Squamous Cell Carcinoma in Never Smokers: An Insight into SMARCB1 Loss
Abstract
1. Introduction
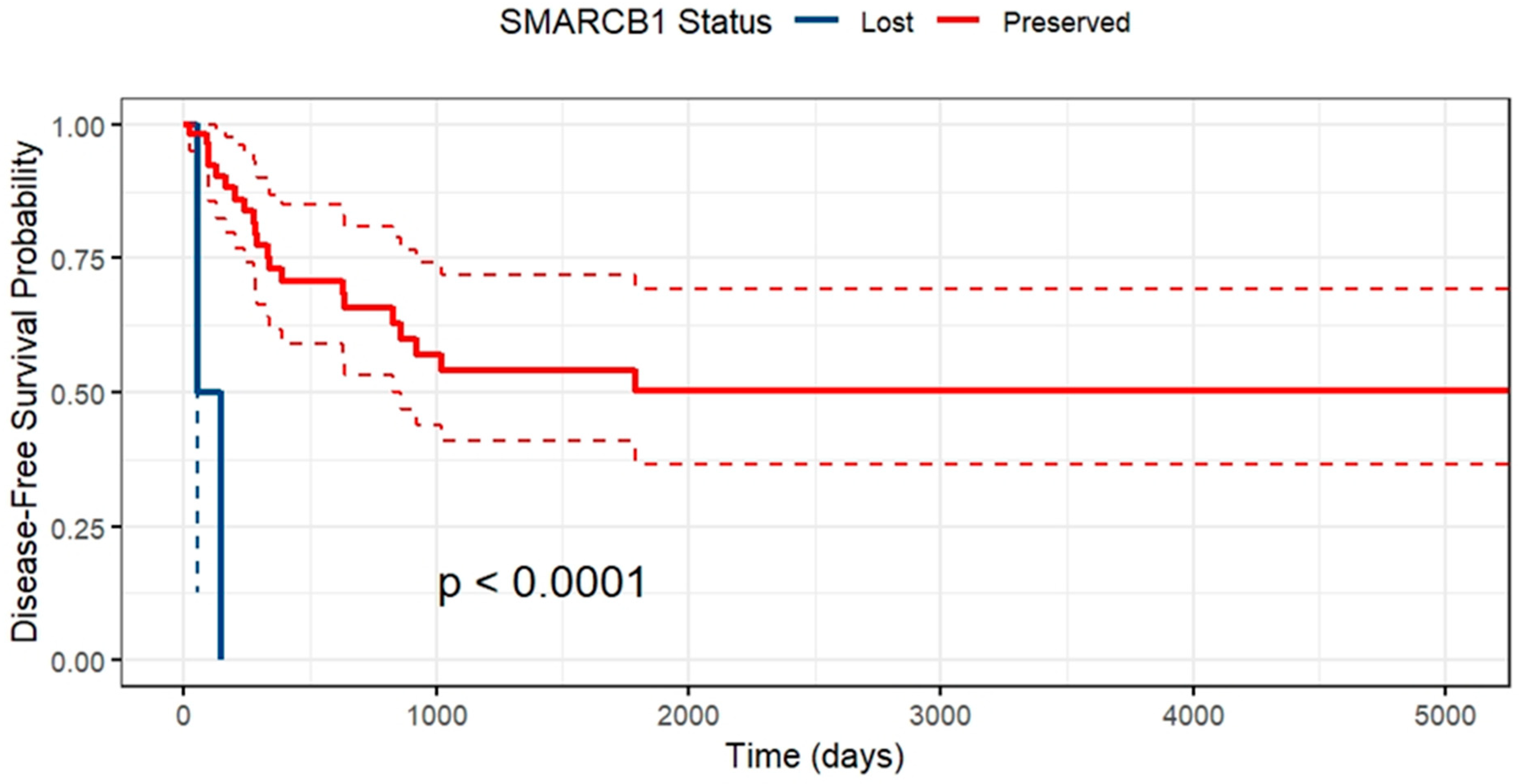
2. Results
3. Discussion
4. Methods and Materials
- Any patient who had undergone radical surgical resection for squamous cell carcinoma of the lung in the time period.
- Never smoking status.
- Non-squamous histology.
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Khuder, S.A. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer 2001, 31, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung Cancer in Never Smokers—A Different Disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Cufari, M.E.; Proli, C.; Sousa, P.D.; Raubenheimer, H.; Sahaf, M.A.; Chavan, H.; Shedden, L.; Niwaz, Z.; Leung, M.; Nicholson, A.G.; et al. Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur. J. Cancer 2017, 84, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhou, C. Lung cancer in never smokers—The East Asian experience. Transl. Lung Cancer Res. 2018, 7, 450–463. [Google Scholar] [CrossRef]
- Shirgaonkar, R.; Mohapatra, P.R.; Panigrahi, M.K.; Mishra, P.; Bhuniya, S.; Sarkar, S.; Girija, A.; Shaik, A.; Mohanty, S.; Moorthy, A. Evaluation of Risk Factors for Lung Cancer Among Never Smokers and Their Association with Common Driver Mutations. Cureus 2024, 16, e56024. [Google Scholar]
- Hill, W.; Lim, E.L.; Weeden, C.E.; Lee, C.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Evans, E.J., Jr.; Moore, D.A.; et al. Lung adenocarcinoma promotion by air pollutants. Nature 2023, 616, 159–167. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, Y.; Fang, Z.; Li, C.; Fang, R.; Gao, B.; Han, X.; Tian, W.; Pao, W.; Chen, H.; et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4616–4620. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Pan, Y.; Li, C.; Shen, L.; Li, Y.; Luo, X.; Ye, T.; Wang, R.; Hu, H.; et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Avila-Tang, E.; Harris, C.C.; Herman, J.G.; Hirsch, F.R.; Pao, W.; Schwartz, A.G.; Vahakangas, K.H.; Samet, J.M. Lung cancer in never smokers: Molecular profiles and therapeutic implications. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5646–5661. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zhang, L.; Zhu, X.; Wang, Z.; Fang, S.; Lin, J.; Wang, J.; Li, N.; Liu, H.; Zhang, X.; et al. Chinese never smokers with adenocarcinoma of the lung are younger and have fewer lymph node metastases than smokers. Respir. Res. 2022, 23, 293. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.; Li, Y.; Martins Rodrigues, F.; Sankararaman, S.; Kadara, H.; Goparaju, C.; Lanc, I.; Pepin, K.; Waqar, S.N.; Morgensztern, D.; et al. Genomic Profiling of Lung Adenocarcinoma in Never-Smokers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3747–3758. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, R.; Pan, Y.; Zhang, Y.; Li, H.; Cheng, C.; Zheng, D.; Zheng, S.; Li, Y.; Shen, X.; et al. Clinical and genetic features of lung squamous cell cancer in never-smokers. Oncotarget 2016, 7, 35979–35988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Joubert, P.; Ansari-Pour, N.; Zhao, W.; Hoang, P.H.; Lokanga, R.; Moye, A.L.; Rosenbaum, J.; Gonzalez-Perez, A.; Martínez-Jiménez, F.; et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat. Genet. 2021, 53, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Kauczor, H.U.; Baird, A.M.; Blum, T.G.; Bonomo, L.; Bostantzoglou, C.; Burghuber, O.; Čepická, B.; Comanescu, A.; Couraud, S.; Devaraj, A.; et al. ESR/ERS statement paper on lung cancer screening. Eur. Respir. J. 2020, 30, 3277–3294. [Google Scholar] [CrossRef]
- Oudkerk, M.; Devaraj, A.; Vliegenthart, R.; Henzler, T.; Prosch, H.; Heussel, C.P.; Bastarrika, G.; Sverzellati, N.; Mascalchi, M.; Delorme, S.; et al. European position statement on lung cancer screening. Lancet Oncol. 2017, 18, e754–e766. [Google Scholar] [CrossRef] [PubMed]
- Baik, C.S.; Pritchard, C.C.; Eaton, K.D.; Chow, L.Q. EGFR mutations in squamous cell lung cancer in never-smokers. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Pathology of Lung Cancer. Clin. Chest. Med. 2011, 32, 669–692. [Google Scholar] [CrossRef]
- Cooper, G.W.; Hong, A.L. SMARCB1-Deficient Cancers: Novel Molecular Insights and Therapeutic Vulnerabilities. Cancers 2022, 14, 3645. [Google Scholar] [CrossRef]
- Versteege, I.; Sévenet, N.; Lange, J.; Rousseau-Merck, M.F.; Ambros, P.; Handgretinger, R.; Aurias, A.; Delattre, O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998, 394, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Naroditsky, I.; Ben-Izhak, O. Primary high-grade myoepithelial carcinoma of the lung: A study of three cases illustrating frequent SMARCB1-deficiency and review of the literature. Ann. Diagn. Pathol. 2021, 53, 151759. [Google Scholar] [CrossRef] [PubMed]
- Del Savio, E.; Maestro, R. Beyond SMARCB1 Loss: Recent Insights into the Pathobiology of Epithelioid Sarcoma. Cells 2022, 11, 2626. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.A.; Burr, M.L.; Williams, B.; Murugasu, A.; Fellowes, A.; John, T.; Christie, M. SMARCB1/INI1-deficient primary lung carcinoma with hepatic metastasis. Pathology 2022, 54, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J. STK11 loss and SMARCB1 deficiency mutation in a dedifferentiated lung cancer patient present response to neo-adjuvant treatment with pembrolizumab and platinum doublet: A case report. Front. Oncol. 2023, 13, 1088534. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.; Chen, L.; Di, M.; Cao, J. SMARCA4-deficient non-small cell lung cancer with an EGFR mutation: A case report. Oncol. Lett. 2023, 26, 513. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.M.; Leroux, M.M.; Fleming, M.D.; Orkin, S.H. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer Cell 2002, 2, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.G.; Wang, X.; Shen, X.; McKenna, E.S.; Lemieux, M.E.; Cho, Y.J.; Koellhoffer, E.C.; Pomeroy, S.L.; Orkin, S.H.; Roberts, C.W.M. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 2010, 18, 316–328. [Google Scholar] [CrossRef]
- Romagosa, C.; Simonetti, S.; López-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Cajal, S.R. p16(Ink4a) overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef]
- Fernandez, P.C.; Frank, S.R.; Wang, L.; Schroeder, M.; Liu, S.; Greene, J.; Cocito, A.; Amati, B. Genomic targets of the human c-Myc protein. Genes Dev. 2003, 17, 1115–1129. [Google Scholar] [CrossRef]
- Sesboue, C.; Le Loarer, F. SWI/SNF-deficient thoraco-pulmonary neoplasms. Semin. Diagn. Pathol. 2021, 38, 183–194. [Google Scholar] [CrossRef]
- Lanzi, C.; Arrighetti, N.; Pasquali, S.; Cassinelli, G. Targeting EZH2 in SMARCB1-deficient sarcomas: Advances and opportunities to potentiate the efficacy of EZH2 inhibitors. Biochem. Pharmacol. 2023, 215, 115727. [Google Scholar] [CrossRef]
- Hoy, S.M. Tazemetostat: First Approval. Drugs 2020, 80, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022, 13, 158–163. [Google Scholar] [CrossRef]
- Wong, J.P.; Todd, J.R.; Finetti, M.A.; McCarthy, F.; Broncel, M.; Vyse, S.; Luczynski, M.T.; Crosier, S.; Ryall, K.A.; Holmes, K.; et al. Dual Targeting of PDGFRα and FGFR1 Displays Synergistic Efficacy in Malignant Rhabdoid Tumors. Cell Rep. 2016, 17, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Krämer, K.F.; Moreno, N.; Frühwald, M.C.; Kerl, K. BRD9 Inhibition, Alone or in Combination with Cytostatic Compounds as a Therapeutic Approach in Rhabdoid Tumors. Int. J. Mol. Sci. 2017, 18, 1537. [Google Scholar] [CrossRef]
- Alimova, I.; Pierce, A.; Danis, E.; Donson, A.; Birks, D.K.; Griesinger, A.; Foreman, N.K.; Santi, M.; Soucek, L.; Venkataraman, S.; et al. Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo. Int. J. Cancer 2019, 144, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.J.; Al-Ibraheemi, A.; Doan, D.; Ward, A.; Clinton, C.M.; Putra, J.; Pinches, R.S.; Kadoch, C.; Chi, S.N.; DuBois, S.G.; et al. Genomic and Immunologic Characterization of INI1-Deficient Pediatric Cancers. Clin. Cancer. Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Postel-Vinay, S. Immunotherapy for SMARCB1-Deficient Sarcomas: Current Evidence and Future Developments. Biomedicines 2022, 10, 650. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Hindi, N.; Grignani, G.; Martinez-Trufero, J.; Redondo, A.; Valverde, C.; Stacchiotti, S.; Lopez-Pousa, A.; D’Ambrosio, L.; Gutierrez, A.; et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: A multicenter, single-arm, phase Ib/II trial. J. Immunother. Cancer 2020, 8, e001561. [Google Scholar] [CrossRef] [PubMed]
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Chi, S. TAZNI: A Phase I/II Combination Trial of Tazemetostat with Nivolumab and Ipilimumab for Children with INI1-Negative or SMARCA4-Deficient Tumors. clinicaltrials.gov. Report No.: NCT05407441; 2023. Available online: https://clinicaltrials.gov/study/NCT05407441 (accessed on 1 January 2024).
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.J.; Cousin, S.; Spalato, M.; Guégan, J.P.; Bessede, A.; Pernot, S.; Italiano, A. Targeting EZH2 to overcome the resistance to immunotherapy in microsatellite stable colorectal cancer: Results from the CAIRE study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 3599. [Google Scholar] [CrossRef]
- Radko-Juettner, S.; Yue, H.; Myers, J.A.; Carter, R.D.; Robertson, A.N.; Mittal, P.; Zhu, Z.; Hansen, B.S.; Donovan, K.A.; Hunkeler, M.; et al. Targeting DCAF5 Suppresses SMARCB1-Mutant Cancer by Stabilizing SWI/SNF. Nature 2024, 628, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. Lond. Engl. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Leffondré, K.; Abrahamowicz, M.; Siemiatycki, J.; Rachet, B. Modeling Smoking History: A Comparison of Different Approaches. Am. J. Epidemiol. 2002, 156, 813–823. [Google Scholar] [CrossRef]
- Survminer R Package: Survival Data Analysis and Visualization-Easy Guides-Wiki-STHDA. Available online: http://www.sthda.com/english/wiki/survminer-r-package-survival-data-analysis-and-visualization (accessed on 20 August 2019).
- GitHub. Ggsurvplot(): Plotting Multiple Surv Objects on the Same Graph · Issue #195 · Kassambara/Survminer. Available online: https://github.com/kassambara/survminer/issues/195 (accessed on 11 May 2020).
- Plotting with Survival Package. Available online: https://cran.r-project.org/web/packages/ggfortify/vignettes/plot_surv.html (accessed on 19 August 2019).
- Presentation-Ready Data Summary and Analytic Result Tables • Gtsummary. Available online: http://www.danieldsjoberg.com/gtsummary/ (accessed on 23 March 2021).
| Patient 1 | Patient 2 |
|---|---|
| 36-year-old male. Never smoker. BMI 31.1. No significant co-morbidities. No history of cancer. | 39-year-old male. Never smoker. BMI 27.4. No significant co-morbidities. No history of cancer. |
| Presented with dull left sided chest pain in September 2022. | Incidental finding of lung mass in right upper lobe after investigation for abdominal pain. |
| Stage 2b NSCLC. | Stage 1B NSCLC. |
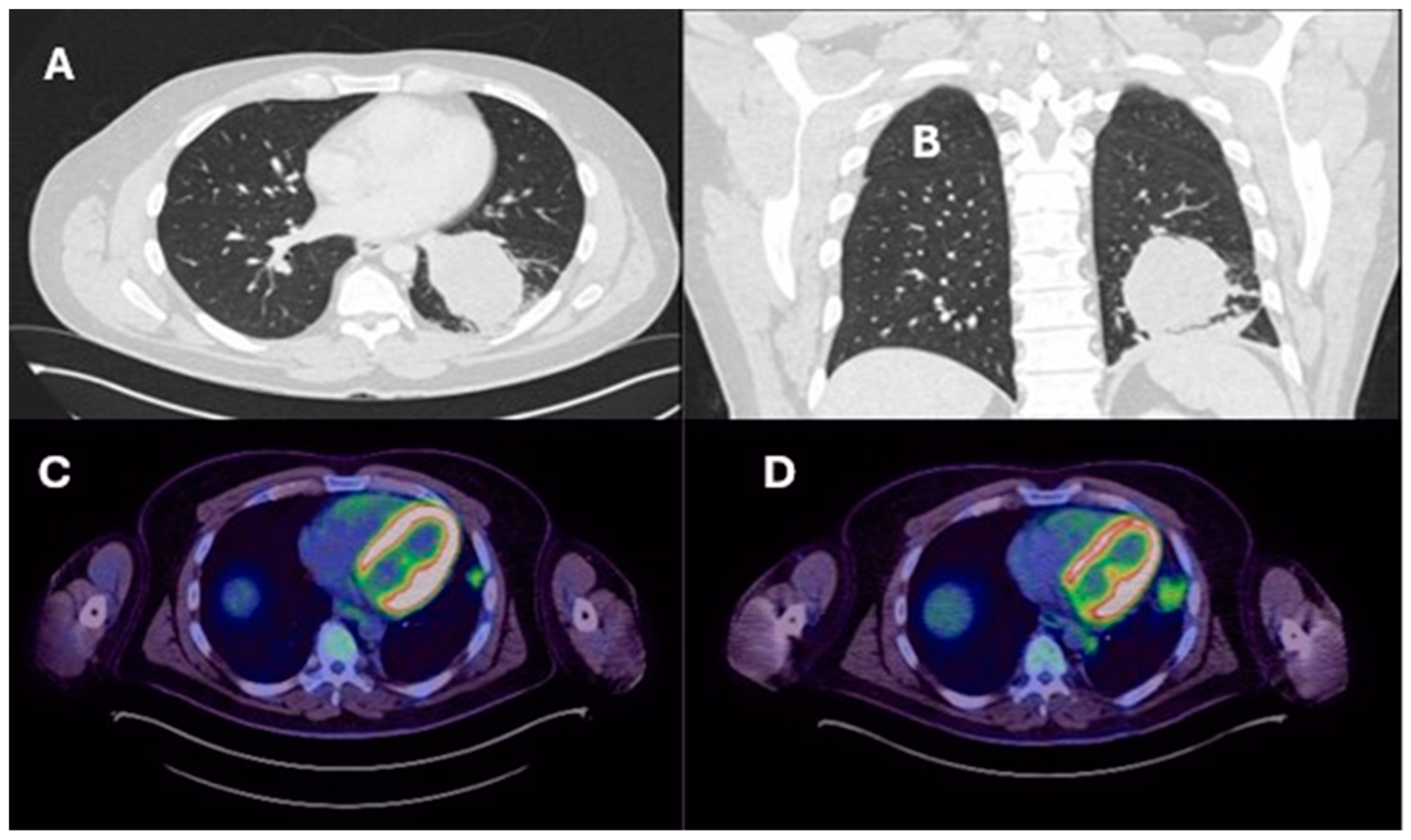
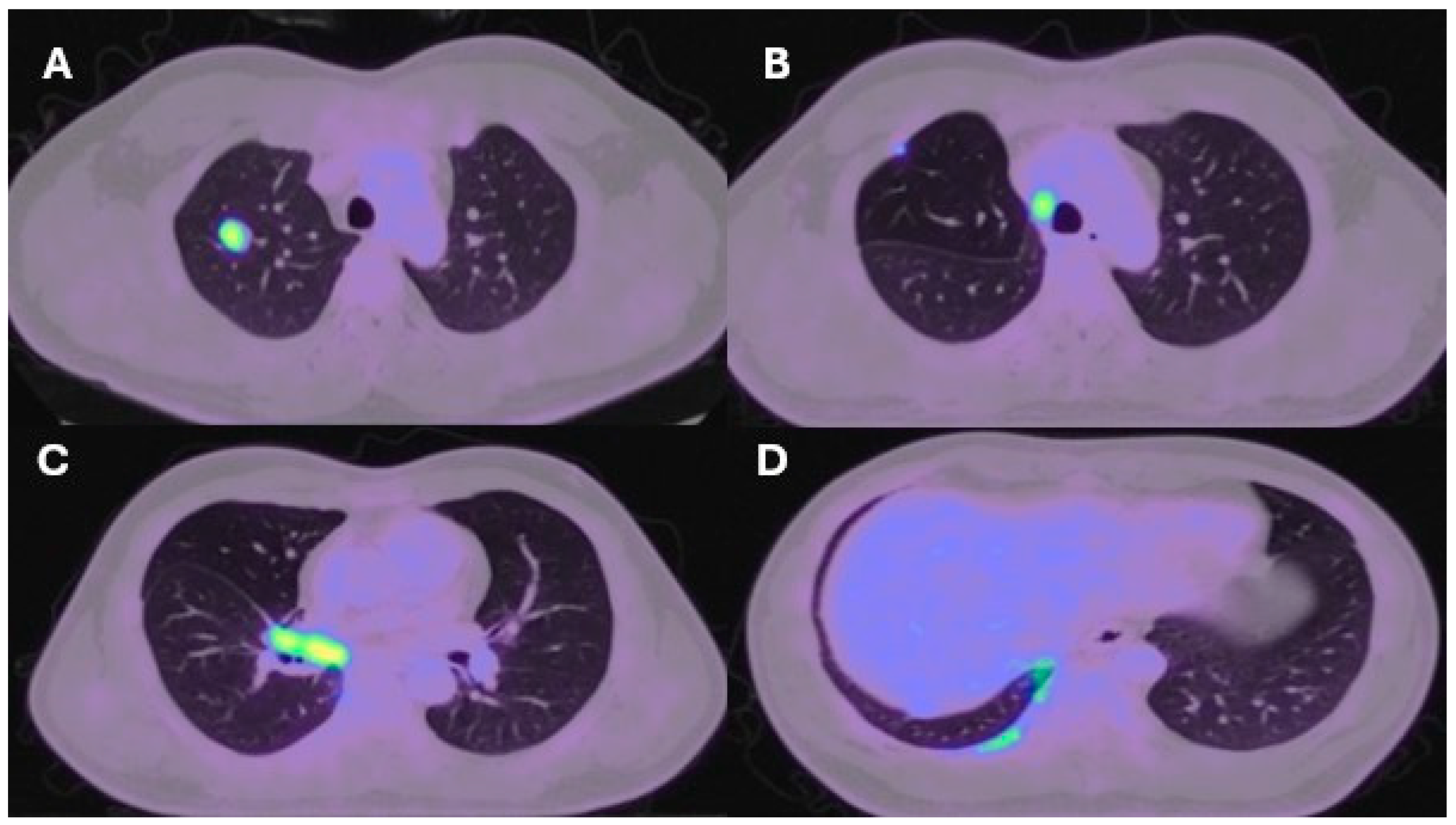
| Lung resection November 2022—Left lower lobectomy + lymph node dissection: pT3pN0 R0 resection. SMARCB1-deficient, PDL1-negative, TTF-1 negative, CD56-positive, cytokeratin positive. Pre-operative CT axial and coronal slices are shown in Figure 2A and Figure 2B, respectively. | Lung resection January 2022—right upper lobectomy + lymph node dissection: pT1cpN0 R0 resection. SMARCB1-deficient, PDL1 negative, TTF-1 negative, CD56-positive, cytokeratin-positive. Pre-operative axial PET slice shown in Figure 3A. |
| Histopathological Features: 58 mm size tumor, lymphovascular and perineural invasion seen, no spread through airways (STAS), no breach of visceral pleura. | Histopathological Features: 21 mm size tumor, no lymphovascular and perineural invasion seen, no spread through airways (STAS), no breach of visceral pleura. |
| Recurrence December 2022—started 4 cycles of Gemcitabine/Cisplatin. Post-operative recurrence demonstrated in PET slices (Figure 2C,D) | Recurrence June 2022—progressive disease noted on CTPA (performed for SOB, pyrexia) at the right hilum, with soft tissue thickening at resection margins. Commenced on Pembrolizumab/Paclitaxel/Cisplatin with systemic intent. Recurrence shown in station 4R (Figure 3B), at the right hilum (Figure 3C) and in the right posterior bony skeleton in ribs 6–9 (Figure 3D). |
| Progressive disease in pleura and mediastinum April 2023—compassionate release form to administer Tazemetostat. | Chemotherapy stopped due to severe allergic reaction to drugs—November 2022. Resolution of soft tissue thickening on PET but with new pleural metastases and bulky paraoesophageal lymphadenopathy. Further maintenance Pembrolizumab only. |
| Increased burden of disease in left hemithorax August 2023—compassionate release of combination checkpoint blockade: Nivolumab/Ipilimumab, Tazemetostat stopped. | Slight progression on CT January 2023. Patient opted for surgical resection of metastatic sites abroad. |
| November 2023—three cycles of checkpoint blockade but developed severe rib pain due to a medial 4th destroying lesion (pure progressive disease), given 20 Gy in 5# of palliative radiotherapy. | Further lung resection March 2023—multiple wedge resections of RUL and RML. Radical lymphadenectomy and resection of tumor deposits in the chest wall, pericardium, and mediastinum and sheath of the SVC. |
| December 2023—all treatment stopped in light of progression and episode of viral pneumonia. Referred to hospice for palliation. | Patient currently under strict surveillance and has relocated abroad. |
| February 2024—Under palliative care team with good symptom control. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.J.; Hemead, H.; Jesani, H.; Bille, A.; Taniere, P.; Middleton, G. Squamous Cell Carcinoma in Never Smokers: An Insight into SMARCB1 Loss. Int. J. Mol. Sci. 2024, 25, 8165. https://doi.org/10.3390/ijms25158165
Patel AJ, Hemead H, Jesani H, Bille A, Taniere P, Middleton G. Squamous Cell Carcinoma in Never Smokers: An Insight into SMARCB1 Loss. International Journal of Molecular Sciences. 2024; 25(15):8165. https://doi.org/10.3390/ijms25158165
Chicago/Turabian StylePatel, Akshay J., Hanan Hemead, Hannah Jesani, Andrea Bille, Philippe Taniere, and Gary Middleton. 2024. "Squamous Cell Carcinoma in Never Smokers: An Insight into SMARCB1 Loss" International Journal of Molecular Sciences 25, no. 15: 8165. https://doi.org/10.3390/ijms25158165
APA StylePatel, A. J., Hemead, H., Jesani, H., Bille, A., Taniere, P., & Middleton, G. (2024). Squamous Cell Carcinoma in Never Smokers: An Insight into SMARCB1 Loss. International Journal of Molecular Sciences, 25(15), 8165. https://doi.org/10.3390/ijms25158165





