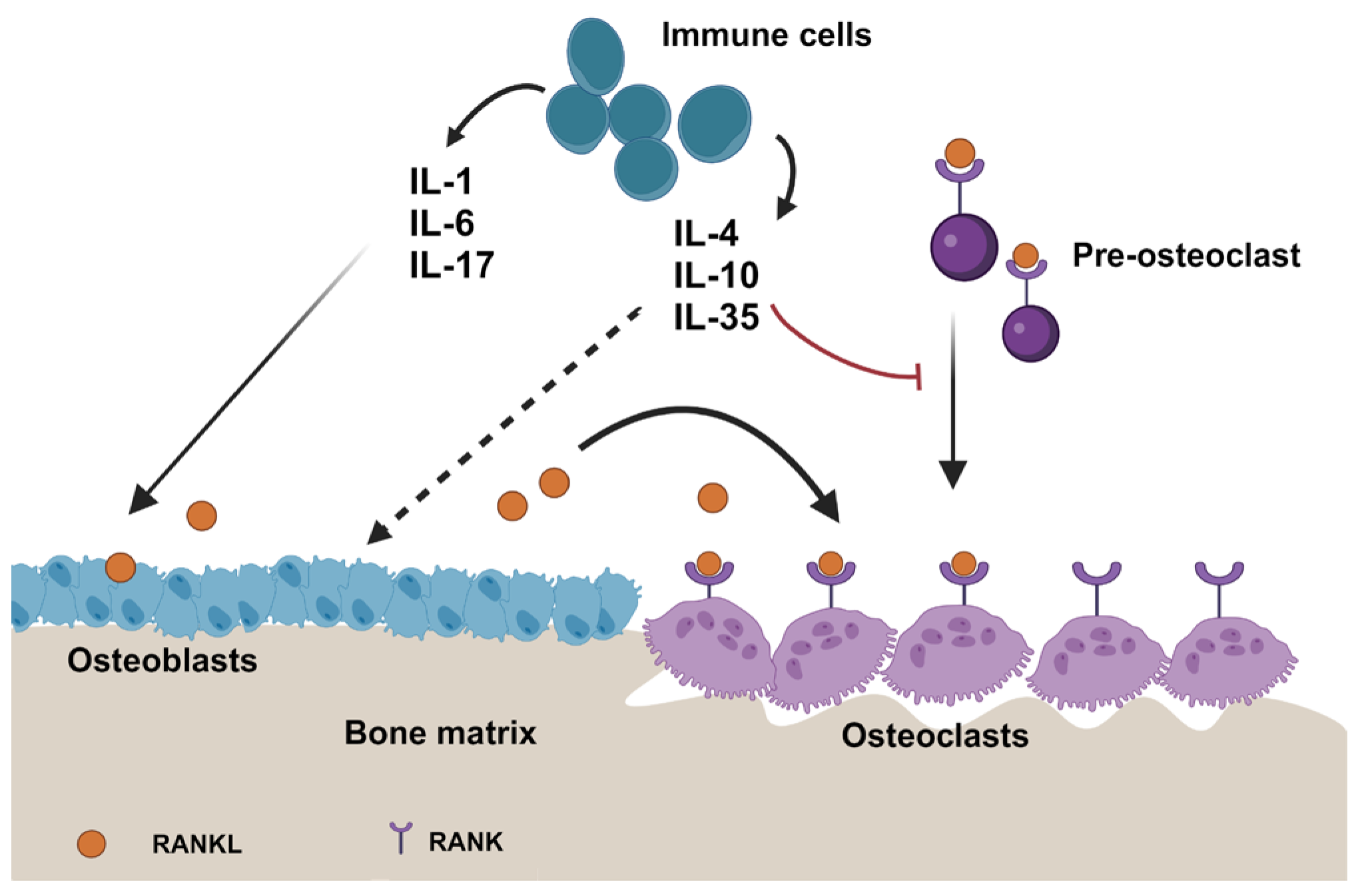
1.4. Interleukins in the Immune Modulation
1.5. Influence of Interleukins on the Bone Microenvironment
1.6. Impact on the Immune Microenvironment in Bone Metastases
2. Interleukins and Cancer
2.1. Pro-Inflammatory Interleukins and Cancer
2.2. Pro-Inflammatory Interleukins and Bone Metastases
2.3. Anti-Inflammatory Interleukins and Cancer
2.4. Anti-Inflammatory Interleukins and Bone Metastases
3. Signaling Pathways Involved
3.1. NF-κB Pathway
3.2. JAK/STAT Pathway
3.3. MAPK Pathway
4. Therapeutic Implications of Targeting Interleukins
4.1. Current Therapeutic Approaches for Bone Metastases
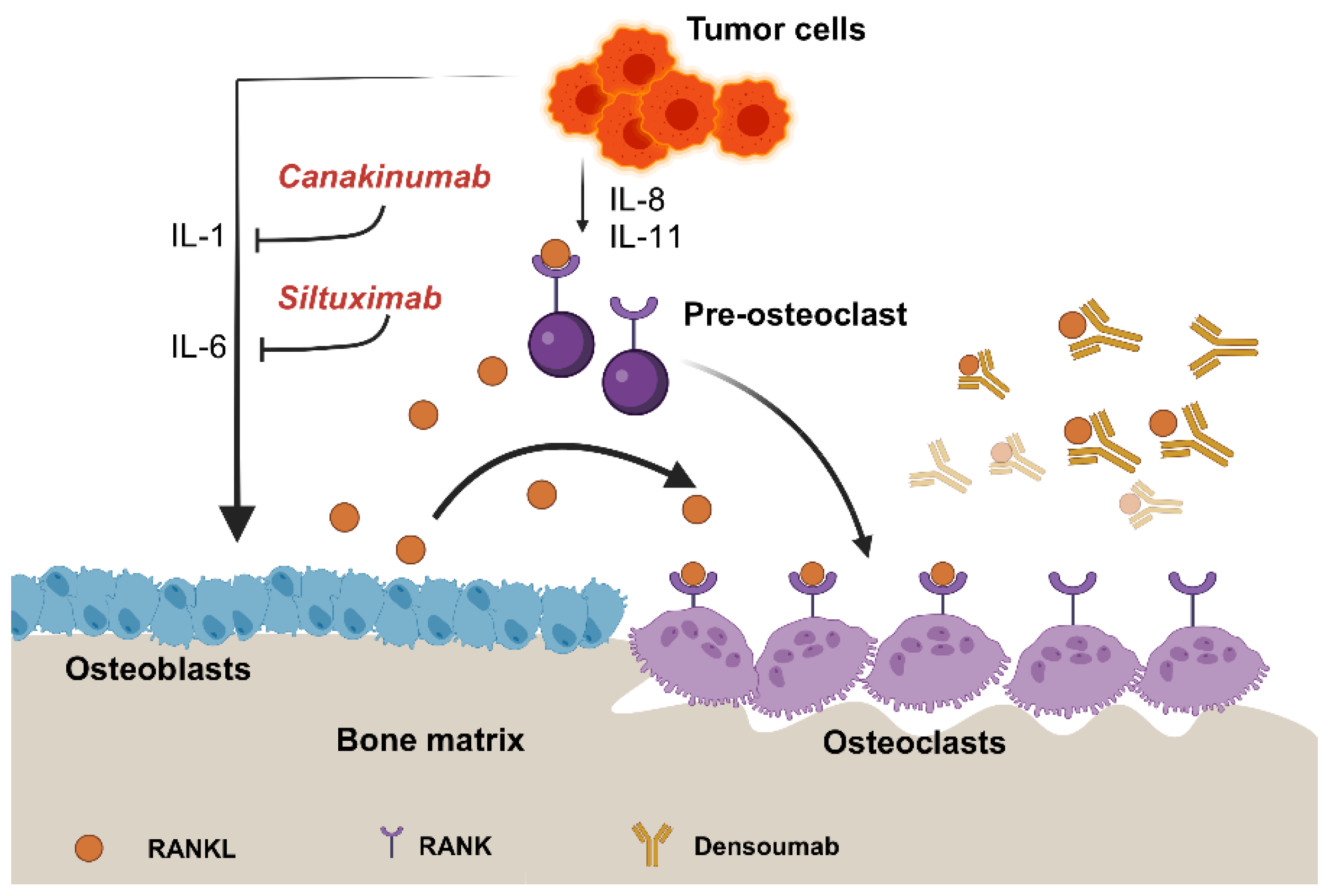
4.2. Overview of Drugs Targeting Interleukins
4.2.1. Tocilizumab
4.2.2. Siltuximab (CNTO 328)
4.2.3. Bimekizumab
4.2.4. ANV419
4.2.5. Canakinumab
4.2.6. Denosumab
4.3. Summary of Clinical Trials and Outcomes
4.4. Combinational Therapies
4.5. Treatment Challenges and Limitations
4.6. Cost Effectiveness
5. Interleukins as Diagnostic and Prognostic Biomarkers
6. Gaps in Current Research and Future Directions
6.1. Research Gaps
6.2. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef]
- Elaasser, B.; Arakil, N.; Mohammad, K.S. Bridging the Gap in Understanding Bone Metastasis: A Multifaceted Perspective. Int. J. Mol. Sci. 2024, 25, 2846. [Google Scholar] [CrossRef]
- Sethakorn, N.; Heninger, E.; Sánchez-de-Diego, C.; Ding, A.B.; Yada, R.C.; Kerr, S.C.; Kosoff, D.; Beebe, D.J.; Lang, J.M. Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers 2022, 14, 757. [Google Scholar] [CrossRef]
- Marino, S.; Idris, A.I. Emerging Therapeutic Targets in Cancer Induced Bone Disease: A Focus on the Peripheral Type 2 Cannabinoid Receptor. Pharmacol. Res. 2017, 119, 391–403. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Salamanna, F.; Borsari, V.; Contartese, D.; Costa, V.; Giavaresi, G.; Fini, M. What Is the Role of Interleukins in Breast Cancer Bone Metastases? A Systematic Review of Preclinical and Clinical Evidence. Cancers 2019, 11, 2018. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Tong, Y.; Cao, Y.; Jin, T.; Huang, Z.; He, Q.; Mao, M. Role of Interleukin-1 Family in Bone Metastasis of Prostate Cancer. Front. Oncol. 2022, 12, 951167. [Google Scholar] [CrossRef]
- Arakil, N.; Akhund, S.A.; Elaasser, B.; Mohammad, K.S. Intersecting Paths: Unraveling the Complex Journey of Cancer to Bone Metastasis. Biomedicines 2024, 12, 1075. [Google Scholar] [CrossRef]
- Hamash, K. Comprehensive Clinical Literature Review of Managing Bone Metastases in Breast Cancer: Focus on Pain and Skeletal-Related Events. Clin. J. Oncol. Nurs. 2023, 27, 615–628. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Thorpe, M.P. Bone Metastases. Cancer J. Sudbury Mass 2024, 30, 202–209. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Yue, X.; Li, X. Bone Serves as a Transfer Station for Secondary Dissemination of Breast Cancer. Bone Res. 2023, 11, 21. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Li, Z.; Wu, Y.; Tong, Z. Comparison of the Clinicopathological Characteristics and Prognosis between Chinese Patients with Breast Cancer with Bone-Only and Non-Bone-Only Metastasis. Oncol. Lett. 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Belezikian, J. Principles of Bone Biology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Granata, V.; Crisafulli, L.; Nastasi, C.; Ficara, F.; Sobacchi, C. Bone Marrow Niches and Tumour Cells: Lights and Shadows of a Mutual Relationship. Front. Immunol. 2022, 13, 884024. [Google Scholar] [CrossRef]
- Wilson, C.; Brown, H.; Holen, I. The Endocrine Influence on the Bone Microenvironment in Early Breast Cancer. Endocr. Relat. Cancer 2016, 23, R567–R576. [Google Scholar] [CrossRef]
- Sohail, A.; Sherin, L.; Butt, S.; Javed, S.; Li, Z.; Iqbal, S.; Beg, O. Role of Key Players in Paradigm Shifts of Prostate Cancer Bone Metastasis. Cancer Manag. Res. 2018, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, J. Role of the Bone Microenvironment in Bone Metastasis of Malignant Tumors—Therapeutic Implications. Cell. Oncol. Dordr. 2020, 43, 751–761. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone Metastasis: The Importance of the Neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Kreps, L.M.; Addison, C.L. Targeting Intercellular Communication in the Bone Microenvironment to Prevent Disseminated Tumor Cell Escape from Dormancy and Bone Metastatic Tumor Growth. Int. J. Mol. Sci. 2021, 22, 2911. [Google Scholar] [CrossRef]
- Russo, S.; Scotto Di Carlo, F.; Gianfrancesco, F. The Osteoclast Traces the Route to Bone Tumors and Metastases. Front. Cell Dev. Biol. 2022, 10, 886305. [Google Scholar] [CrossRef]
- Dai, R.; Liu, M.; Xiang, X.; Xi, Z.; Xu, H. Osteoblasts and Osteoclasts: An Important Switch of Tumour Cell Dormancy during Bone Metastasis. J. Exp. Clin. Cancer Res. 2022, 41, 316. [Google Scholar] [CrossRef]
- Renema, N.; Navet, B.; Heymann, M.-F.; Lezot, F.; Heymann, D. RANK–RANKL Signalling in Cancer. Biosci. Rep. 2016, 36, e00366. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.J.; Devarakonda, S.; Govindan, R. Bone Metastases in Non-Small Cell Lung Cancer: A Narrative Review. J. Thorac. Dis. 2022, 14, 1696–1712. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Hempel, H.A.; De Marzo, A.M. Inflammation and Cancer. In Advances in Experimental Medicine and Biology; Aggarwal, B.B., Ed.; Springer: Basel, Switzerland; Heidelberg, Germany, 2014. [Google Scholar]
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The Role of the Bone Microenvironment in Skeletal Metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chow, S.-O.; Boernert, K.; Basel, D.; Mikuscheva, A.; Kim, S.; Fong-Yee, C.; Trivedi, T.; Buttgereit, F.; Sutherland, R.L.; et al. Direct Crosstalk between Cancer and Osteoblast Lineage Cells Fuels Metastatic Growth in Bone via Auto-Amplification of IL-6 and RANKL Signaling Pathways. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.-J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra Are Key Regulators of the Inflammatory Response to RNA Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Beringer, A.; Miossec, P. IL-17 and TNF-α Co-Operation Contributes to the Proinflammatory Response of Hepatic Stellate Cells. Clin. Exp. Immunol. 2019, 198, 111–120. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-Inflammatory and Immune-Regulatory Cytokines in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Nikovics, K.; Favier, A.-L.; Rocher, M.; Mayinga, C.; Gomez, J.; Dufour-Gaume, F.; Riccobono, D. In Situ Identification of Both IL-4 and IL-10 Cytokine-Receptor Interactions during Tissue Regeneration. Cells 2023, 12, 1522. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2022, 24, 641. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in Cancer: From Biology to Therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The Family of the Interleukin-1 Receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef] [PubMed]
- Nitz, R.; Lokau, J.; Aparicio-Siegmund, S.; Scheller, J.; Garbers, C. Modular Organization of Interleukin-6 and Interleukin-11 α-Receptors. Biochimie 2015, 119, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Brändlein, S.; Ehrhardt, I.; Krause, S.; Luttmann, W. Interleukin-4- and Interleukin-13 Receptors Trigger Distinct JAK/STAT Activation Patterns in Mouse Lymphocytes. Signal Transduct. 2003, 3, 26–32. [Google Scholar] [CrossRef]
- Foxwell, B.M.J.; Barrett, K.; Feldmann, M. Cytokine Receptors: Structure and Signal Transduction. Clin. Exp. Immunol. 2008, 90, 161–169. [Google Scholar] [CrossRef]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 Axis for Targeted Immune Modulation. Immunol. Rev. 2023, 320, 29–57. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Bendelac, A.; Watson, C.; Hu-Li, J.; Paul, W.E. Role of NK1.1 + T Cells in a T H 2 Response and in Immunoglobulin E Production. Science 1995, 270, 1845–1847. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate Production of TH2 Cytokines by Adipose Tissue-Associated c-Kit+Sca-1+ Lymphoid Cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Nonaka, M.; Nonaka, R.; Woolley, K.; Adelroth, E.; Miura, K.; Okhawara, Y.; Glibetic, M.; Nakano, K.; O’Byrne, P.; Dolovich, J. Distinct Immunohistochemical Localization of IL-4 in Human Inflamed Airway Tissues. IL-4 Is Localized to Eosinophils in Vivo and Is Released by Peripheral Blood Eosinophils. J. Immunol. Baltim. 1995, 155, 3234–3244. [Google Scholar] [CrossRef]
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent Advances in Understanding the Role of IL-4 Signaling. Fac. Rev. 2021, 10, 71. [Google Scholar] [CrossRef]
- Rani, L.; Kumar, A.; Karhade, J.; Pandey, G.; Guha, A.; Mishra, G.C.; Wani, M.R. IL-3 Regulates the Differentiation of Pathogenic Th17 Cells. Eur. J. Immunol. 2022, 52, 1842–1858. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Liu, D.; Zhang, H.; Yang, J.; Shen, H.; Xia, L.; Yao, L.; Lu, J. Interleukin-35 Protein Inhibits Osteoclastogenesis and Attenuates Collagen-Induced Arthritis in Mice. J. Cell. Physiol. 2024. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Gan, X.; Liu, J.; Bao, C.; He, C. Roles of IL-11 in the Regulation of Bone Metabolism. Front. Endocrinol. 2023, 14, 1290130. [Google Scholar] [CrossRef]
- Dai, Z.; Chen, Y.; He, E.; Wang, H.; Guo, W.; Wu, Z.; Huang, K.; Zhao, Q. Interleukin-19 Promotes Bone Resorption by Suppressing Osteoprotegerin Expression in BMSCs in a Lipopolysaccharide-Induced Bone Loss Mouse Model. Bone Jt. Res. 2023, 12, 691–701. [Google Scholar] [CrossRef]
- Hou, J.; Xu, P.; Zhong, Y.; Zhou, Z.; Zhang, W. Interleukin-21 Knockout Reduces Bone Loss in Ovariectomized Mice by Inhibiting Osteoclastogenesis. Biosci. Biotechnol. Biochem. 2023, 87, 1265–1273. [Google Scholar] [CrossRef]
- Kelly Smith, J. Exercise, Interleukins and Bone Homeostasis. Integr. Mol. Med. 2016, 3, 802–804. [Google Scholar] [CrossRef]
- Haider, M.-T.; Ridlmaier, N.; Smit, D.J.; Taipaleenmäki, H. Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2021, 22, 2898. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Nakaminami, Y.; Takahata, Y.; Hata, K.; Nishimura, R. Activation and Function of NLRP3 Inflammasome in Bone and Joint-Related Diseases. Int. J. Mol. Sci. 2022, 23, 5365. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The Mechanism of Osteoclast Differentiation Induced by IL-1. J. Immunol. Baltim. 2009, 183, 1862–1870. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Fujikado, N.; Manaka, H.; Yasuda, H.; Iwakura, Y. IL-1 Plays an Important Role in the Bone Metabolism under Physiological Conditions. Int. Immunol. 2010, 22, 805–816. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Samuel, S.G.; Schroder, K.; Lévesque, J.-P.; Alexander, K.A. Inflammasomes and the IL-1 Family in Bone Homeostasis and Disease. Curr. Osteoporos. Rep. 2022, 20, 170–185. [Google Scholar] [CrossRef]
- Landuzzi, L.; Ruzzi, F.; Pellegrini, E.; Lollini, P.-L.; Scotlandi, K.; Manara, M.C. IL-1 Family Members in Bone Sarcomas. Cells 2024, 13, 233. [Google Scholar] [CrossRef]
- Liang, J.D.; Hock, J.M.; Sandusky, G.E.; Santerre, R.F.; Onyia, J.E. Immunohistochemical Localization of Selected Early Response Genes Expressed in Trabecular Bone of Young Rats given hPTH 1-34. Calcif. Tissue Int. 1999, 65, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T. IL-6 Is Produced by Osteoblasts and Induces Bone Resorption. J. Immunol. Baltim. 1990, 145, 3297–3303. [Google Scholar] [CrossRef]
- Briso, E.M.; Dienz, O.; Rincon, M. Cutting Edge: Soluble IL-6R Is Produced by IL-6R Ectodomain Shedding in Activated CD4 T Cells. J. Immunol. Baltim. 2008, 180, 7102–7106. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, M.; Rubert, M.; Montero, M.; de la Piedra, C. Effects of Cyclosporine, Tacrolimus, and Rapamycin on Osteoblasts. Transplant. Proc. 2017, 49, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Modur, V.; Li, Y.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Retrograde Inflammatory Signaling from Neutrophils to Endothelial Cells by Soluble Interleukin-6 Receptor Alpha. J. Clin. Investig. 1997, 100, 2752–2756. [Google Scholar] [CrossRef]
- Tawara, K.; Oxford, J.T.; Jorcyk, C.L. Clinical Significance of Interleukin (IL)-6 in Cancer Metastasis to Bone: Potential of Anti-IL-6 Therapies. Cancer Manag. Res. 2011, 3, 177–189. [Google Scholar] [CrossRef]
- Liu, X.-H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. The Role of the Interleukin-6/Gp130 Signaling Pathway in Bone Metabolism. Vitam. Horm. 2006, 74, 341–355. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Combination of IL-6 and sIL-6R Differentially Regulate Varying Levels of RANKL-Induced Osteoclastogenesis through NF-κB, ERK and JNK Signaling Pathways. Sci. Rep. 2017, 7, 41411. [Google Scholar] [CrossRef]
- Feng, W.; Yang, P.; Liu, H.; Zhang, F.; Li, M. IL-6 Promotes Low Concentration of RANKL-Induced Osteoclastic Differentiation by Mouse BMMs through Trans-Signaling Pathway. J. Mol. Histol. 2022, 53, 599–610. [Google Scholar] [CrossRef]
- Hao, Z.; Ma, Y.; Wu, J.; Li, X.; Chen, H.; Shen, J.; Wang, H. Osteocytes Regulate Osteoblast Differentiation and Osteoclast Activity through Interleukin-6 under Mechanical Loading. RSC Adv. 2017, 7, 50200–50209. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Bakker, A.D.; Kulkarni, R.N.; Klein-Nulend, J.; Lems, W.F. IL-6 Alters Osteocyte Signaling toward Osteoblasts but Not Osteoclasts. J. Dent. Res. 2014, 93, 394–399. [Google Scholar] [CrossRef]
- Lazzaro, L.; Tonkin, B.A.; Poulton, I.J.; McGregor, N.E.; Ferlin, W.; Sims, N.A. IL-6 Trans-Signalling Mediates Trabecular, but Not Cortical, Bone Loss after Ovariectomy. Bone 2018, 112, 120–127. [Google Scholar] [CrossRef]
- Huang, R.-L.; Sun, Y.; Ho, C.-K.; Liu, K.; Tang, Q.-Q.; Xie, Y.; Li, Q. IL-6 Potentiates BMP-2-Induced Osteogenesis and Adipogenesis via Two Different BMPR1A-Mediated Pathways. Cell Death Dis. 2018, 9, 144. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, S.; Chen, K.; Yuan, S.; Hu, J.; Wang, H. IL-17A Regulates Autophagy and Promotes Osteoclast Differentiation through the ERK/mTOR/Beclin1 Pathway. PLoS ONE 2023, 18, e0281845. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Chothani, S.; Viswanathan, S.; Goh, J.W.T.; Lim, W.-W.; Cook, S.A. IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues. Int. J. Mol. Sci. 2022, 23, 8900. [Google Scholar] [CrossRef]
- McCoy, E.M.; Hong, H.; Pruitt, H.C.; Feng, X. IL-11 Produced by Breast Cancer Cells Augments Osteoclastogenesis by Sustaining the Pool of Osteoclast Progenitor Cells. BMC Cancer 2013, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. Osteotropic Agents Regulate the Expression of Osteoclast Differentiation Factor and Osteoprotegerin in Osteoblastic Stromal Cells. Endocrinology 1998, 139, 4743. [Google Scholar] [CrossRef] [PubMed]
- Romas, E.; Udagawa, N.; Zhou, H.; Tamura, T.; Saito, M.; Taga, T.; Hilton, D.J.; Suda, T.; Ng, K.W.; Martin, T.J. The Role of Gp130-Mediated Signals in Osteoclast Development: Regulation of Interleukin 11 Production by Osteoblasts and Distribution of Its Receptor in Bone Marrow Cultures. J. Exp. Med. 1996, 183, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.C.; Poulton, I.J.; McGregor, N.E.; Ho, P.W.; Allan, E.H.; Quach, J.M.; Martin, T.J.; Sims, N.A. Sustained RANKL Response to Parathyroid Hormone in Oncostatin M Receptor-Deficient Osteoblasts Converts Anabolic Treatment to a Catabolic Effect in Vivo. J. Bone Miner. Res. 2012, 27, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.; Shi, Z.; Xu, D.; Luo, S.; Siegal, G.P.; Feng, X.; Wei, S. Interleukin-4 Inhibits RANKL-Induced NFATc1 Expression via STAT6: A Novel Mechanism Mediating Its Blockade of Osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3385–3392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin Inhibits Osteoclastogenesis through Modulation of Helper T Cells-Secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, X.; Moreno, J.L.; Farber, D.L.; Keegan, A.D. NF-κB Signaling Participates in Both RANKL- and IL-4-Induced Macrophage Fusion: Receptor Cross-Talk Leads to Alterations in NF-κB Pathways. J. Immunol. Baltim. 2011, 187, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Qiu, M.; Chen, J. IL-4 Modulates Macrophage Polarization in Ankylosing Spondylitis. Cell. Physiol. Biochem. 2015, 35, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Feng, X.; He, Y.; Gao, Y.; Yang, S.; Shao, Z.; Yang, C.; Wang, H.; Ye, Z. IL-4 Administration Exerts Preventive Effects via Suppression of Underlying Inflammation and TNF-α-Induced Apoptosis in Steroid-Induced Osteonecrosis. Osteoporos. Int. 2016, 27, 1827–1837. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 Inhibits Bone Resorption: A Potential Therapeutic Strategy in Periodontitis and Other Bone Loss Diseases. BioMed Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef]
- Park-Min, K.-H.; Ji, J.-D.; Antoniv, T.; Reid, A.C.; Silver, R.B.; Humphrey, M.B.; Nakamura, M.; Ivashkiv, L.B. IL-10 Suppresses Calcium-Mediated Costimulation of Receptor Activator NF-Kappa B Signaling during Human Osteoclast Differentiation by Inhibiting TREM-2 Expression. J. Immunol. Baltim. 2009, 183, 2444–2455. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, C.; Chen, L.; Endo, Y.; Sun, Y.; Zhou, W.; Hu, Y.; Hu, L.; Chen, D.; Xue, H.; et al. IL-10 Induces MC3T3-E1 Cells Differentiation towards Osteoblastic Fate in Murine Model. J. Cell. Mol. Med. 2020, 24, 1076–1086. [Google Scholar] [CrossRef]
- Fujioka, K.; Kishida, T.; Kukida, Y.; Nagahara, H.; Fujii, W.; Murakami, K.; Seno, T.; Yamamoto, A.; Kohno, M.; Mazda, O.; et al. SAT0562 Directly Reprogrammed Osteoblasts Genetically Engineered to Produce Interleukin-10 Significantly Suppress Osteoclastgenesis. Ann. Rheum. Dis. 2014, 73 (Suppl. 2), 794. [Google Scholar] [CrossRef]
- Meng, B.; Wu, D.; Cheng, Y.; Huang, P.; Liu, Y.; Gan, L.; Liu, C.; Cao, Y. Interleukin-20 Differentially Regulates Bone Mesenchymal Stem Cell Activities in RANKL-induced Osteoclastogenesis through the OPG/RANKL/RANK Axis and the NF-κB, MAPK and AKT Signalling Pathways. Scand. J. Immunol. 2020, 91, e12874. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Yuan, L.; Yao, L.; Yang, J.; Xia, L.; Shen, H.; Lu, J. Interleukin-35 Is Involved in Angiogenesis/Bone Remodeling Coupling Through T Helper 17/Interleukin-17 Axis. Front. Endocrinol. 2021, 12, 642676. [Google Scholar] [CrossRef]
- Yago, T.; Nanke, Y.; Kawamoto, M.; Kobashigawa, T.; Yamanaka, H.; Kotake, S. IL-35 Inhibits Human Osteoclastogenesis from Monocytes Induced by Receptor-Activator of NF-κB Ligand. Cent. Eur. J. Immunol. 2018, 43, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Lu, J. Interleukin-35 Promote Osteogenesis and Inhibit Adipogenesis: Role of Wnt/β-Catenin and PPARγ Signaling Pathways. Inflammation 2023, 46, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Rousselle, A.V. Gp130 Cytokine Family and Bone Cells. Cytokine 2000, 12, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The Dual Role of IL-6-Type Cytokines on Bone Remodeling and Bone Tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Aboelnazar, S.; Ghoneim, H.; Shalaby, T.; Sorour, S.; Osman, E.M. Modulatory Effect of Interleukin-2 Loaded Chitosan Nano Sphere on Regulatory T Cell Activity in Streptozotocin-Induced Diabetic Mice. Int. Immunopharmacol. 2024, 132, 112019. [Google Scholar] [CrossRef]
- Kim, N.; Yi, E.; Lee, E.; Park, H.J.; Kim, H.S. Interleukin-2 Is Required for NKp30-Dependent NK Cell Cytotoxicity by Preferentially Regulating NKp30 Expression. Front. Immunol. 2024, 15, 1388018. [Google Scholar] [CrossRef]
- Gallardo-Vera, F.; Tapia-Rodriguez, M.; Diaz, D.; Fortoul Van Der Goes, T.; Montaño, L.F.; Rendón-Huerta, E.P. Vanadium Pentoxide Increased PTEN and Decreased SHP1 Expression in NK-92MI Cells, Affecting PI3K-AKT-mTOR and Ras-MAPK Pathways. J. Immunotoxicol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Ko, E.; Yoon, T.; Lee, Y.; Kim, J.; Park, Y.-B. ADSC Secretome Constrains NK Cell Activity by Attenuating IL-2-Mediated JAK-STAT and AKT Signaling Pathway via Upregulation of CIS and DUSP4. Stem Cell Res. Ther. 2023, 14, 329. [Google Scholar] [CrossRef]
- Shouse, A.N.; LaPorte, K.M.; Malek, T.R. Interleukin-2 Signaling in the Regulation of T Cell Biology in Autoimmunity and Cancer. Immunity 2024, 57, 414–428. [Google Scholar] [CrossRef]
- Cheng, M.L.; Fong, L. Effects of RANKL-Targeted Therapy in Immunity and Cancer. Front. Oncol. 2014, 3, 329. [Google Scholar] [CrossRef]
- Da Cunha, B.R.; Domingos, C.; Stefanini, A.C.B.; Henrique, T.; Polachini, G.M.; Castelo-Branco, P.; Tajara, E.H. Cellular Interactions in the Tumor Microenvironment: The Role of Secretome. J. Cancer 2019, 10, 4574–4587. [Google Scholar] [CrossRef]
- He, N.; Jiang, J. Contribution of Immune Cells to Bone Metastasis Pathogenesis. Front. Endocrinol. 2022, 13, 1019864. [Google Scholar] [CrossRef]
- Zhou, J.; Tulotta, C.; Ottewell, P.D. IL-1β in Breast Cancer Bone Metastasis. Expert Rev. Mol. Med. 2022, 24, e11. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; Lefley, D.V.; Moore, C.K.; Amariutei, A.E.; Spicer-Hadlington, A.R.; Quayle, L.A.; Hughes, R.O.; Ahmed, K.; Cookson, V.; Evans, C.A.; et al. IL-1B Drives Opposing Responses in Primary Tumours and Bone Metastases; Harnessing Combination Therapies to Improve Outcome in Breast Cancer. Npj Breast Cancer 2021, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Zhang, Y.; Zheng, C.; Huang, Q.; Lu, J.; Pulver, E.M.; Houthuijzen, J.; Hutten, S.; Luo, R.; He, J.; et al. Metastasis of Breast Cancer to Bones Alters the Tumor Immune Microenvironment. Eur. J. Med. Res. 2023, 28, 119. [Google Scholar] [CrossRef]
- Zhang, C.S.; Kim, H.; Mullins, G.; Tyryshkin, K.; LeBrun, D.P.; Elliott, B.E.; Greer, P.A. Interleukin-4 Expressed By Neoplastic Cells Provokes an Anti-Metastatic Myeloid Immune Response. J. Clin. Cell. Immunol. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Ito, S.-E.; Shirota, H.; Kasahara, Y.; Saijo, K.; Ishioka, C. IL-4 Blockade Alters the Tumor Microenvironment and Augments the Response to Cancer Immunotherapy in a Mouse Model. Cancer Immunol. Immunother. CII 2017, 66, 1485–1496. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Z. Immune Modulation of Metastatic Niche Formation in the Bone. Front. Immunol. 2021, 12, 765994. [Google Scholar] [CrossRef]
- Lee, H.-M.; Lee, H.-J.; Chang, J.-E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef]
- Wang, W.; Dong, G.; Yang, Z.; Li, S.; Li, J.; Wang, L.; Zhu, Q.; Wang, Y. Single-Cell Analysis of Tumor Microenvironment and Cell Adhesion Reveals That Interleukin-1 Beta Promotes Cancer Cell Proliferation in Breast Cancer. Anim. Models Exp. Med. 2024. [Google Scholar] [CrossRef]
- Song, M.; Tang, Y.; Cao, K.; Qi, L.; Xie, K. Unveiling the Role of Interleukin-6 in Pancreatic Cancer Occurrence and Progression. Front. Endocrinol. 2024, 15, 1408312. [Google Scholar] [CrossRef]
- Ara, T.; DeClerck, Y.A. Interleukin-6 in Bone Metastasis and Cancer Progression. Eur. J. Cancer 2010, 46, 1223–1231. [Google Scholar] [CrossRef]
- Elgohary, S.; El Tayebi, H.M. Inflammasomes in Breast Cancer: The Ignition Spark of Progression and Resistance? Expert Rev. Mol. Med. 2023, 25, e22. [Google Scholar] [CrossRef]
- Steiner, G.E.; Newman, M.E.; Paikl, D.; Stix, U.; Memaran-Dagda, N.; Lee, C.; Marberger, M.J. Expression and Function of Pro-inflammatory Interleukin IL-17 and IL-17 Receptor in Normal, Benign Hyperplastic, and Malignant Prostate. Prostate 2003, 56, 171–182. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Ferraretto, A.; Lombardi, G. Interleukin 11 (IL-11): Role(s) in Breast Cancer Bone Metastases. Biomedicines 2021, 9, 659. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and Its Expanding Biological Functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Sethi, N.; Kang, Y. Unravelling the Complexity of Metastasis—Molecular Understanding and Targeted Therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone Metastasis: Mechanisms, Therapies, and Biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Tulotta, C.; Lefley, D.V.; Freeman, K.; Gregory, W.M.; Hanby, A.M.; Heath, P.R.; Nutter, F.; Wilkinson, J.M.; Spicer-Hadlington, A.R.; Liu, X.; et al. Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone Microenvironment. Clin. Cancer Res. 2019, 25, 2769–2782. [Google Scholar] [CrossRef]
- Eyre, R.; Alférez, D.G.; Santiago-Gómez, A.; Spence, K.; McConnell, J.C.; Hart, C.; Simões, B.M.; Lefley, D.; Tulotta, C.; Storer, J.; et al. Microenvironmental IL1β Promotes Breast Cancer Metastatic Colonisation in the Bone via Activation of Wnt Signalling. Nat. Commun. 2019, 10, 5016. [Google Scholar] [CrossRef]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 Drives Breast Cancer Growth and Bone Metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Wang, H.; Tang, H.; Yuan, S.; Liang, C.; Li, Y.; Zhu, S.; Chen, K. IL-17A Deficiency Inhibits Lung Cancer-Induced Osteoclastogenesis by Promoting Apoptosis of Osteoclast Precursor Cells. PLoS ONE 2024, 19, e0299028. [Google Scholar] [CrossRef]
- Lehrer, S.; Diamond, E.J.; Mamkine, B.; Stone, N.N.; Stock, R.G. Serum Interleukin-8 Is Elevated in Men with Prostate Cancer and Bone Metastases. Technol. Cancer Res. Treat. 2004, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Margulies, A.G.; Walser, B.; Akel, N.S.; Bhattacharrya, S.; Skinner, R.A.; Swain, F.; Ramani, V.; Mohammad, K.S.; Wessner, L.L.; et al. Tumor-Derived Interleukin-8 Stimulates Osteolysis Independent of the Receptor Activator of Nuclear Factor-κB Ligand Pathway. Cancer Res. 2005, 65, 11001–11009. [Google Scholar] [CrossRef] [PubMed]
- Hamidullah; Changkija, B.; Konwar, R. Role of Interleukin-10 in Breast Cancer. Breast Cancer Res. Treat. 2012, 133, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-C.; Chen, W.-Y.; Lee, K.-D.; Tsai, Y.-C. Tumor-Infiltrating Leukocytes Suppress Local Inflammation Via Interleukin-1 Receptor Antagonist in a Syngeneic Prostate Cancer Model. Biology 2020, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Kawakami, K.; Stepensky, V.A.; Maki, R.A.; Robin, H.; Muller, W.; Husain, S.R.; Puri, R.K. Interleukin 4 Receptor on Human Lung Cancer: A Molecular Target for Cytotoxin Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 3503–3511. [Google Scholar]
- LaMarche, N.M.; Hegde, S.; Park, M.D.; Maier, B.B.; Troncoso, L.; Le Berichel, J.; Hamon, P.; Belabed, M.; Mattiuz, R.; Hennequin, C.; et al. An IL-4 Signalling Axis in Bone Marrow Drives pro-Tumorigenic Myelopoiesis. Nature 2024, 625, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hunter, C.A. The Immunobiology of Interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef] [PubMed]
- Sénécal, V.; Deblois, G.; Beauseigle, D.; Schneider, R.; Brandenburg, J.; Newcombe, J.; Moore, C.S.; Prat, A.; Antel, J.; Arbour, N. Production of IL-27 in Multiple Sclerosis Lesions by Astrocytes and Myeloid Cells: Modulation of Local Immune Responses. Glia 2016, 64, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Jaiswal, A.R.; Ager, C.R.; Chin, R.; Chen, C.-H.; Budhani, P.; Ai, M.; Reilley, M.J.; Sebastian, M.M.; Hong, D.S.; et al. Activation of 4-1BB on Liver Myeloid Cells Triggers Hepatitis via an Interleukin-27-Dependent Pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Ciummo, S.L.; Sorrentino, C.; Fieni, C.; Di Carlo, E. Interleukin-30 Subverts Prostate Cancer-Endothelium Crosstalk by Fostering Angiogenesis and Activating Immunoregulatory and Oncogenic Signaling Pathways. J. Exp. Clin. Cancer Res. 2023, 42, 336. [Google Scholar] [CrossRef] [PubMed]
- Peluzzo, A.M.; Autieri, M.V. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef]
- Mangashetti, L.S.; Khapli, S.M.; Wani, M.R. IL-4 Inhibits Bone-Resorbing Activity of Mature Osteoclasts by Affecting NF-κB and Ca2+ Signaling. J. Immunol. 2005, 175, 917–925. [Google Scholar] [CrossRef]
- Stein, N.C.; Kreutzmann, C.; Zimmermann, S.-P.; Niebergall, U.; Hellmeyer, L.; Goettsch, C.; Schoppet, M.; Hofbauer, L.C. Interleukin-4 and Interleukin-13 Stimulate the Osteoclast Inhibitor Osteoprotegerin by Human Endothelial Cells through the STAT6 Pathway. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 750–758. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, H.; Jing, Z.; Hong-Hua, W.; Ben-Jing, S.; Li-Ting, W.; Li-Juan, Y.; Wei, X.; Xia, K.; Juan, W.; et al. IL4/IL4R Signaling Promotes the Osteolysis in Metastatic Bone of CRC through Regulating the Proliferation of Osteoclast Precursors. Mol. Med. Camb. Mass 2021, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Uhl, C.; Nyirenda, T.; Siegel, D.S.; Lee, W.Y.; Zilberberg, J. Natural Killer Cells Activity against Multiple Myeloma Cells Is Modulated by Osteoblast-Induced IL-6 and IL-10 Production. Heliyon 2022, 8, e09167. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, C.; Ciummo, S.L.; Cipollone, G.; Caputo, S.; Bellone, M.; Di Carlo, E. Interleukin-30/IL27p28 Shapes Prostate Cancer Stem-like Cell Behavior and Is Critical for Tumor Onset and Metastasization. Cancer Res. 2018, 78, 2654–2668. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, C.; D’Antonio, L.; Ciummo, S.L.; Fieni, C.; Landuzzi, L.; Ruzzi, F.; Vespa, S.; Lanuti, P.; Lotti, L.V.; Lollini, P.L.; et al. CRISPR/Cas9-Mediated Deletion of Interleukin-30 Suppresses IGF1 and CXCL5 and Boosts SOCS3 Reducing Prostate Cancer Growth and Mortality. J. Hematol. Oncol. 2022, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Errani, C.; Gillies, R.; Mercatali, L.; Ibrahim, T.; Tamanti, J.; Baldini, N.; Avnet, S. Acid-Induced Inflammatory Cytokines in Osteoblasts: A Guided Path to Osteolysis in Bone Metastasis. Front. Cell Dev. Biol. 2021, 9, 678532. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-κB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Deka, K.; Li, Y. Transcriptional Regulation during Aberrant Activation of NF-κB Signalling in Cancer. Cells 2023, 12, 788. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbas, M.; Ullah, M.F.; Aziz, M.H.; Beylerli, O.; Alam, M.A.; Syed, M.A.; Uddin, S.; Ahmad, A. Long Non-Coding RNAs Regulated NF-κB Signaling in Cancer Metastasis: Micromanaging by Not so Small Non-Coding RNAs. Semin. Cancer Biol. 2022, 85, 155–163. [Google Scholar] [CrossRef]
- Aqdas, M.; Sung, M.-H. NF-κB Dynamics in the Language of Immune Cells. Trends Immunol. 2023, 44, 32–43. [Google Scholar] [CrossRef]
- Martin, E.W.; Pacholewska, A.; Patel, H.; Dashora, H.; Sung, M.-H. Integrative Analysis Suggests Cell Type-Specific Decoding of NF-κB Dynamics. Sci. Signal. 2020, 13, eaax7195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bonomi, P.D. Immune System Disorder and Cancer-Associated Cachexia. Cancers 2024, 16, 1709. [Google Scholar] [CrossRef]
- Preedy, M.K.; White, M.R.H.; Tergaonkar, V. Cellular Heterogeneity in TNF/TNFR1 Signalling: Live Cell Imaging of Cell Fate Decisions in Single Cells. Cell Death Dis. 2024, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Jornayvaz, F.R. Interleukin-18 in Metabolism: From Mice Physiology to Human Diseases. Front. Endocrinol. 2022, 13, 971745. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Corbett, M.; Ramessur, R.; Marshall, D.; Acencio, M.L.; Ostaszewski, M.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Haddad, S.; Jensen, A.H.M.; et al. Biomarkers of Systemic Treatment Response in People with Psoriasis: A Scoping Review. Br. J. Dermatol. 2022, 187, 494–506. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Genno, S.; Tomita, K.; Nishida, S. RANK/RANKL Axis Promotes Migration, Invasion, and Metastasis of Osteosarcoma via Activating NF-κB Pathway. Exp. Cell Res. 2024, 436, 113978. [Google Scholar] [CrossRef]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef]
- Kumar, N.; Kuang, L.; Villa, R.; Kumar, P.; Mishra, J. Mucosal Epithelial Jak Kinases in Health and Diseases. Mediat. Inflamm. 2021, 2021, 6618924. [Google Scholar] [CrossRef]
- Fasouli, E.S.; Katsantoni, E. JAK-STAT in Early Hematopoiesis and Leukemia. Front. Cell Dev. Biol. 2021, 9, 669363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Wang, Q.; Meng, G.; Lv, X.; Zhou, H.; Li, W.; Zhang, J. The Relationship between microRNAs and the STAT3-Related Signaling Pathway in Cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317719869. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving Cognition of the JAK-STAT Signaling Pathway: Autoimmune Disorders and Cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The Molecular Details of Cytokine Signaling via the JAK/STAT Pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ryan, N.; Nedungadi, D.; Lamenza, F.; Swingler, M.; Siddiqui, A.; Satoskar, A.; Upadhaya, P.; Pietrzak, M.; Oghumu, S. STAT1 Is Regulated by TRIM24 and Promotes Immunosuppression in Head and Neck Squamous Carcinoma Cells, but Enhances T Cell Antitumour Immunity in the Tumour Microenvironment. Br. J. Cancer 2022, 127, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Targeting STAT3 Signaling Pathway in Colorectal Cancer. Biomedicines 2021, 9, 1016. [Google Scholar] [CrossRef]
- Damerau, A.; Gaber, T.; Ohrndorf, S.; Hoff, P. JAK/STAT Activation: A General Mechanism for Bone Development, Homeostasis, and Regeneration. Int. J. Mol. Sci. 2020, 21, 9004. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The Role of IL-6/JAK2/STAT3 Signaling Pathway in Cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Godoi, M.A.; Camilli, A.C.; Gonzales, K.G.A.; Costa, V.B.; Papathanasiou, E.; Leite, F.R.M.; Guimarães-Stabili, M.R. JAK/STAT as a Potential Therapeutic Target for Osteolytic Diseases. Int. J. Mol. Sci. 2023, 24, 10290. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Horiuchi, S.; Topley, N.; Yamamoto, N.; Fuller, G.M. The Soluble Interleukin 6 Receptor: Mechanisms of Production and Implications in Disease. FASEB J. 2001, 15, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Grosinger, A.J.; Alcorn, S.R. An Update on the Management of Bone Metastases. Curr. Oncol. Rep. 2024, 26, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Tan, S.-L.; Takano, J.; Ohsawa, K.; Hasada, I.; Hanasaki, A.; Ito, I.; Mihara, M.; Nishida, K. Tocilizumab, a Humanized Anti-IL-6R Antibody, as an Emerging Therapeutic Option for Rheumatoid Arthritis: Molecular and Cellular Mechanistic Insights. Int. Rev. Immunol. 2015, 34, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Torne, C.; Ortiz, M.D.A.; Moya, P.; Hernandez, M.V.; Reina, D.; Castellvi, I.; De Agustin, J.J.; Fuente, D.D.L.; Corominas, H.; Sanmarti, R.; et al. The Combination of IL-6 and Its Soluble Receptor Is Associated with the Response of Rheumatoid Arthritis Patients to Tocilizumab. Semin. Arthritis Rheum. 2018, 47, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Hamaguchi, T.; Nagao, N.; Kato, S.; Iino, T.; Nakamura, T.; Sudo, A. Interleukin-6 Receptor Inhibitor Suppresses Bone Metastases in a Breast Cancer Cell Line. Breast Cancer Tokyo Jpn. 2018, 25, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, B. Siltuximab (CNTO 328): A Promising Option for Human Malignancies. Drug Des. Devel. Ther. 2015, 9, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.; Kulbe, H.; Chakravarty, P.; Leader, D.; Vassileva, V.; Leinster, D.A.; Thompson, R.; Schioppa, T.; Nemeth, J.; Vermeulen, J.; et al. Interleukin-6 as a Therapeutic Target in Human Ovarian Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Jemec, G.B.E.; Sayed, C.J.; Kirby, J.S.; Prens, E.; Ingram, J.R.; Garg, A.; Gottlieb, A.B.; Szepietowski, J.C.; Bechara, F.G.; et al. Efficacy and Safety of Bimekizumab in Patients with Moderate-to-Severe Hidradenitis Suppurativa (BE HEARD I and BE HEARD II): Two 48-Week, Randomised, Double-Blind, Placebo-Controlled, Multicentre Phase 3 Trials. Lancet 2024, 403, 2504–2519. [Google Scholar] [CrossRef]
- Shah, M.; Maroof, A.; Gikas, P.; Mittal, G.; Keen, R.; Baeten, D.; Shaw, S.; Roberts, S.J. Dual Neutralisation of IL-17F and IL-17A with Bimekizumab Blocks Inflammation-Driven Osteogenic Differentiation of Human Periosteal Cells. RMD Open 2020, 6, e001306. [Google Scholar] [CrossRef]
- van der Heijde, D.; Gensler, L.S.; Deodhar, A.; Baraliakos, X.; Poddubnyy, D.; Kivitz, A.; Farmer, M.K.; Baeten, D.; Goldammer, N.; Coarse, J.; et al. Dual Neutralisation of Interleukin-17A and Interleukin-17F with Bimekizumab in Patients with Active Ankylosing Spondylitis: Results from a 48-Week Phase IIb, Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Study. Ann. Rheum. Dis. 2020, 79, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Singh, K.B.; Prakash, R.; Singh, D. Functional Block of IL-17 Cytokine Promotes Bone Healing by Augmenting FOXO1 and ATF4 Activity in Cortical Bone Defect Model. Osteoporos. Int. 2017, 28, 2207–2220. [Google Scholar] [CrossRef]
- Läubli, H.; Alonso, G.; Lopez, J.S.; Calvo, E.; Sanchez Perez, V.; Di Blasi, D.; Nair, A.; Richter, K.; Huber, C.; Egli, N.; et al. A Phase I/II Study of ANV419, a Selective IL-2R-Beta-Gamma Targeted Antibody-IL-2 Fusion Protein, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2022, 40 (Suppl. 16), e21552. [Google Scholar] [CrossRef]
- Mathiot, L.; Combarel, D.; Cagnat, J.; Delahousse, J.; Ouali, K.; Marabelle, A.; Loriot, Y.; Ponce, S.; Champiat, S.; Broutin, S.; et al. Phase 1 First-in-Human Dose-Escalation Study of ANV419 in Patients with Relapsed/Refractory Advanced Solid Tumors. J. Immunother. Cancer 2024, 12, e008847. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W.M. Treating Inflammation by Blocking Interleukin-1 in a Broad Spectrum of Diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Tran, T.H.; Pham, J.T.; Shafeeq, H.; Manigault, K.R.; Arya, V. Role of Interleukin-1 Inhibitors in the Management of Gout. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 744–753. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A. Canakinumab: A Guide to Its Use in Acute Gouty Arthritis Flares. BioDrugs 2013, 27, 401–406. [Google Scholar] [CrossRef]
- Zhou, J.; Down, J.M.; George, C.N.; Murphy, J.; Lefley, D.V.; Tulotta, C.; Alsharif, M.A.; Leach, M.; Ottewell, P.D. Novel Methods of Targeting IL-1 Signalling for the Treatment of Breast Cancer Bone Metastasis. Cancers 2022, 14, 4816. [Google Scholar] [CrossRef] [PubMed]
- Cadieux, B.; Coleman, R.; Jafarinasabian, P.; Lipton, A.; Orlowski, R.Z.; Saad, F.; Scagliotti, G.V.; Shimizu, K.; Stopeck, A. Experience with Denosumab (XGEVA®) for Prevention of Skeletal-Related Events in the 10 Years after Approval. J. Bone Oncol. 2022, 33, 100416. [Google Scholar] [CrossRef]
- Burkiewicz, J.S.; Scarpace, S.L.; Bruce, S.P. Denosumab in Osteoporosis and Oncology. Ann. Pharmacother. 2009, 43, 1445–1455. [Google Scholar] [CrossRef]
- Kim, A.S.; Taylor, V.E.; Castro-Martinez, A.; Dhakal, S.; Zamerli, A.; Mohanty, S.; Xiao, Y.; Simic, M.K.; Wen, J.; Chai, R.; et al. Temporal Patterns of Osteoclast Formation and Activity Following Withdrawal of RANKL Inhibition. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2024, 39, 484–497. [Google Scholar] [CrossRef]
- Chen, C.-H.; Liao, H.-T.; Chen, H.-A.; Yen, Y.-N.; Chen, C.-H. Denosumab Use Reduces Risk of Rheumatoid Arthritis in Patients with Osteoporosis. Clin. Exp. Rheumatol. 2024, 42, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Trovarelli, G.; Rizzo, A.; Cerchiaro, M.; Pala, E.; Angelini, A.; Ruggieri, P. The Evaluation and Management of Lung Metastases in Patients with Giant Cell Tumors of Bone in the Denosumab Era. Curr. Oncol. 2024, 31, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Marquez, J.; Chen, B.K.; Kim, D.M.; Cheng, M.L.; Liu, E.V.; Yang, H.; Zhang, L.; Sinha, M.; Cheung, A.; et al. Immune Modulation with RANKL Blockade through Denosumab Treatment in Patients with Cancer. Cancer Immunol. Res. 2024, 12, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant Denosumab in Postmenopausal Patients with Hormone Receptor-Positive Breast Cancer (ABCSG-18): Disease-Free Survival Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Cadarette, S.M.; Ban, J.K.; Lipscombe, L.; Narod, S.A.; Kotsopoulos, J. Denosumab and Breast Cancer Risk in Postmenopausal Women: A Population-Based Cohort Study. Br. J. Cancer 2018, 119, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hu, D.; Zhang, Y.; Ma, C.; Shen, L.; Shuai, B. Current Comprehensive Understanding of Denosumab (the RANKL Neutralizing Antibody) in the Treatment of Bone Metastasis of Malignant Tumors, Including Pharmacological Mechanism and Clinical Trials. Front. Oncol. 2023, 13, 1133828. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, Double-Blind Study of Denosumab versus Zoledronic Acid in the Treatment of Bone Metastases in Patients with Advanced Cancer (Excluding Breast and Prostate Cancer) or Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef]
- Bonnelye, E.; Juárez, P. Targeting Bone Metastasis in Cancers. Cancers 2021, 13, 4490. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus Zoledronic Acid in Bone Disease Treatment of Newly Diagnosed Multiple Myeloma: An International, Double-Blind, Double-Dummy, Randomised, Controlled, Phase 3 Study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant Denosumab in Early Breast Cancer (D-CARE): An International, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Yu, H.; Zhu, Z.; Li, W.; Huang, X. A Retrospective Analysis of Denosumab for the Treatment of Bone Metastases in Chinese Patients with Breast Cancer. Clin. Med. Insights Oncol. 2023, 17, 11795549231182266. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Chen, Z.; Zeng, A.; Zhang, H.; Yu, Y.; Wei, S.; Li, Q.; Wang, X.; Wang, X.; et al. Efficacy and Safety of Denosumab Biosimilar QL1206 Versus Denosumab in Patients with Bone Metastases from Solid Tumors: A Randomized Phase III Trial. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2023, 37, 259–269. [Google Scholar] [CrossRef]
- Morita, T.; Shima, Y.; Fujimoto, K.; Tsuboi, H.; Saeki, Y.; Narazaki, M.; Ogata, A.; Kumanogoh, A. Anti-Receptor Activator of Nuclear Factor κB Ligand Antibody Treatment Increases Osteoclastogenesis-Promoting IL-8 in Patients with Rheumatoid Arthritis. Int. Immunol. 2019, 31, 277–285. [Google Scholar] [CrossRef]
- Tan, D.S.W.; Felip, E.; de Castro, G.; Solomon, B.J.; Greystoke, A.; Cho, B.C.; Cobo, M.; Kim, T.M.; Ganguly, S.; Carcereny, E.; et al. Canakinumab Versus Placebo in Combination with First-Line Pembrolizumab Plus Chemotherapy for Advanced Non-Small-Cell Lung Cancer: Results From the CANOPY-1 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Adam, Z.; Šedivá, A.; Zeman, D.; Fojtík, Z.; Petrášová, H.; Diatková, J.; Tomíška, M.; Král, Z.; Treglerová, J.; Peřina, V.; et al. Successful Treatment of SAPHO Syndrome (Chronic Nonbacterial Osteomyelitis and Acne) with Anakinra and Denosumab. Case Report and Review of Therapy. Vnitř. Lékařství 2023, 69, E4–E14. [Google Scholar] [CrossRef]
- Singh, K.B.; Rai, R.; Khanka, S.; Singh, D. Discontinuation of PTH Therapy Amplifies Bone Loss by Increasing Oxidative Stress: An Event Ameliorated by Sequential IL-17 Neutralizing Antibody Therapy. Biomed. Pharmacother. 2022, 145, 112390. [Google Scholar] [CrossRef]
- Albahdal, A.S.; Alotaibi, A.M.; Alanazi, M.A.; Abanmy, N.; Alwhaibi, M.; AlRuthia, Y. Cost-Consequence Analysis of Tocilizumab versus Adalimumab and Etanercept among Rheumatoid Arthritis Patients in Saudi Arabia: A Single-Center Study. Cost Eff. Resour. Alloc. CE 2024, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Shupo, F.; Abrams, K.R.; Ademi, Z.; Wayi-Wayi, G.; Zibelnik, N.; Kirchmann, M.; Rutherford, C.; Makarounas-Kirchmann, K. Cost-Effectiveness Analysis of Siltuximab for Australian Public Investment in the Rare Condition Idiopathic Multicentric Castleman Disease. PharmacoEconomics-Open 2023, 7, 777–792. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Iuliani, M.; Simonetti, S.; Ribelli, G.; Napolitano, A.; Pantano, F.; Vincenzi, B.; Tonini, G.; Santini, D. Current and Emerging Biomarkers Predicting Bone Metastasis Development. Front. Oncol. 2020, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, F.; Ma, Y.; Luo, Y.; Zhang, Y.; Yang, N.; Liu, M.; Liu, H.; Li, J. Potential Biomarkers for the Early Detection of Bone Metastases. Front. Oncol. 2023, 13, 1188357. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Yuan, L.; Tan, Z.; Zhang, X.; Hambly, B.D.; Bao, S.; Tao, K. IL-38 Promotes the Development of Prostate Cancer. Front. Immunol. 2024, 15, 1384416. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xu, Y.; Mo, Z.; Zhu, T.; Dong, H.; Zhou, W.; Xia, Q. Interleukin-25 as a Potential Biomarker in Lung Metastasis of Hepatocellular Carcinoma with HBV History in Chinese Patients: A Single Center, Case-Control Study. Int. J. Med. Sci. 2024, 21, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-D.; Chen, M.-Z.; Yang, D.-F.; Hu, S.-B.; Zheng, D.-D. IL-6 Significantly Correlated with the Prognosis in Low-Grade Glioma and the Mediating Effect of Immune Microenvironment. Medicine 2024, 103, e38091. [Google Scholar] [CrossRef]
- Ding, Y.; Yi, J.; Shan, Y.; Gu, J.; Sun, Z.; Lin, J. Low Expression of Interleukin-1 Receptor Antagonist Correlates with Poor Prognosis via Promoting Proliferation and Migration and Inhibiting Apoptosis in Oral Squamous Cell Carcinoma. Cytokine 2024, 179, 156595. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.B.; Borba, M.A.S.M.; Fernandes, M.S.S.; Filgueira, T.O.; Martins, D.B.G.; Filho, J.L.L.; Castoldi, A.; Souto, F.O. Interleukin-33 Expression on Treatment Outcomes and Prognosis in Brazilian Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Int. J. Mol. Sci. 2023, 24, 16326. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Kishida, T.; Kouro, T.; Igarashi, Y.; Takebe, S.; Yamamoto, S.; Kondo, T.; Koizumi, M.; Terao, H.; Suzuki, T.; et al. MMP1, IL-1β, sTNFR-1, and IL-6 Are Prognostic Factors for Patients with Unresectable or Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Int. J. Clin. Oncol. 2024, 29, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Lacroix, M.; Lespagnard, L.; Larsimont, D.; Paesmans, M.; Body, J.-J. Interleukins-6 and -11 Expression in Primary Breast Cancer and Subsequent Development of Bone Metastases. Cancer Lett. 2001, 169, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bhadresha, K.P.; Patel, M.; Jain, N.K.; Rawal, R.M. A Predictive Biomarker Panel for Bone Metastases: Liquid Biopsy Approach. J. Bone Oncol. 2021, 29, 100374. [Google Scholar] [CrossRef]
- Habberstad, R.; Aass, N.; Mollnes, T.E.; Damås, J.K.; Brunelli, C.; Rossi, R.; Garcia-Alonso, E.; Kaasa, S.; Klepstad, P. Inflammatory Markers and Radiotherapy Response in Patients with Painful Bone Metastases. J. Pain Symptom Manag. 2022, 64, 330–339. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Agent | Target Interleukin | Mechanism of Action | Clinical Application | Challenges/Limitations |
|---|---|---|---|---|
| Tocilizumab | IL-6 | Inhibits IL-6 receptor | Rheumatoid arthritis, potential in reducing bone metastases | Infections, liver enzyme elevation |
| Siltuximab | IL-6 | Neutralizes IL-6 bioactivity | Prostate cancer, ovarian cancer | Resistance, cytokine release syndrome |
| Bimekizumab | IL-17A and IL-17F | Dual inhibition of IL-17A and IL-17F | Psoriatic arthritis, potential in bone healing | Long-term safety, high cost |
| ANV419 | IL-2Rβγ | Selective IL-2Rβγ binding agonist | Advanced solid tumors | Grade 1/2 adverse events |
| Canakinumab | IL-1β | IL-1β inhibition | Rheumatoid arthritis, potential in bone metastases | Expensive, risk of infections |
| Denosumab | RANKL | Inhibits RANK/RANKL interaction | Bone metastases from solid tumors | Osteonecrosis of the jaw, hypocalcemia |
| Drug | Dose | Clinical Effect | Side Effects | Clinical Study | Animal Study |
|---|---|---|---|---|---|
| Tocilizumab | 8 mg/kg IV every 4 weeks | Reduces inflammation, improves symptoms in RA and other conditions | Infections, elevated liver enzymes | Effective in reducing bone metastases in preclinical studies; used in RA and Castleman’s disease; investigated in other conditions (Crohn’s disease, SLE) | Reduced bone metastases in animal models of breast cancer; inhibited cell survival and reduced expressions of Stat3, VEGF, and RANK in MDA-231 cells |
| Siltuximab | 11 mg/kg IV every 3 weeks | Neutralizes IL-6, reduces tumor cell survival, enhances chemotherapy effects | Infections, neutropenia, thrombocytopenia | Inhibits proliferation of prostate cancer cells in vitro; extends disease stability; reduces levels of TNF-α, IL-1, CCL2, CXCL12, and VEGF | Inhibits androgen-dependent prostate cancer progression in mice; reduces cachexia levels in prostate cancer models |
| Bimekizumab | 160–320 mg SC every 4 weeks | Dual neutralization of IL-17A and IL-17F, improves bone health | Infections, nasopharyngitis, oral candidiasis | Effective in hidradenitis suppurativa and spondyloarthritis; improves key outcomes such as disease activity, functional status, and quality of life | Inhibits osteogenic differentiation and bone formation in human periosteum-derived cell models; improves bone healing and regeneration in osteoporotic models |
| ANV419 | 243 µg/kg IV every 2 weeks | Enhances tumor-killing capabilities of immune cells, minimizes immunosuppressive cell activation | Chills, low-grade fever | Phase I/II study showed well-tolerated, disease stabilization in solid tumors; phase I study demonstrated antitumor activity with stable disease and partial response in advanced tumors | Stimulates immune cells selectively, enhancing tumor-killing capabilities while minimizing activation of immunosuppressive cells |
| Canakinumab | 150–300 mg SC every 4 weeks | Reduces inflammation, slows bone metastasis progression | Infections, neutropenia, thrombocytopenia | Ongoing trials for various solid tumor malignancies; reduces metastasis and bone tumor growth in preclinical studies | Inhibits breast cancer growth and bone metastasis in preclinical models; reduces metastasis and metastatic outgrowth in bone with VX765 and Anakinra |
| Denosumab | 120 mg SC every 4 weeks | Inhibits bone resorption, delays skeletal-related events, improves quality of life | Hypocalcemia, osteonecrosis of the jaw | Effective in delaying skeletal-related events in breast and prostate cancer; superior to bisphosphonates in delaying skeletal-related events | Increases serum IL-8 levels, promoting osteoclast formation even without RANKL activity; combination with IL inhibitors may enhance treatment outcomes |
| Interleukin | Type | Role | Diagnostic Biomarker | Prognostic Biomarker |
|---|---|---|---|---|
| IL-1 | Pro-inflammatory | Promotes osteoclastogenesis, tumor proliferation, angiogenesis | Elevated IL-1 levels in patient serum indicate active inflammation and metastasis | High IL-1 levels correlate with increased tumor burden and poor prognosis |
| IL-6 | Pro-inflammatory | Promotes osteoclastogenesis, tumor proliferation, angiogenesis | Elevated IL-6 levels indicate tumor activity and systemic inflammation | High IL-6 levels correlate with advanced disease and poor prognosis |
| IL-8 | Pro-inflammatory | Stimulates osteoclastogenesis, promotes angiogenesis | Elevated IL-8 levels indicate active metastatic process | High IL-8 levels correlate with increased metastatic potential and poor prognosis |
| IL-11 | Pro-inflammatory | Promotes osteoclastogenesis and bone degradation | Elevated IL-11 levels are associated with active bone resorption | High IL-11 levels correlate with bone metastasis and worse clinical outcomes |
| IL-17 | Pro-inflammatory | Enhances osteoclastogenesis, promotes tumor cell survival | Elevated IL-17 levels indicate aggressive disease | High IL-17 levels correlate with poor survival and increased bone destruction |
| IL-18 | Pro-inflammatory | Modulates tumor microenvironment and tumor development | Elevated IL-18 levels are associated with tumor progression | High IL-18 levels correlate with poor patient outcomes |
| IL-4 | Anti-inflammatory | Inhibits osteoclast activity, modulates immune responses | Elevated IL-4 levels indicate anti-inflammatory response | High IL-4 levels correlate with better clinical outcomes and reduced bone resorption |
| IL-10 | Anti-inflammatory | Inhibits osteoclast activity, promotes immune regulation | Elevated IL-10 levels indicate anti-inflammatory state | High IL-10 levels correlate with reduced tumor progression and better prognosis |
| IL-13 | Anti-inflammatory | Inhibits osteoclast activity, modulates immune responses | Elevated IL-13 levels indicate anti-inflammatory environment | High IL-13 levels correlate with better prognosis and reduced metastatic potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawalibi, A.; Alosaimi, A.A.; Mohammad, K.S. Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments. Int. J. Mol. Sci. 2024, 25, 8163. https://doi.org/10.3390/ijms25158163
Dawalibi A, Alosaimi AA, Mohammad KS. Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments. International Journal of Molecular Sciences. 2024; 25(15):8163. https://doi.org/10.3390/ijms25158163
Chicago/Turabian StyleDawalibi, Ahmad, Amal Ahmed Alosaimi, and Khalid S. Mohammad. 2024. "Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments" International Journal of Molecular Sciences 25, no. 15: 8163. https://doi.org/10.3390/ijms25158163
APA StyleDawalibi, A., Alosaimi, A. A., & Mohammad, K. S. (2024). Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments. International Journal of Molecular Sciences, 25(15), 8163. https://doi.org/10.3390/ijms25158163








