Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice
Abstract
1. Introduction
2. Results
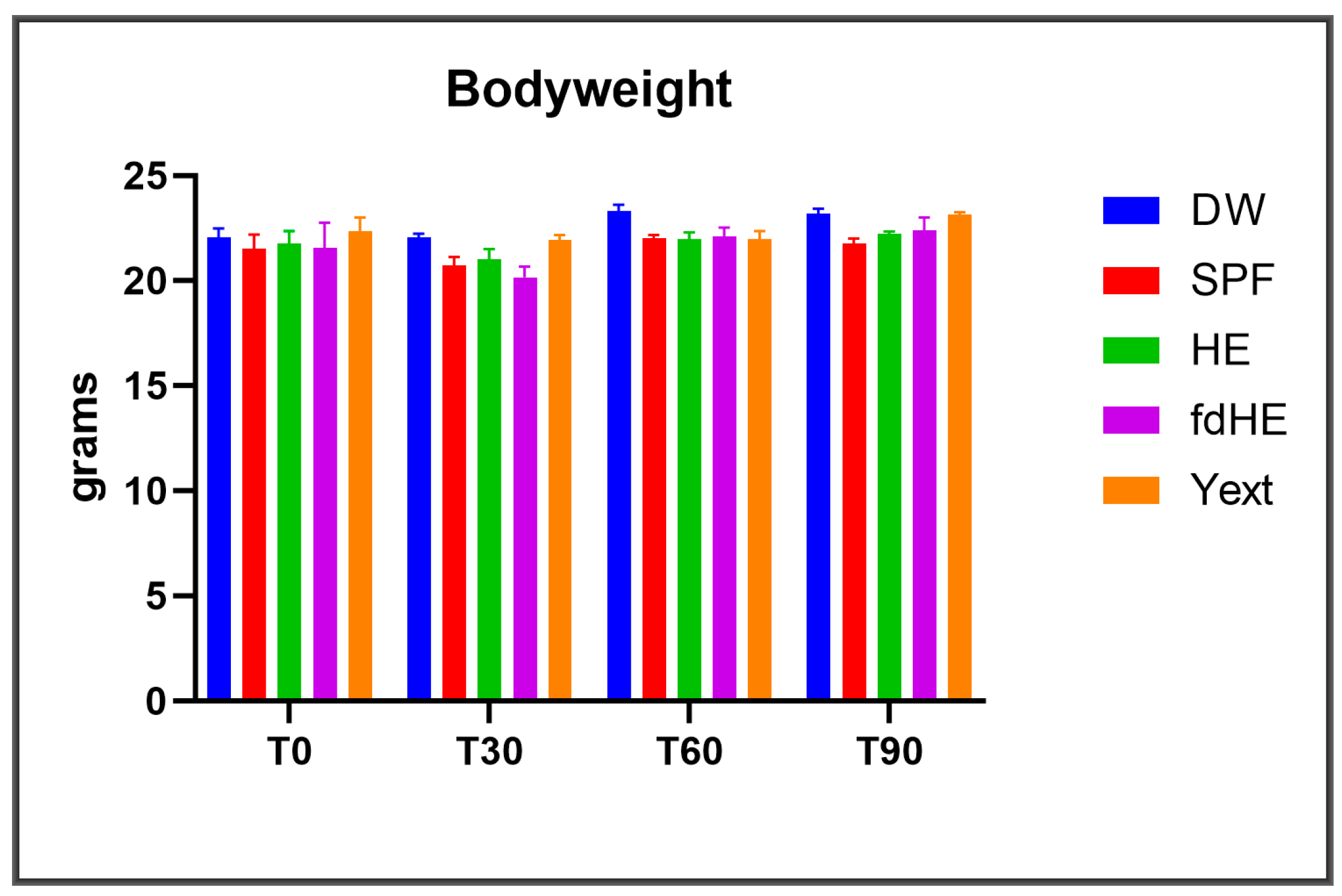
2.1. Clinical Observations
2.2. Histopathology Results
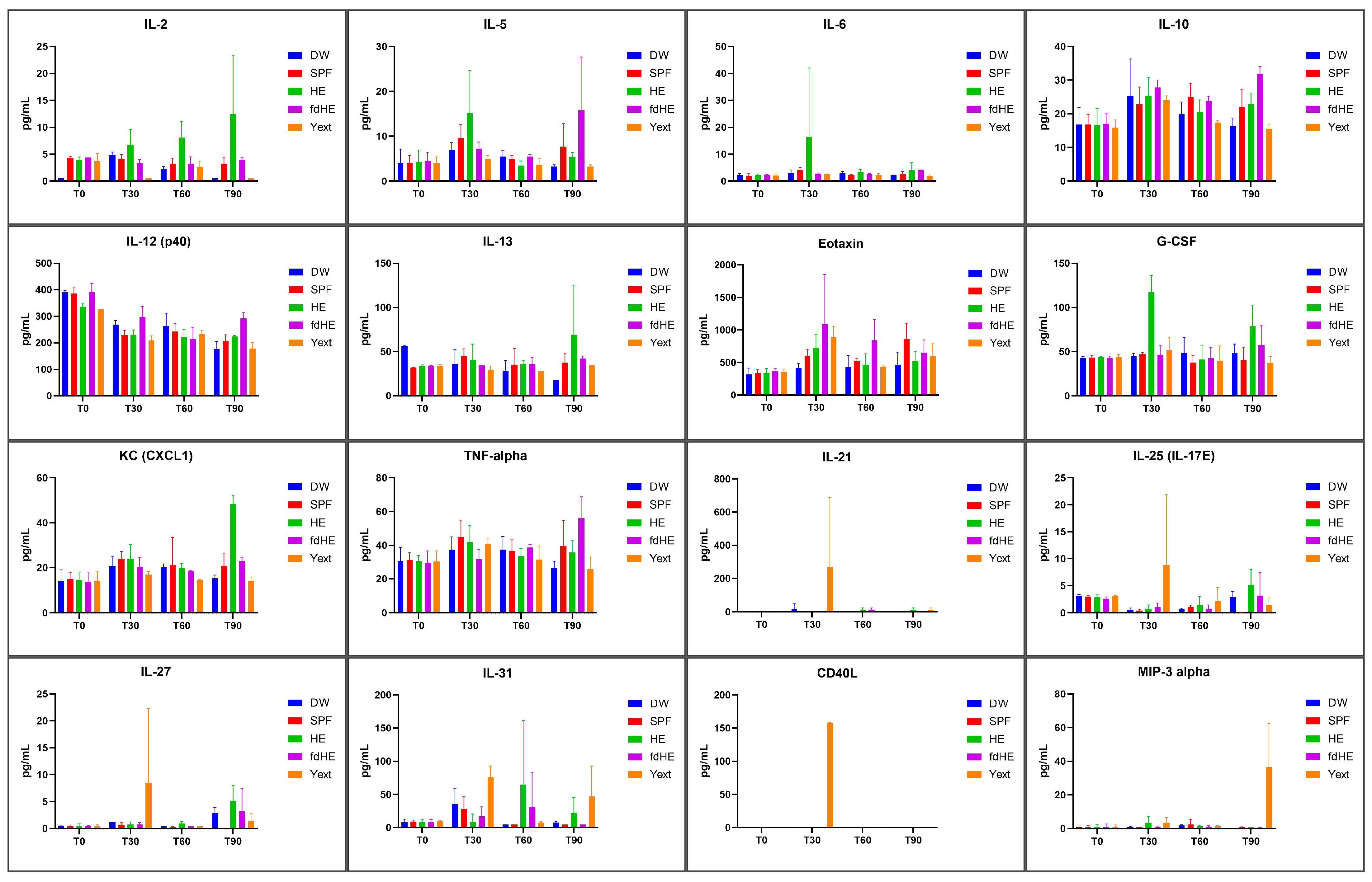
2.3. Serum Cytokines
2.4. Serum Biochemistry
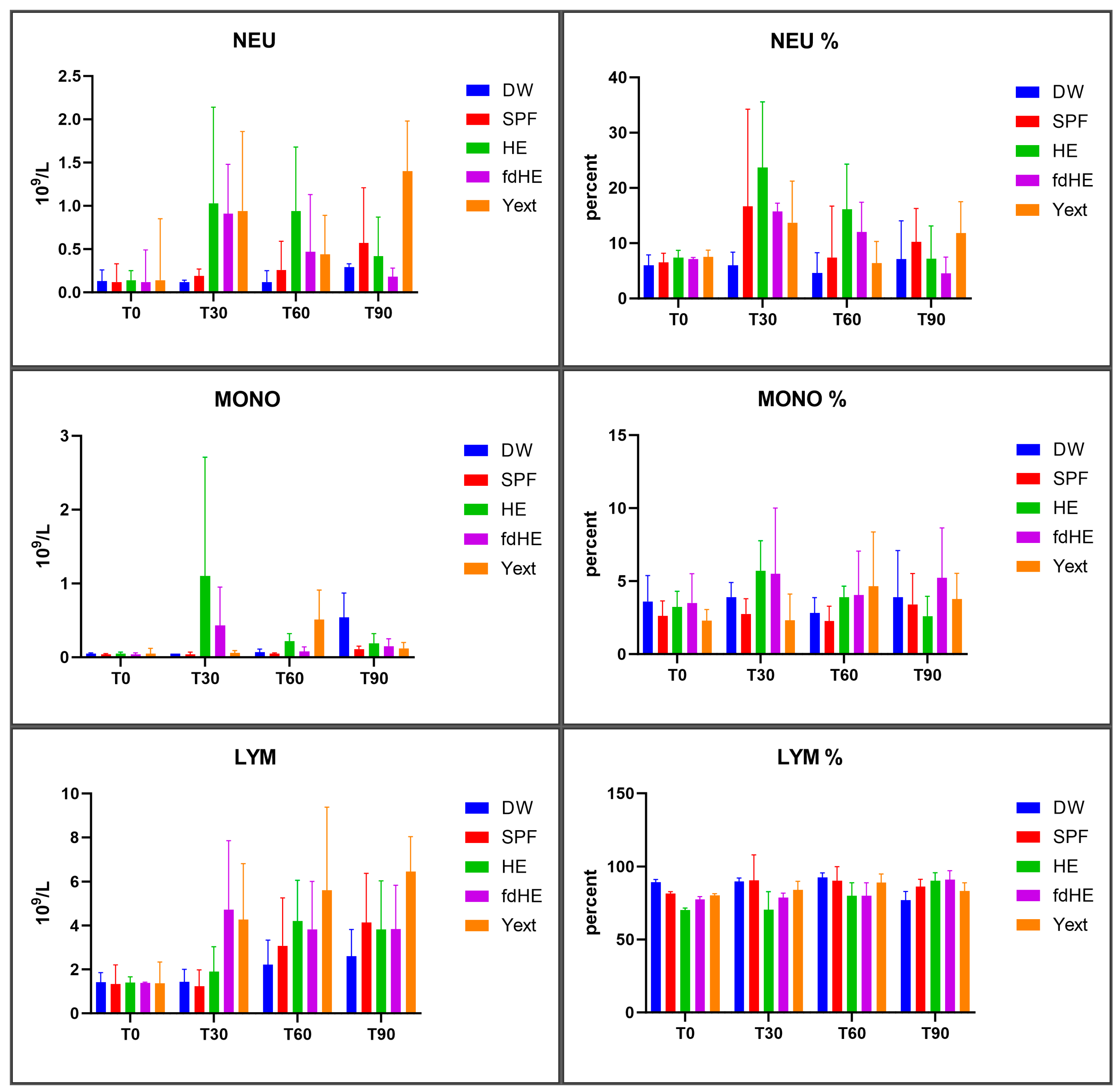
2.5. Hematology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Administered Formulations
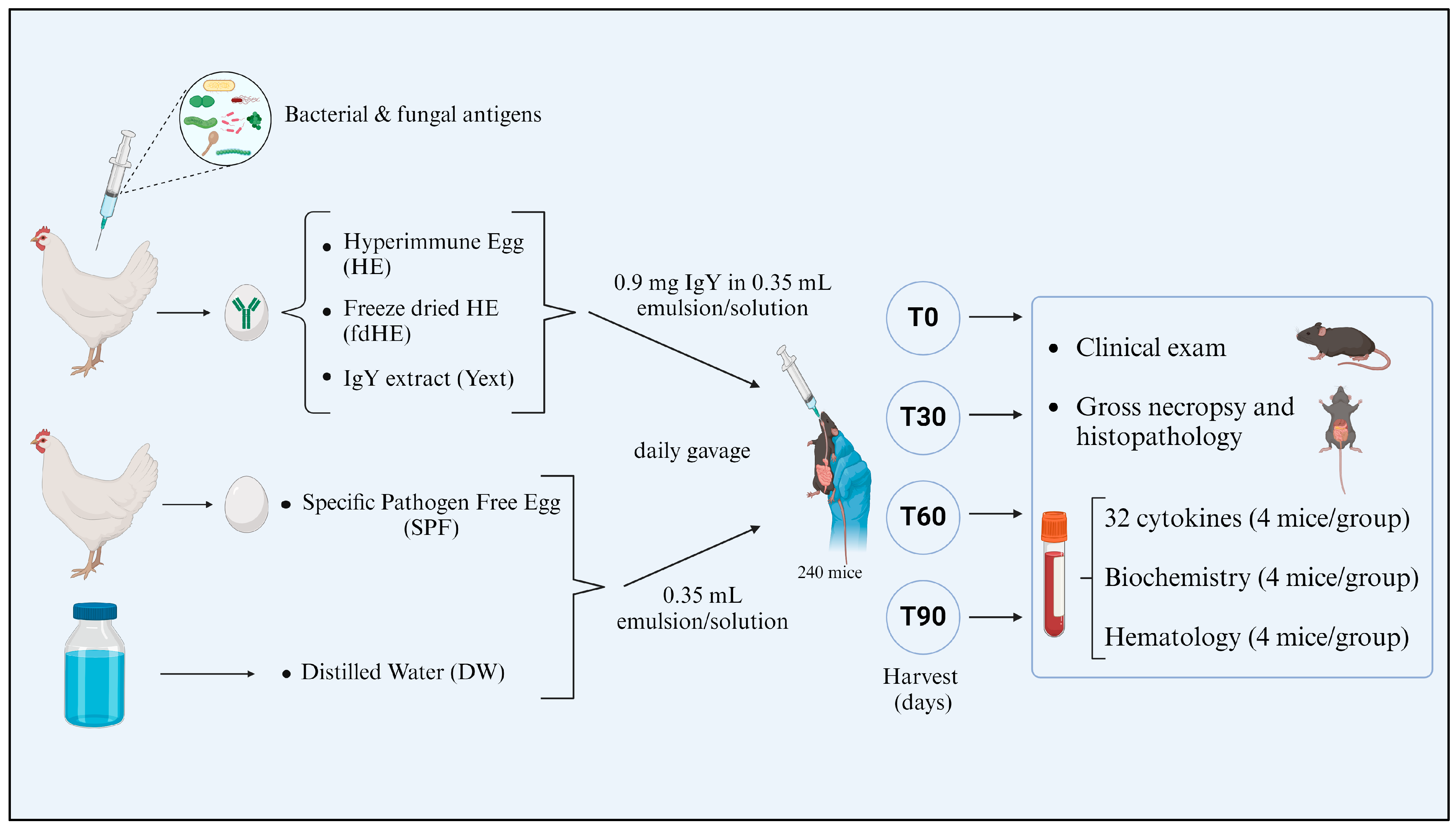
4.3. Experimental Design
- AED = Animal Equivalent Dose,
- HD = Human Dose
- 12.3 = Conversion factor for mice.
4.4. Histopathology Investigations
4.5. Serum Cytokines Profiles
4.6. Serum Biochemistry Investigations
4.7. Hematology Investigations
4.8. Statistical Analysis
4.9. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, K.L. Hyperimmune Eggs Capture Natural Immune Support. Altern. Complement. Ther. 2000, 6, 118–124. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Wu, R.; Chelliappan, B.; Zhang, X. Trends in Industrialization and Commercialization of IgY Technology. Front. Immunol. 2022, 13, 991931. [Google Scholar] [CrossRef]
- Kühlmann, R.; Wiedemann, V.; Schmidt, P.; Wanke, R.; Linckh, E.; Lösch, U. Chicken Egg Antibodies for Prophylaxis and Therapy of Infectious Intestinal Diseases. I. Immunization and Antibody Determination. Zentralbl Vet. B 1988, 35, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Carlander, D.; Stålberg, J.; Larsson, A. Chicken Antibodies: A Clinical Chemistry Perspective. Upsala J. Med. Sci. 1999, 104, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Peralta, R.C.; Umeda, K.; Hashi, T.; Icatlo, F.C.; Kuroki, M.; Ikemori, Y.; Kodama, Y. Prevention of Fatal Salmonellosis in Neonatal Calves, Using Orally Administered Chicken Egg Yolk Salmonella-Specific Antibodies. Am. J. Vet. Res. 1998, 59, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.M.; Domingo, R.; Sandhu, J. Oral Delivery of Antibodies. Clin. Pharmacokinet. 1997, 32, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Carlander, D.; Kollberg, H.; Wejåker, P.-E.; Larsson, A. Peroral Immunotheraphy with Yolk Antibodies for the Prevention and Treatment of Enteric Infections. Immunol. Res. 2000, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, J.; Martel, A.; Canessa, S.; Van Rysselberghe, N.; De Zutter, L.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F.; Garmyn, A. Reducing Campylobacter Jejuni Colonization in Broiler Chickens by In-Feed Supplementation with Hyperimmune Egg Yolk Antibodies. Sci. Rep. 2019, 9, 8931. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Eradication of Small Intestinal Bacterial Overgrowth Reduces Symptoms of Irritable Bowel Syndrome. Off. J. Am. Coll. Gastroenterol. ACG 2000, 95, 3503–3506. [Google Scholar] [CrossRef]
- Schade, R.; Calzado, E.G.; Sarmiento, R.; Chacana, P.A.; Porankiewicz-Asplund, J.; Terzolo, H.R. Chicken Egg Yolk Antibodies (IgY-Technology): A Review of Progress in Production and Use in Research and Human and Veterinary Medicine. Altern. Lab. Anim. 2005, 33, 129–154. [Google Scholar] [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of Chicken Egg Yolk Antibodies (IgY) against Diarrhea in Domesticated Animals: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e97716. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Kovacs-Nolan, J. Chicken Egg Yolk Antibodies as Therapeutics in Enteric Infectious Disease: A Review. J. Med. Food 2002, 5, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.N.; Sunwoo, H.H.; Menninen, K.; Sim, J.S. In Vitro Studies of Chicken Egg Yolk Antibody (IgY) against Salmonella Enteritidis and Salmonella Typhimurium. Poult. Sci. 2002, 81, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Tsubokura, K.; Berndtson, E.; Bogstedt, A.; Kaijser, B.; Kim, M.; Ozeki, M.; Hammarström, L. Oral Administration of Antibodies as Prophylaxis and Therapy in Campylobacter Jejuni-Infected Chickens. Clin. Exp. Immunol. 1997, 108, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for Potential Diagnostic and Therapeutic Applications in Infectious Diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken Egg Yolk Antibodies (IgY) as Non-Antibiotic Production Enhancers for Use in Swine Production: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wen, Y.; Yang, F.; He, P. Chicken Egg Yolk Antibody (IgY) Protects Mice Against Enterotoxigenic Escherichia Coli Infection Through Improving Intestinal Health and Immune Response. Front. Cell. Infect. Microbiol. 2021, 11, 662710. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Montoya, C.A. Dietary Alternatives to In-Feed Antibiotics, Gut Barrier Function and Inflammation in Piglets Post-Weaning: Where Are We Now? Anim. Feed Sci. Technol. 2021, 274, 114836. [Google Scholar] [CrossRef]
- Han, S.; Yu, H.; Yang, F.; Qiao, S.; He, P. Effect of Dietary Supplementation with Hyperimmunized Hen Egg Yolk Powder on Diarrhoea Incidence and Intestinal Health of Weaned Pigs. Food Agric. Immunol. 2019, 30, 333–348. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Wang, X.; Zhen, Y.; Thacker, P.A.; Wang, L.; Shi, M.; Zhao, J.; Zong, Y.; Wang, N.; et al. Chicken Egg Yolk Antibodies (IgY) Modulate the Intestinal Mucosal Immune Response in a Mouse Model of Salmonella Typhimurium Infection. Int. Immunopharmacol. 2016, 36, 305–314. [Google Scholar] [CrossRef]
- Scheraiber, M.; Grześkowiak, Ł.; Zentek, J.; Barbosa, F.F.; Félix, A.P.; da Silva, A.V.F. Inclusion of IgY in a Dog’s Diet Has Moderate Impact on the Intestinal Microbial Fermentation. J. Appl. Microbiol. 2019, 127, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, A.-C.; Vacaras, V.; Nistor, C.; Vacaras, C.; Strilciuc, S.; Muresanu, D.F. The Effect of Multiple Sclerosis Therapy on Gut Microbiota Dysbiosis: A Longitudinal Prospective Study. Microb. Cell 2024, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, H.C.; Adalsteinsson, Ö.; Kagen, L. Administration to Arthritis Patients of a Dietary Supplement Containing Immune Egg: An Open-Label Pilot Study. J. Med. Food 1998, 1, 171–179. [Google Scholar] [CrossRef]
- Polanowski, A.; Zabłocka, A.; Sosnowska, A.; Janusz, M.; Trziszka, T. Immunomodulatory Activity Accompanying Chicken Egg Yolk Immunoglobulin Y. Poult. Sci. 2012, 91, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, A.; Bednarz, R.; Pacewicz, M.; Georgiades, J.A.; Wilusz, T.; Polanowski, A. Colostrum from Different Mammalian Species—A Rich Source of Colostrinin. Int. Dairy J. 2008, 18, 204–209. [Google Scholar] [CrossRef]
- Piasecki, E.; Inglot, A.D.; Winiarska, M.; Krukowska, K.; Janusz, M.; Lisowski, J. Coincidence between Spontaneous Release of Interferon and Tumor Necrosis Factor by Colostral Leukocytes and the Production of a Colostrinine by Human Mammary Gland after Normal Delivery. Arch. Immunol. Ther. Exp. 1997, 45, 109–117. [Google Scholar]
- Muhammad Yusoff, F.; Wong, K.K.; Mohd Redzwan, N. Th1, Th2, and Th17 Cytokines in Systemic Lupus Erythematosus. Autoimmunity 2020, 53, 8–20. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Liu, J.; Liu, Y.; Zhao, C.; Cai, H.; Lei, W.; Ma, J.; Fan, H.; Zhou, J.; et al. The Correlations between Th1 and Th2 Cytokines in Human Alveolar Echinococcosis. BMC Infect. Dis. 2020, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Mirshafiey, A. Th17 Cell, the New Player of Neuroinflammatory Process in Multiple Sclerosis. Scand. J. Immunol. 2011, 74, 1–13. [Google Scholar] [CrossRef]
- Martins, A.; Han, J.; Kim, S.O. The Multifaceted Effects of Granulocyte Colony-Stimulating Factor in Immunomodulation and Potential Roles in Intestinal Immune Homeostasis. IUBMB Life 2010, 62, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Foster, P.S. Stop Press: Eosinophils Drafted to Join the Th17 Team. Immunity 2015, 43, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Guglani, L.; Khader, S.A. Th17 Cytokines in Mucosal Immunity and Inflammation. Curr. Opin. HIV AIDS 2010, 5, 120–127. [Google Scholar] [CrossRef]
- Deng, C.; Peng, N.; Tang, Y.; Yu, N.; Wang, C.; Cai, X.; Zhang, L.; Hu, D.; Ciccia, F.; Lu, L. Roles of IL-25 in Type 2 Inflammation and Autoimmune Pathogenesis. Front. Immunol. 2021, 12, 691559. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Yoshimoto, T.; Yasuda, K.; Mizuguchi, J.; Nakanishi, K. IL-27 Suppresses Th2 Cell Development and Th2 Cytokines Production from Polarized Th2 Cells: A Novel Therapeutic Way for Th2-Mediated Allergic Inflammation1. J. Immunol. 2007, 179, 4415–4423. [Google Scholar] [CrossRef]
- Koguchi, Y.; Buenafe, A.C.; Thauland, T.J.; Gardell, J.L.; Bivins-Smith, E.R.; Jacoby, D.B.; Slifka, M.K.; Parker, D.C. Preformed CD40L Is Stored in Th1, Th2, Th17, and T Follicular Helper Cells as Well as CD4+8− Thymocytes and Invariant NKT Cells but Not in Treg Cells. PLoS ONE 2012, 7, e31296. [Google Scholar] [CrossRef] [PubMed]
- Castellani, M.L.; Felaco, P.; Galzio, R.J.; Tripodi, D.; Toniato, E.; De Lutiis, M.A.; Fulcheri, M.; Caraffa, A.; Antinolfi, P.; Tetè, S.; et al. IL-31 a Th2 Cytokine Involved in Immunity and Inflammation. Int. J. Immunopathol. Pharmacol. 2010, 23, 709–713. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hong, J.-H.; Glimcher, L.H. IL-2 Production in Developing Th1 Cells Is Regulated by Heterodimerization of RelA and T-Bet and Requires T-Bet Serine Residue 508. J. Exp. Med. 2005, 202, 1289–1300. [Google Scholar] [CrossRef]
- Korbecki, J.; Gąssowska-Dobrowolska, M.; Wójcik, J.; Szatkowska, I.; Barczak, K.; Chlubek, M.; Baranowska-Bosiacka, I. The Importance of CXCL1 in Physiology and Noncancerous Diseases of Bone, Bone Marrow, Muscle and the Nervous System. Int. J. Mol. Sci. 2022, 23, 4205. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 Cytokines and Asthma the Role of Interleukin-5 in Allergic Eosinophilic Disease. Respir. Res. 2001, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Marks, E.; Naudin, C.; Nolan, G.; Goggins, B.J.; Burns, G.; Mateer, S.W.; Latimore, J.K.; Minahan, K.; Plank, M.; Foster, P.S.; et al. Regulation of IL-12p40 by HIF Controls Th1/Th17 Responses to Prevent Mucosal Inflammation. Mucosal Immunol. 2017, 10, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-P.; Kim, Y.-S.; Kim, O.Y.; Kim, Y.-M.; Jeon, S.G.; Roh, T.-Y.; Park, J.-S.; Gho, Y.S.; Kim, Y.-K. TNF-Alpha Is a Key Mediator in the Development of Th2 Cell Response to Inhaled Allergens Induced by a Viral PAMP Double-Stranded RNA. Allergy 2012, 67, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhong, Z.; Jiang, H.; Chen, H.; Lyu, J.; Zhou, L. Th17-Associated Cytokines Multiplex Testing Indicates the Potential of Macrophage Inflammatory Protein-3 Alpha in the Diagnosis of Biliary Atresia. Cytokine 2019, 116, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How Informative Is the Mouse for Human Gut Microbiota Research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Rana, J.; Muñoz, M.M.; Biswas, M. Oral Tolerance to Prevent Anti-Drug Antibody Formation in Protein Replacement Therapies. Cell Immunol. 2022, 382, 104641. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.B.; Biswas, M.; Terhorst, C.; Daniell, H.; Herzog, R.W.; Piñeros, A.R. Role of Orally Induced Regulatory T Cells in Immunotherapy and Tolerance. Cell Immunol. 2021, 359, 104251. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.M.; Weiner, H.L. History and Mechanisms of Oral Tolerance. Semin. Immunol. 2017, 30, 3–11. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Pinheiro-Rosa, N.; Torres, L.; Oliveira, M.d.A.; Andrade-Oliveira, M.F.; Guimarães, M.A.d.F.; Coelho, M.M.; Alves, J.d.L.; Maioli, T.U.; Faria, A.M.C. Oral Tolerance as Antigen-Specific Immunotherapy. Immunother. Adv. 2021, 1, ltab017. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.O.P.T.; Guedes, M.I.F. Egg Yolk Antibodies (IgY) and Their Applications in Human and Veterinary Health: A Review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Akita, E.M.; Nakai, S. Production and Purification of Fab’ Fragments from Chicken Egg Yolk Immunoglobulin Y (IgY). J. Immunol. Methods 1993, 162, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, P.; Chen, Y.; Ma, S. Intact anti-LPS IgY Is Found in the Blood after Intragastric Administration in Mice. FEBS Open Bio 2019, 9, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Sevcik, C.; D’Suze, G.; Salazar, V.; Díaz, P.; Vázquez, H. Horse IgG- and Ostrich IgY-F(Ab’)2 Groups Have Different Affinities for Mice Erythrocytes and Lymphocytes. Implications for Avian Immunoglobulin Therapeutic Usefulness. Toxicon 2012, 60, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Akita, E.M.; Jang, C.B.; Kitts, D.D.; Nakai, S. Evaluation of Allergenicity of Egg Yolk Immunoglobulin Y and Other Egg Proteins by Passive Cutaneous Anaphylaxis. Food Agric. Immunol. 1999, 11, 191–201. [Google Scholar] [CrossRef]
- Farah, R.; Khamisy-Farah, R. Significance of MPV, RDW with the Presence and Severity of Metabolic Syndrome. Exp. Clin. Endocrinol. Diabetes 2015, 123, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Gao, P. Increased Red Cell Distribution Width Predicts Severity of Drug-Induced Liver Injury: A Retrospective Study. Sci. Rep. 2021, 11, 773. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red Blood Cell Distribution Width: A Simple Parameter with Multiple Clinical Applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Salvagno, G.L.; Guidi, G.C. Increased Red Blood Cell Distribution Width (RDW) Is Associated with Higher Glycosylated Hemoglobin (HbA1c) in the Elderly. Clin. Lab. 2014, 60, 2095–2098. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, S.; Vimalraj, S.; Thangavelu, L. The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules 2021, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- Tahtaci, M.; Yurekli, O.T.; Bolat, A.D.; Balci, S.; Akin, F.E.; Buyukasik, N.S.; Ersoy, O. Increased Mean Platelet Volume Is Related to Histologic Severity of Primary Biliary Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1382. [Google Scholar] [CrossRef] [PubMed]
- Ozhan, H.; Aydin, M.; Yazici, M.; Yazgan, O.; Basar, C.; Gungor, A.; Onder, E. Mean Platelet Volume in Patients with Non-Alcoholic Fatty Liver Disease. Platelets 2010, 21, 29–32. [Google Scholar] [CrossRef]
- van Niekerk, G.; Davis, T.; Patterton, H.-G.; Engelbrecht, A.-M. How Does Inflammation-Induced Hyperglycemia Cause Mitochondrial Dysfunction in Immune Cells? Bioessays 2019, 41, e1800260. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Jania, B.; Andraszek, K. Application of Native Agarose Gel Electrophoresis of Serum Proteins in Veterinary Diagnostics. J. Vet. Res. 2016, 60, 501–508. [Google Scholar] [CrossRef]
- Całkosiński, I.; Majda, J.; Terlecki, G.; Gostomska-Pampuch, K.; Małolepsza-Jarmołowska, K.; Sobolewska, S.; Całkosińska, A.; Kumala, A.; Gamian, A. Dynamic Analysis of Changes of Protein Levels and Selected Biochemical Indices in Rat Serum in the Course of Experimental Pleurisy. Inflammation 2016, 39, 1076–1089. [Google Scholar] [CrossRef][Green Version]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef] [PubMed]
- Torché, A.-M.; Le Dimna, M.; Le Corre, P.; Mesplède, A.; Le Gal, S.; Cariolet, R.; Le Potier, M.-F. Immune Responses after Local Administration of IgY Loaded-PLGA Microspheres in Gut-Associated Lymphoid Tissue in Pigs. Vet. Immunol. Immunopathol. 2006, 109, 209–217. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.; Pohler, E.; Moraga, I. Molecular and Cellular Factors Determining the Functional Pleiotropy of Cytokines. FEBS J. 2023, 290, 2525–2552. [Google Scholar] [CrossRef] [PubMed]
- Frick, V.O.; Rubie, C.; Keilholz, U.; Ghadjar, P. Chemokine/Chemokine Receptor Pair CCL20/CCR6 in Human Colorectal Malignancy: An Overview. World J. Gastroenterol. 2016, 22, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.L. CC, C, and CX3C Chemokines. In Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: New York, NY, USA, 2003; pp. 255–263. ISBN 978-0-12-341103-7. [Google Scholar]
- Alfaro, S.; Acuña, V.; Ceriani, R.; Cavieres, M.F.; Weinstein-Oppenheimer, C.R.; Campos-Estrada, C. Involvement of Inflammation and Its Resolution in Disease and Therapeutics. Int. J. Mol. Sci. 2022, 23, 10719. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The Resolution of Inflammation: Principles and Challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Ferguson, L.R. Susceptibility to Chronic Inflammation: An Update. Arch. Toxicol. 2017, 91, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs Modulate the Inflammatory Response to Carbohydrate Restricted Diets in Overweight Men. Nutr. Metab. 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Tannock, L.R.; O’Brien, K.D.; Knopp, R.H.; Retzlaff, B.; Fish, B.; Wener, M.H.; Kahn, S.E.; Chait, A. Cholesterol Feeding Increases C-Reactive Protein and Serum Amyloid a Levels in Lean Insulin-Sensitive Subjects. Circulation 2005, 111, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, Inflammation and Innate Immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Yokooji, T.; Hamura, K.; Matsuo, H. Intestinal Absorption of Lysozyme, an Egg-White Allergen, in Rats: Kinetics and Effect of NSAIDs. Biochem. Biophys. Res. Commun. 2013, 438, 61–65. [Google Scholar] [CrossRef]
- van Splunter, M.; van Hoffen, E.; Floris-Vollenbroek, E.G.; Timmerman, H.; de Bos, E.L.; Meijer, B.; Ulfman, L.H.; Witteman, B.; Wells, J.M.; Brugman, S.; et al. Oral Cholera Vaccination Promotes Homing of IgA+ Memory B Cells to the Large Intestine and the Respiratory Tract. Mucosal Immunol. 2018, 11, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Hind, L.E.; Huttenlocher, A. Neutrophil Reverse Migration and a Chemokinetic Resolution. Dev. Cell 2018, 47, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the Function and Fate of Neutrophils in Sterile Injury and Repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ochi, H.; Abraham, M.; Ishikawa, H.; Frenkel, D.; Yang, K.; Basso, A.S.; Wu, H.; Chen, M.-L.; Gandhi, R.; Miller, A.; et al. Oral CD3-Specific Antibody Suppresses Autoimmune Encephalomyelitis by Inducing CD4+ CD25- LAP+ T Cells. Nat. Med. 2006, 12, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Marth, T.; Ring, S.; Schulte, D.; Klensch, N.; Strober, W.; Kelsall, B.L.; Stallmach, A.; Zeitz, M. Antigen-Induced Mucosal T Cell Activation Is Followed by Th1 T Cell Suppression in Continuously Fed Ovalbumin TCR-Transgenic Mice. Eur. J. Immunol. 2000, 30, 3478–3486. [Google Scholar] [CrossRef]
- Farazuddin, M.; Landers, J.J.; Janczak, K.W.; Lindsey, H.K.; Finkelman, F.D.; Baker, J.R.; O’Konek, J.J. Mucosal Nanoemulsion Allergy Vaccine Suppresses Alarmin Expression and Induces Bystander Suppression of Reactivity to Multiple Food Allergens. Front. Immunol. 2021, 12, 599296. [Google Scholar] [CrossRef]
- Ilan, Y. Immune Rebalancing by Oral Immunotherapy: A Novel Method for Getting the Immune System Back on Track. J. Leukoc. Biol. 2019, 105, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.M.; Nakagaki, B.N.; Moreira, T.G.; Lopes, J.R.; Kuhn, C.; Tatematsu, B.K.; Boulenouar, S.; Maghzi, A.-H.; Rubino, S.; Menezes, G.B.; et al. Γδ T Cell-Secreted XCL1 Mediates Anti-CD3-Induced Oral Tolerance. J. Immunol. 2019, 203, 2621–2629. [Google Scholar] [CrossRef]
- Paiatto, L.N.; Silva, F.G.D.; Bier, J.; Brochetto-Braga, M.R.; Yamada, Á.T.; Tamashiro, W.M.S.C.; Simioni, P.U. Oral Tolerance Induced by OVA Intake Ameliorates TNBS-Induced Colitis in Mice. PLoS ONE 2017, 12, e0170205. [Google Scholar] [CrossRef]
- Navarro, S.; Lazzari, A.; Kanda, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V. Bystander Immunotherapy as a Strategy to Control Allergen-Driven Airway Inflammation. Mucosal Immunol. 2015, 8, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, H.I.; Moore, G.; Wnorowski, G. Inhibition of Diarrhea by Immune Egg. J. Nutraceuticals Funct. Med. Foods 2000, 3, 47–53. [Google Scholar] [CrossRef]
- Karge, W.H.; Deluca, J.P.; Marchitelli, L.J.; Champagne, C.; Tulley, R.; Rood, J.; Paulos, M.A.; Lieberman, H.R. Pilot Study on the Effect of Hyperimmune Egg Protein on Elevated Cholesterol Levels and Cardiovascular Risk Factors. J. Med. Food 1999, 2, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.H.; Monaghan, T.; Sellers, J.; Estrada, C.; Moghaddam, M. Effect of Avian Immunoglobulin on Post-Exercise Muscle Damage and Muscular Soreness. J. Hum. Sport Exerc. 2019, 14, 802–812. [Google Scholar] [CrossRef]
- Surcel, M.; Munteanu, A.; Isvoranu, G.; Ibram, A.; Caruntu, C.; Constantin, C.; Neagu, M. Unconventional Therapy with IgY in a Psoriatic Mouse Model Targeting Gut Microbiome. J. Pers. Med. 2021, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Constantin, C.; Munteanu, A.N.; Costea, D.A.; Isvoranu, G.; Codrici, E.; Popescu, I.D.; Tănase, C.; Ibram, A.; Neagu, M. Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis. J. Pers. Med. 2023, 13, 1556. [Google Scholar] [CrossRef]
- Esterházy, D.; Canesso, M.C.C.; Mesin, L.; Muller, P.A.; de Castro, T.B.R.; Lockhart, A.; ElJalby, M.; Faria, A.M.C.; Mucida, D. Compartmentalized Gut Lymph Node Drainage Dictates Adaptive Immune Responses. Nature 2019, 569, 126–130. [Google Scholar] [CrossRef]
- Houston, S.A.; Cerovic, V.; Thomson, C.; Brewer, J.; Mowat, A.M.; Milling, S. The Lymph Nodes Draining the Small Intestine and Colon Are Anatomically Separate and Immunologically Distinct. Mucosal Immunol. 2016, 9, 468–478. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Colonic Antigen Administration Induces Significantly Higher Humoral Levels of Colonic and Vaginal IgA, and Serum IgG Compared to Oral Administration. Vaccine 2008, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Pereira e Silva, A.; Marmello, B.O.; Soares, J.R.A.; Mazza-Guimaraes, I.; Teixeira, G.A.P.B. Induction of Food Tolerance Is Dependent on Intestinal Inflammatory State. Immunol. Lett. 2021, 234, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and Resolution of Periodontal Inflammation and Its Systemic Impact. Periodontol. 2000 2015, 69, 255–273. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 489354. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Jae, S.Y.; Kurl, S.; Kauhanen, J.; Laukkanen, J.A. Inflammation, Sauna Bathing, and All-Cause Mortality in Middle-Aged and Older Finnish Men: A Cohort Study. Eur. J. Epidemiol. 2022, 37, 1225–1231. [Google Scholar] [CrossRef]
- Patrick, R.P.; Johnson, T.L. Sauna Use as a Lifestyle Practice to Extend Healthspan. Exp. Gerontol. 2021, 154, 111509. [Google Scholar] [CrossRef]
- Behzadi, P.; Gravel, H.; Neagoe, P.-E.; Barry, H.; Sirois, M.G.; Gagnon, D. Impact of Finnish Sauna Bathing on Circulating Markers of Inflammation in Healthy Middle-Aged and Older Adults: A Crossover Study. Complement. Ther. Med. 2020, 52, 102486. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Leite, A.d.O.F.; Bento Torres Neto, J.; dos Reis, R.R.; Sobral, L.L.; de Souza, A.C.P.; Trévia, N.; de Oliveira, R.B.; Lins, N.A.d.A.; Diniz, D.G.; Diniz, J.A.P.; et al. Unwanted Exacerbation of the Immune Response in Neurodegenerative Disease: A Time to Review the Impact. Front. Cell. Neurosci. 2021, 15, 749595. [Google Scholar] [CrossRef] [PubMed]
- Brunell, A.E.; Lahesmaa, R.; Autio, A.; Thotakura, A.K. Exhausted T Cells Hijacking the Cancer-Immunity Cycle: Assets and Liabilities. Front. Immunol. 2023, 14, 1151632. [Google Scholar] [CrossRef] [PubMed]
- Pătrașcu, I.V.; Chiurciu, V.; Chiurciu, C.; Topilescu, G. Procedure to Obtain and Use Hen Egg Immunoglobulins (IgY). RO129645 A0 2014, 39, 30. [Google Scholar]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]



| Group | HE | fdHE | Yext | |
|---|---|---|---|---|
| Time | ||||
| T30 | IL-6: p = 0.0172 (vs. DW) p = 0.0296 (vs. SPF) p = 0.0177 (vs. T0) p = 0.0205 (vs. T60) p = 0.0279 (vs. T90) G-CSF: p < 0.0001 (vs. DW) p < 0.0001 (vs. SPF) p < 0.0001 (vs. T0) p < 0.0001 (vs. T60) p = 0.0004 (vs. T90) | Eotaxin: p = 0.0010 (vs. DW) p = 0.0295 (vs. SPF) p = 0.0002 (vs. T0) p = 0.0436 (vs. T90) | IL-21: p = 0.0034 (vs. DW) p = 0.0016 (vs. SPF) p = 0.0010 (vs. T0) p = 0.0010 (vs. T60) p = 0.0018 (vs. T90) IL-25 (IL-17E): p = 0.0064 (vs. DW) p = 0.0054 (vs. SPF) p = 0.0298 (vs. T60) p = 0.0127 (vs. T90) IL-27: p = 0.0226 (vs. DW) p = 0.0124 (vs. SPF) p = 0.0058 (vs. T0) p = 0.0058 (vs. T60) p = 0.0198 (vs. T90) CD40L: p < 0.0001 (vs. DW) p < 0.0001 (vs. SPF) p < 0.0001 (vs. T0) p < 0.0001 (vs. T60) p < 0.0001 (vs. T90) | |
| T60 | IL-31: p = 0.0351 (vs. DW) p = 0.0351 (vs. SPF) p = 0.0378 (vs. T0) p = 0.0374 (vs. T30) | – | – | |
| T90 | IL-2: p < 0.0001 (vs. DW) p < 0.0001 (vs. SPF) p = 0.0002 (vs. T0) p = 0.0206 (vs. T30) IL-13: p = 0.0001 (vs. DW) p = 0.0370 (vs. SPF) p = 0.0087 (vs. T0) p = 0.0177 (vs. T60) G-CSF: p = 0.0101 (vs. DW) p = 0.0005 (vs. SPF) p = 0.0012 (vs. T0) p = 0.0004 (vs. T30) p = 0.0005 (vs. T60) KC (CXCL1): p < 0.0001 (vs. DW) p < 0.0001 (vs. SPF) p < 0.0001 (vs. T0) p < 0.0001 (vs. T30) p < 0.0001 (vs. T60) | IL-5: p = 0.0002 (vs. DW) p = 0.0338 (vs. SPF) p = 0.0006 (vs. T0) p = 0.0134 (vs. T30) p = 0.0019 (vs. T60) IL-10: p < 0.0001 (vs. DW) p = 0.0115 (vs. SPF) p = 0.0006 (vs. T0) p = 0.0381 (vs. T60) IL-12 (p40): p < 0.0001 (vs. DW) p = 0.0001 (vs. SPF) p < 0.0001 (vs. T0) p = 0.0003 (vs. T60) TNFα: p < 0.0001 (vs. DW) p = 0.0276 (vs. SPF) p < 0.0001 (vs. T0) p = 0.0002 (vs. T30) p = 0.0117 (vs. T60) | MIP-3α: p < 0.0001 (vs. DW) p < 0.0001 (vs. SPF) p < 0.0001 (vs. T0) p < 0.0001 (vs. T30) p < 0.0001 (vs. T60) | |
| Group | HE | fdHE | Yext | |
|---|---|---|---|---|
| Time | ||||
| T30 | IL-6: Th2 *, Th17 [27,28,29,30] G-CSF: Th17 [31] | Eotaxin: Th2, Th17 [28,32] | IL-21: Th17 [30,33] IL-25 (IL-17E): Th2 [30,34] IL-27: Th1, Th2 [35] CD40L: Th1, Th2, Th17 [36] | |
| T60 | IL-31: Th2 [37] | – | – | |
| T90 | IL-2: Th1, Th2 [27,38] IL-13: Th2 [27,28] G-CSF: Th17 [31] KC (CXCL1): Th17 [39] | IL-5: Th2 [40,41] IL-10: Th2 [27] IL-12 (p40): Th1, Th17 [42] TNFα: Th1, Th2, Th17 [27,30,43] | MIP-3α: Th17 [44] | |
| Time | Group | RBC (1012/L) | HGB (g/dL) | HCT (%) | MCV (fl) | MCH (pg) | MCHC (g/dL) | RDWc (%) | RDWs (%) | PLT (109/L) | PCT (%) | MPV (fl) | PDWc (%) | PDWs (fl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | DW | 9.56 ±0.12 | 13.20 ±0.76 | 40.92 ±1.91 | 41.15 ±0.56 | 13.13 ±0.71 | 31.12 ±0.23 | 19.53 ±0.16 | 31.12 ±0.88 | 283.00 ±43.10 | 0.15 ±0.78 | 6.01 ±0.18 | 27.00 ±0.22 | 6.67 ±0.22 |
| SPF | 9.72 ±1.16 | 14.12 ±0.32 | 42.38 ±0.89 | 42.11 ±0.62 | 13.23 ±0.43 | 32.14 ±0.56 | 19.43 ±0.63 | 30.93 ±0.10 | 291.00 ±32.10 | 0.16 ±0.03 | 6.00 ±0.11 | 28.01 ±0.21 | 7.00 ±0.08 | |
| HE | 9.43 ±1.34 | 12.54 ±0.14 | 41.08 ±1.76 | 41.31 ±0.78 | 13.00 ±0.83 | 31.44 ±0.72 | 19.45 ±0.73 | 31.33 ±0.12 | 287.00 ±42.00 | 0.15 ±0.39 | 6.00 ±0.22 | 27.08 ±0.14 | 6.80 ±0.03 | |
| fdHE | 9.62 ±1.34 | 13.48 ±0.19 | 42.62 ±0.86 | 42.00 ±0.18 | 13.08 ±0.61 | 31.83 ±0.64 | 19.13 ±0.13 | 30.33 ±0.87 | 286.00 ±12.00 | 0.15 ±0.39 | 6.00 ±0.12 | 27.43 ±0.19 | 6.56 ±0.18 | |
| Yext | 9.71 ±1.34 | 12.48 ±0.19 | 43.28 ±0.86 | 42.00 ±0.35 | 13.33 ±0.41 | 31.52 ±0.56 | 19.51 ±0.11 | 31.21 ±0.24 | 293.00 ±12.00 | 0.15 ±0.32 | 6.20 ±0.18 | 27.23 ±0.19 | 6.57 ±0.24 | |
| T30 | DW | 9.69 ±0.74 | 13.22 ±0.59 | 42.97 ±0.70 | 42.75 ±0.49 | 13.55 ±0.26 | 31.75 ±0.45 | 19.50 ±0.44 | 31.99 ±0.88 | 286.00 ±58.40 | 0.16 ±0.03 | 6.17 ±0.14 | 27.00 ±0.49 | 6.82 ±0.24 |
| SPF | 9.96 ±1.37 | 14.15 ±0.15 | 43.21 ±1.64 | 42.05 ±0.71 | 13.37 ±0.12 | 32.10 ±0.48 | 19.50 ±0.43 | 30.67 ±0.30 | 347.00 ±85.50 | 0.20 ±0.04 | 6.05 ±0.08 | 28.70 ±0.60 | 7.70 ±0.34 | |
| HE | 9.84 ±0.48 | 12.97 ±0.51 | 40.08 ±1.85 | 41.75 ±0.82 | 13.17 ±0.28 | 31.77 ±0.32 | 19.25 ±0.28 | 30.03 ±0.34 | 354.00 ±29.40 | 0.21 ±0.01 | 6.17 ±0.19 | 28.15 ±0.62 | 7.37 ±0.86 | |
| fdHE | 10.27 ±0.54 | 14.17 ±0.39 | 43.36 ±1.36 | 42.25 ±0.82 | 13.85 ±0.45 | 32.77 ±0.54 | 19.55 ±0.57 | 31.05 ±0.61 | 412.00 ±32.00 | 0.27 ±0.09 | 6.70 ±0.21 | 28.95 ±1.01 | 7.87 ±0.29 | |
| Yext | 10.49 ±0.55 | 14.40 ±0.53 | 44.17 ±2.26 | 42.25 ±0.43 | 13.70 ±0.25 | 32.07 ±0.57 | 19.67 ±0.83 | 31.45 ±1.21 | 349.00 ±66.50 | 0.21 ±0.03 | 6.12 ±0.17 | 29.47 ±2.53 | 8.27 ±1.70 | |
| T60 | DW | 9.81 ±0.42 | 13.52 ±0.36 | 43.24 ±0.97 | 43.25 ±0.82 | 13.45 ±0.26 | 31.20 ±0.33 | 18.82 ±0.43 | 30.87 ±0.64 | 417.50 ±52.47 | 0.26 ±0.05 | 6.30 ±0.24 | 28.00 ±0.45 | 7.20 ±0.24 |
| SPF | 10.51 ±0.49 | 14.20 ±0.45 | 44.50 ±1.69 | 42.50 ±0.50 | 13.52 ±0.29 | 31.85 ±0.55 | 19.25 ±0.61 | 30.73 ±0.91 | 440.00 ±24.48 | 0.27 ±0.02 | 6.15 ±0.21 | 28.40 ±0.45 | 7.20 ±0.24 | |
| HE | 10.30 ±0.42 | 14.00 ±0.36 | 44.56 ±1.26 | 43.25 ±1.20 | 13.52 ±0.34 | 31.45 ±0.26 | 19.27 ±0.41 | 31.11 ±0.69 | 399.00 ±43.03 | 0.24 ±0.04 | 5.90 ±0.10 | 28.07 ±1.75 | 7.30 ±1.02 | |
| fdHE | 10.35 ±0.60 | 13.67 ±0.75 | 43.12 ±1.94 | 43.00 ±0.70 | 13.60 ±0.20 | 31.72 ±0.49 | 20.75 ±1.05 | 33.77 ±2.45 | 361.25 ±41.44 | 0.24 ±0.08 | 6.10 ±0.12 | 28.80 ±1.06 | 7.77 ±0.65 | |
| Yext | 10.45 ±0.40 | 13.25 ±0.37 | 41.95 ±0.89 | 43.00 ±1.22 | 13.55 ±0.18 | 31.50 ±0.67 | 19.00 ±0.52 | 30.85 ±0.35 | 434.00 ±55.21 | 0.27 ±0.03 | 6.20 ±0.27 | 28.80 ±1.40 | 7.77 ±0.84 | |
| T90 | DW | 9.21 ±0.50 | 13.20 ±0.64 | 37.38 ±2.58 | 40.50 ±1.11 | 14.35 ±0.22 | 35.35 ±0.83 | 18.10 ±0.33 | 27.70 ±0.40 | 236.75 ±10.25 | 0.16 ±0.07 | 6.55 ±0.61 | 32.57 ±3.09 | 10.50 ±2.37 |
| SPF | 10.55 ±0.30 | 15.25 ±0.45 | 41.34 ±1.21 | 39.25 ±1.29 | 14.45 ±0.30 | 37.07 ±1.23 | 19.00 ±0.27 | 28.30 ±0.87 | 471.25 ±27.18 | 0.29 ±0.16 | 6.17 ±0.08 | 28.82 ±0.78 | 2.77 ±0.48 | |
| HE | 10.42 ±0.48 | 14.77 ±0.87 | 42.01 ±1.95 | 40.00 ±1.34 | 14.17 ±0.25 | 35.12 ±0.55 | 18.87 ±0.44 | 28.70 ±0.66 | 351.00 ±16.34 | 0.21 ±0.09 | 6.27 ±0.19 | 30.52 ±1.58 | 8.87 ±1.04 | |
| fdHE | 10.62 ±0.39 | 14.85 ±0.45 | 42.94 ±1.13 | 40.25 ±0.43 | 14.17 ±0.21 | 34.57 ±0.59 | 18.87 ±0.40 | 28.70 ±0.34 | 313.00 ±10.53 | 0.20 ±0.05 | 6.47 ±0.30 | 31.72 ±1.94 | 9.67 ±1.27 | |
| Yext | 10.45 ±0.11 | 14.45 ±0.29 | 42.36 ±0.93 | 40.05 ±0.50 | 13.85 ±0.16 | 34.12 ±0.19 | 18.35 ±0.51 | 27.90 ±0.83 | 466.75 ±38.27 | 0.29 ±0.02 | 6.29 ±0.02 | 29.45 ±1.32 | 8.15 ±0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastasa, V.; Minea, B.; Pasca, A.-S.; Bostanaru-Iliescu, A.-C.; Stefan, A.-E.; Gologan, D.; Capota, R.; Foia, L.-G.; Mares, M. Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 8701. https://doi.org/10.3390/ijms25168701
Nastasa V, Minea B, Pasca A-S, Bostanaru-Iliescu A-C, Stefan A-E, Gologan D, Capota R, Foia L-G, Mares M. Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice. International Journal of Molecular Sciences. 2024; 25(16):8701. https://doi.org/10.3390/ijms25168701
Chicago/Turabian StyleNastasa, Valentin, Bogdan Minea, Aurelian-Sorin Pasca, Andra-Cristina Bostanaru-Iliescu, Alina-Elena Stefan, Daniela Gologan, Robert Capota, Liliana-Georgeta Foia, and Mihai Mares. 2024. "Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice" International Journal of Molecular Sciences 25, no. 16: 8701. https://doi.org/10.3390/ijms25168701
APA StyleNastasa, V., Minea, B., Pasca, A.-S., Bostanaru-Iliescu, A.-C., Stefan, A.-E., Gologan, D., Capota, R., Foia, L.-G., & Mares, M. (2024). Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice. International Journal of Molecular Sciences, 25(16), 8701. https://doi.org/10.3390/ijms25168701








