The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs
Abstract
1. Introduction
2. Oestrogen Characteristics (Type of Oestrogens, Synthesis, and Production)
3. Oestrogen Receptors
4. The Role of Oestrogen in the Physiology of Women and Men
5. Oestrogens and Bones
6. Oestrogens and the Cardiovascular System
7. Oestrogens and Brain
8. Oestrogen and Immunity
9. Exogenous Sources of Endocrinologically Active Oestrogen Derivates Compounds
9.1. Bisphenols
9.1.1. The Influence of Bisphenols on Cancer Development
9.1.2. The Influence of Bisphenols on Endocrine Disorders
9.1.3. The Influence of Bisphenols on the Male Reproduction System
9.2. Phthalates
9.3. Other EDCs
| Gene | Regulation: Up/Down ↑/↓ | Cancerogenesis Progression: |
|---|---|---|
| ADORA1 | ↑ | excessive proliferation [140] |
| CELSR2 | ↑ | migration, invasion, adhesion, metastasis [141] |
| DDIT4 | ↑ | suppression of mTOR activity, excessive proliferation, apoptosis disorders [134,142] |
| FOSL2 AP-1 | ↑ | participates in activating the metastasis cascade [143] |
| HSPA13 | ↑ | TANK stabilisation, proliferation, migration [144] |
| IER3 | ↑ | apoptosis disruption [145] |
| IGF1R | ↑ | inhibition of apoptosis, proliferation, enhanced ER activation [146,147] |
| JUN AP-1 | ↑ | invasion, migration, metastasis [143] |
| PGR | ↑ | abnormal and excessive cell growth [148] |
| RUNX2 | ↑ | bone metastasis, impaired function and development of osteoblasts [149] |
| SLC7A2 | ↑ | inflammation and oxidative stress caused by increased NO synthesis, but inhibited invasion and migration [150,151] |
| SLC7A5 | ↑ | provides the tumour with access to amino acid development and proliferation, activates the mTORC1 pathway [152] |
| SLC7A11 | ↑ | protection of cancer cells from oxygen radicals, delivery of glucose and glutamine to cancer cells [153] |
| STC2 | ↑ | increased proliferation and viability regulation of the MAPK pathway [154] |
| BCAS3 | ↓ | overexpressed gene in breast cancer (inhibits apoptosis, enhances proliferation) gets silenced [155] |
| PHF19 | ↓ | silencing of proliferation and migration, increased apoptosis [156,157] |
| PRKCD (suppressor gene) | ↓ | accelerated development of cancer cells [158] |
9.4. Phytoestrogens
9.4.1. 8-Prenylonaringenin
9.4.2. Genistein
9.4.3. DHEA
10. Oestrogen-Related Diseases
10.1. Cancerogenesis
10.1.1. Breast Cancer
10.1.2. Endometrial Cancer
10.2. Venous Thromboembolism
10.3. Autoimmune Diseases
11. Exogenous Oestrogen Therapies and the Risk of Breast Cancer
12. Oestrogen and Cortisol
13. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym/Abbreviation | Explanation |
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| 2-OHE2 | 2-hydroxy oestradiol |
| 4-OHE2 | 4-hydroxy oestradiol |
| 8-PN | 8-Prenylnaringenin |
| ACTH | adrenocorticotropic hormone |
| AhR | aryl hydrocarbon receptor |
| AR | androgen receptors |
| BCP | benzyl cyclohexyl phthalate |
| BOP | butyl octyl phthalate |
| BPA | bisphenol A |
| BPS | bisphenol S |
| CBG | cortisol-binding globulin |
| DDT | dichlorodiphenyltrichloroethane |
| DHEP | di(2-ethylhexyl) phthalate |
| E1 | oestrone |
| E2 | oestradiol (17β-oestradiol) |
| E3 | oestriol |
| E4 | oestetrol |
| ECS | endocannabinoid system |
| EE | ethinyl oestradiol |
| EDCs | chemicals disrupt endocrine functions |
| Erα/ESR1 | oestrogen receptor 1/oestrogen receptor α |
| Erβ/ESR2 | oestrogen receptor 2/oestrogen receptor β |
| EREs | oestrogen response elements |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone |
| GM-CSF | colony-stimulating factor |
| GR | glucocorticoid receptors |
| GPER | G protein-coupled oestrogen receptor 1 |
| HRT | hormone replacement therapy |
| HSP | heat shock proteins |
| IFNγ | interferonγ |
| IL-1, IL-6 | interleukin 1 and 6 |
| IL-8 | interleukin 8 |
| IGF-1 | insulin-like growth factor 1 |
| MAPK | mitogen-activated protein kinase |
| MMPs/TIMPs | matrix metalloproteinases and enhance the action of MMP inhibitors |
| MS | multiple sclerosis |
| NO | nitric oxide |
| NP | 4-para-nonylphenol |
| OPEs | organic phosphoric acid esters |
| PCBs | polychlorinated biphenyls |
| PCOS | polycystic ovary syndrome |
| PET | positron emission tomography |
| PTH | parathormone |
| RA | rheumatoid arthritis |
| RFLP | restriction fragment length polymorphism |
| SAD | seasonal affective disorder |
| SLE | systemic lupus erythematosus |
| SNPs | single nucleotide polymorphisms |
| TH | thyroid hormone (triiodothyronine, T3) |
| TGFβ | transforming growth factor β |
| T3 | triiodothyronine |
| TNFα | tumour necrosis factor α |
| TPHP | triphenyl phosphate |
| TOCP | tri-o-cresyl phosphate |
| VEGF | vascular endothelial growth factor |
| VTE | venous thromboembolism (includes DVT and PE) |
| VMN | hypothalamus’s ventromedial nucleus |
References
- Sawicka, E.; Woźniak, A.; Drąg-Zalesińska, M.; Piwowar, A. Effect of estrogens and their metabolites genotoxicity on the pathogenesis and progression of estrogen-dependent breast cancer. Adv. Hyg. Exp. Med. 2019, 73, 909–919. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of Estrogens Present in Environment on Health and Welfare of Animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef]
- Delgado, B.J.; Lopez-Ojeda, W. Estrogen. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Wang, X.; Ha, D.; Yoshitake, R.; Chan, Y.S.; Sadava, D.; Chen, S. Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts. Int. J. Mol. Sci. 2021, 22, 8798. [Google Scholar] [CrossRef]
- Forma, E.; Szymczak, A.; Krześlak, A. Wybrane ksenoestrogeny i ich wpływ na zdrowie człowieka. Folia Medica Lodz. 2013, 40, 79–97. [Google Scholar]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Krishnan, V.; Heath, H.; Bryant, H.U. Mechanism of action of estrogens and selective estrogen receptor modulators. Vitam Horm. 2000, 60, 123–147. [Google Scholar]
- Hess, R.A.; Bunick, D.; Lee, K.H.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509–512. [Google Scholar] [CrossRef]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Davis, S.; Jones, M. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer 1999, 6, 131–137. [Google Scholar] [CrossRef]
- Diel, P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol. Lett. 2002, 127, 217–224. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- MacDonald, P.C.; Madden, J.D.; Brenner, P.F.; Wilson, J.D.; Siiteri, P.K. Origin of estrogen in normal men and in women with testicular feminization. J. Clin. Endocrinol. Metab. 1979, 49, 905–916. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J. Are all estrogens the same? Maturitas 2004, 47, 269–275. [Google Scholar] [CrossRef]
- Cui, J.Y. Regulation of estrogen synthesis and metabolism. J. Cell Biochem. 2004, 93, 233–247. [Google Scholar]
- Grumbach, M.M.; Auchus, R.J. Estrogen: Consequences and implications of human mutations in synthesis and action. J. Clin. Endocrinol. Metab. 1999, 84, 4677–4694. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Bianco, J.J.; Ellem, S.J.; McPherson, S.J. Oestrogens and prostate cancer. Endocr. Relat. Cancer 2003, 10, 187–191. [Google Scholar] [CrossRef][Green Version]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase—A brief overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the regulation of estrogen biosynthesis--some new perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef]
- Azcoitia, I.; Mendez, P.; Garcia-Segura, L.M. Aromatase in the Human Brain. Androg. Clin. Res. Ther. 2021, 2, 189–202. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kim, T.; Kanno, H.; Noguchi, T.; Yamamoto, T.; Okada, H. Growth suppression of MCF-7 human breast cancer cells by aromatase inhibitors: A new system for aromatase inhibitor screening. J. Steroid. Biochem. Mol. Biol. 1993, 44, 667–670. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45 (Suppl. S3), S116–S124. [Google Scholar] [CrossRef]
- Flouriot, G.; Brand, H.; Denger, S.; Metivier, R.; Kos, M.; Reid, G.; Sonntag-Buck, V.; Gannon, F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000, 19, 4688–4700. [Google Scholar] [CrossRef]
- Paige, L.A.; Christensen, D.J.; Grøn, H.; Norris, J.D.; Gottlin, E.B.; Padilla, K.M.; Chang, C.Y.; Ballas, L.M.; Hamilton, P.T.; McDonnell, D.P.; et al. Estrogen receptor (ER) modulators each induce distinct conformational changes in ER alpha and ER beta. Proc. Natl. Acad. Sci. USA 1999, 96, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Steroid hormones are multifunctional messengers to the brain. Trends Endocrinol. Metab. 1991, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- McKenna, N.J.; Lanz, R.B.; O’Malley, B.W. Nuclear receptor coregulators: Cellular and molecular biology. Endocr. Rev. 1999, 20, 321–344. [Google Scholar]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Connor, C.E.; Wijayaratne, A.; Chang, C.Y.; Norris, J.D. Definition of the molecular and cellular mechanisms underlying the tissue-selective agonist/antagonist activities of selective estrogen receptor modulators. Recent Prog. Horm. Res. 2002, 57, 295–316. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Kim, H.T.; Hilsenbeck, S.; Cuba, V.; Tsimelzon, A.; Brown, P.H. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: Identification of estrogen-induced/activator protein-1-dependent genes. Mol. Endocrinol. 2005, 19, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Breborowicz, J.; Filas, V.; Moczko, J.; Godlewski, D.; Stawicka, M.; Kaleta, R. Expression of estrogen receptors alfa and beta (ERa and ERb) in hereditary breast cancer. J. Clin. Oncol. 2005, 23 (Suppl. S16), 550. [Google Scholar] [CrossRef]
- Jakacka, M.; Ito, M.; Weiss, J.; Chien, P.Y.; Gehm, B.D.; Jameson, J.L. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J. Biol. Chem. 2001, 276, 13615–13621. [Google Scholar] [CrossRef]
- Gustafsson, J.A. Estrogen receptor beta--a new dimension in estrogen mechanism of action. J. Endocrinol. 1999, 163, 379–383. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Roy, S.K. Expression of estrogen receptor α 36 (ESR36) in the hamster ovary throughout the estrous cycle: Effects of gonadotropins. PLoS ONE 2013, 8, e58291. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; van Pottelberg, I. Estradiol in elderly men. Aging Male 2002, 5, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Janulis, L.; Hess, R.A.; Bunick, D.; Nitta, H.; Janssen, S.; Asawa, Y.; Bahr, J.M. Mouse epididymal sperm contain active P450 aromatase which decreases as sperm traverse the epididymis. J. Androl. 1996, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Frenette, G.; Leclerc, P.; D’amours, O.; Sullivan, R. Estrogen sulfotransferase is highly expressed along the bovine epididymis and is secreted into the intraluminal environment. J. Androl. 2009, 30, 580–589. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.A. Environmental signaling: From environmental estrogens to endocrine-disrupting chemicals and beyond. Andrology 2016, 4, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Carpino, A.; Rago, V.; Pezzi, V.; Carani, C.; Andò, S. Detection of aromatase and estrogen receptors (ERalpha, ERbeta1, ERbeta2) in human Leydig cell tumor. Eur. J. Endocrinol. 2007, 157, 239–244. [Google Scholar] [CrossRef]
- Arden, N.K.; Spector, T.D.; Badurski, J.E. (Eds.) Osteoporoza: Aktualny Stan Wiedzy; Borgis: Warszawa, Poland, 2000. [Google Scholar]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Invest. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Patlak, M. Bone builders: The discoveries behind preventing and treating osteoporosis. FASEB J. 2001, 15, 1677E-E. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Cann, C.E.; Ettinger Gordan, G.S. Quantitative computed tomography of vertebral spongiosa: A sensitive method for detecting early bone loss after oophorectomy. Ann. Intern. Med. 1982, 97, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef]
- Eastell, R.; Yergey, A.L.; Vieira, N.E.; Cedel, S.L.; Kumar, R.; Riggs, B.L. Interrelationship among vitamin D metabolism, true calcium absorption, parathyroid function, and age in women: Evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin D action. J. Bone Miner. Res. 1991, 6, 125–132. [Google Scholar] [CrossRef]
- Nicolas, V.; Prewett, A.; Bettica, P.; Mohan, S.; Finkelman, R.D.; Baylink, D.J.; Farley, J.R. Age-related decreases in insulin-like growth factor-I and transforming growth factor-beta in femoral cortical bone from both men and women: Implications for bone loss with aging. J. Clin. Endocrinol. Metab. 1994, 78, 1011–1016. [Google Scholar]
- Khosla, S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. USA 2013, 68, 1226–1235. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Prestwood, K.M.; Kenny, A.M.; Kleppinger, A.; Kulldorff, M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: A randomized controlled trial. JAMA 2003, 290, 1042–1048. [Google Scholar] [CrossRef]
- McKane, W.R.; Khosla, S.; Burritt, M.F.; Kao, P.C.; Wilson, D.M.; Ory, S.J.; Riggs, B.L. Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause--a clinical research center study. J. Clin. Endocrinol. Metab. 1995, 80, 3458–3464. [Google Scholar] [PubMed]
- Khosla, S.; Atkinson, E.J.; Melton, L.J., 3rd; Riggs, B.L. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: A population-based study. J. Clin. Endocrinol. Metab. 1997, 82, 1522–1527. [Google Scholar] [PubMed]
- Prince, R.L. Counterpoint: Estrogen effects on calcitropic hormones and calcium homeostasis. Endocr. Rev. 1994, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Barger-Lux, M.J.; Davies, K.M.; Heaney, R.P. Calcium supplementation does not augment bone gain in young women consuming diets moderately low in calcium. J. Nutr. 2005, 135, 2362–2366. [Google Scholar] [CrossRef][Green Version]
- Gallagher, J.C.; Riggs, B.L.; Eisman, J.; Hamstra, A.; Arnaud, S.B.; DeLuca, H.F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: Effect of age and dietary calcium. J. Clin. Investig. 1979, 64, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J. 3rd. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef]
- Bikle, D.D.; Halloran, B.P.; Harris, S.T.; Portale, A.A. Progestin antagonism of estrogen stimulated 1,25-dihydroxyvitamin D levels. J. Clin. Endocrinol. Metab. 1992, 75, 519–523. [Google Scholar] [PubMed]
- Gennari, C.; Agnusdei, D.; Nardi, P.; Civitelli, R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J. Clin. Endocrinol. Metab. 1990, 71, 1288–1293. [Google Scholar] [CrossRef]
- Nordin, B.E.; Need, A.G.; Morris, H.A.; O’Loughlin, P.D.; Horowitz, M. Effect of age on calcium absorption in postmenopausal women. Am. J. Clin. Nutr. 2004, 80, 998–1002. [Google Scholar] [CrossRef]
- Cosman, F.; Nieves, J.; Horton, J.; Shen, V.; Lindsay, R. Effects of estrogen on response to edetic acid infusion in postmenopausal osteoporotic women. J. Clin. Endocrinol. Metab. 1994, 78, 939–943. [Google Scholar]
- Järvinen, T.L.; Sievänen, H.; Khan, K.M.; Heinonen, A.; Kannus, P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 2008, 336, 124–126. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Wu, Q.; Oltmann, S.; Konaniah, E.S.; Umetani, M.; Korach, K.S.; Thomas, G.D.; Mineo, C.; Yuhanna, I.S.; Kim, S.H.; et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J. Clin. Investig. 2010, 120, 2319–2330. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Darblade, B.; Pendaries, C.; Krust, A.; Dupont, S.; Fouque, M.J.; Rami, J.; Chambon, P.; Bayard, F.; Arnal, J.F. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ. Res. 2002, 90, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, H.; Barman, S.A.; Liu, A.T.; Sellers, M.; Stallone, J.N.; Prossnitz, E.R.; White, R.E.; Han, G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E882–E888. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, S.H.; Carver, K.A.; Prossnitz, E.R.; Chappell, M.C. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2. Lewis female rat. J. Cardiovasc. Pharmacol. 2011, 57, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Bopassa, J.C.; Eghbali, M.; Toro, L.; Stefani, E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H16–H23. [Google Scholar] [CrossRef] [PubMed]
- Mean, W.D.I. Nongenomic, Estrogen Receptor–Mediated Activation of Endothelial Nitric Oxide Synthase. Circ. Res. 2000, 87, 956–960. [Google Scholar]
- Ortmann, J.; Veit, M.; Zingg, S.; Di Santo, S.; Traupe, T.; Yang, Z.; Völzmann, J.; Dubey, R.K.; Christen, S.; Baumgartner, I. Estrogen receptor-α but not -β or GPER inhibits high glucose-induced human VSMC proliferation: Potential role of ROS and ERK. J. Clin. Endocrinol. Metab. 2011, 96, 220–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parashar, S.; Katz, R.; Smith, N.L.; Arnold, A.M.; Vaccarino, V.; Wenger, N.K.; Gottdiener, J.S. Race, gender, and mortality in adults > or 65 years of age with incident heart failure (from the Cardiovascular Health Study). Am. J. Cardiol. 2009, 103, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.L.; Lin, L.; Wang, Y.; Knowlton, A.A. Effect of ovariectomy on cardiac gene expression: Inflammation and changes in SOCS gene expression. Physiol. Genom. 2008, 32, 254–263. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc. Res. 2010, 85, 719–728. [Google Scholar] [CrossRef]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Stoffel-Wagner, B.; Watzka, M.; Schramm, J.; Bidlingmaier, F.; Klingmüller, D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J. Steroid. Biochem. Mol. Biol. 1999, 70, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hosoya, T.; Onoe, K.; Takashima, T.; Tanaka, M.; Ishii, A.; Nakatomi, Y.; Tazawa, S.; Takahashi, K.; Doi, H.; et al. Association between aromatase in human brains and personality traits. Sci. Rep. 2018, 8, 16841. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 1990, 15, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogenic effects on memory in women. Ann. N. Y. Acad. Sci. 1994, 743, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Klaiber, E.L.; Broverman, D.M.; Vogel, W.; Peterson, L.G.; Snyder, M.B. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology 1997, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Small, G.W.; Hamilton, S.H.; Bystritsky, A.; Nemeroff, C.B.; Meyers, B.S. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am. J. Geriatr. Psychiatry 1997, 5, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Halbreich, U.; Kahn, L.S. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs 2001, 15, 797–817. [Google Scholar] [CrossRef]
- Smith, S. The Effects of Oestrogen and Progesterone on GABA and Glutamate Responses at Extrahypothalamic Sites; Thieme Medical: New York, NY, USA, 1991; pp. 87–94. [Google Scholar]
- Spence, R.D.; Voskuhl, R.R. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocr. 2012, 33, 105–115. [Google Scholar] [CrossRef]
- Smith, C.C.; Vedder, L.C.; McMahon, L.L. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S130–S142. [Google Scholar] [CrossRef]
- Fugger, H.N.; Foster, T.C.; Gustafsson, J.; Rissman, E.F. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000, 883, 258–264. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M.; et al. German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Armstrong, S.M.; Matt, K.S.; Mattox, J.H. The application of positron emission tomography to the study of the normal menstrual cycle. Hum. Reprod. 1996, 11, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Haan, M.; Byers, A.; Tangen, C.; Kuller, L. Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology 2000, 54, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Craft, S.; Baker, L.D.; Raskind, M.A.; Birnbaum, R.S.; Lofgreen, C.P.; Veith, R.C.; Plymate, S.R. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: Results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999, 24, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Genovese, T.; Mazzon, E.; Esposito, E.; Di Paola, R.; Muià, C.; Crisafulli, C.; Peli, A.; Bramanti, P.; Chaudry, I.H. Effect of 17beta-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock 2008, 29, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Ansar Ahmed, S.; Penhale, W.J.; Talal, N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am. J. Pathol. 1985, 121, 531–551. [Google Scholar]
- Khalaj, A.J.; Yoon, J.; Nakai, J.; Winchester, Z.; Moore, S.M.; Yoo, T.; Martinez-Torres, L.; Kumar, S.; Itoh, N.; Tiwari-Woodruff, S.K. Estrogen receptor (ER) β expression in oligodendrocytes is required for attenuato attenuateisease by an ERβ ligand. Proc. Natl. Acad. Sci. USA 2013, 110, 19125–19130. [Google Scholar] [CrossRef]
- Perl, A.; Nagy, G.; Koncz, A.; Gergely, P.; Fernandez, D.; Doherty, E.; Telarico, T.; Bonilla, E.; Phillips, P.E. Molecular mimicry and immunomodulation by the HRES-1 endogenous retrovirus in SLE. Autoimmunity 2008, 41, 287–297. [Google Scholar] [CrossRef]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, A.; Fadini, G.P.; Tedesco, S.; Cappellari, R.; Vegeto, E.; Maggi, A.; Avogaro, A.; Bolego, C.; Cignarella, A. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J. Clin. Endocrinol. Metab. 2015, 100, E50–E58. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet, Y.M.; Marriott, I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology 2009, 150, 3877–3884. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.; Melenotte, C.; Lakbar, I.; Mezouar, S.; Devaux, C.; Raoult, D.; Bendiane, M.K.; Leone, M.; Mège, J.L. Sexual Dimorphism and Gender in Infectious Diseases. Front. Immunol. 2021, 12, 698121. [Google Scholar] [CrossRef]
- Horng, H.C.; Chang, W.H.; Yeh, C.C.; Huang, B.S.; Chang, C.P.; Chen, Y.J.; Tsui, K.H.; Wang, P.H. Estrogen Effects on Wound Healing. Int. J. Mol. Sci. 2017, 18, 2325. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M.A. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Nielsen, S.; Nielsen, B.S.; Pedersen, S.H.; Vyberg, M. Estrogen Receptor-α Quantification in Breast Cancer: Concordance Between Immunohistochemical Assays and mRNA-In Situ Hybridization for ESR1 Gene. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 347–353. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Bergman, Å.; Heindel, J.J.; Jobling, S.; Kidd, K.; Zoeller, T.R. World Health Organization. In State of the Science of Endocrine Disrupting Chemicals 2012; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ashrap, P.; Sánchez, B.N.; Téllez-Rojo, M.M.; Basu, N.; Tamayo-Ortiz, M.; Peterson, K.E.; Meeker, J.D.; Watkins, D.J. In utero and peripubertal metals exposure in relation to reproductive hormones and sexual maturation and progression among girls in Mexico City. Environ. Res. 2019, 177, 108630. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, M.C.; Mares, C.; Petca, R.C.; Sandru, F.; Popescu, R.I.; Mehedintu, C.; Petca, A. Carcinogenic effects of bisphenol A in breast and ovarian cancers. Oncol. Lett. 2020, 20, 282. [Google Scholar] [CrossRef]
- Fauconnier, M.B.; Albert, C.; Tondreau, A.; Maumy, L.; Rouzier, R.; Bonneau, C. Bisphénol A et cancer du sein: État des lieux des connaissances et méta-analyse. Bull. Cancer 2023, 110, 151–159. [Google Scholar] [CrossRef]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic. Acids. Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Chambo, D.; Kemp, C.; Costa, A.M.; Souza, N.C.; Guerreiro da Silva, I.D. Polymorphism in CYP17, GSTM1 and the progesterone receptor genes and its relationship with mammographic density. Braz. J. Med. Biol. Res. 2009, 42, 323–329. [Google Scholar] [CrossRef]
- Li, J.; Eriksson, L.; Humphreys, K.; Czene, K.; Liu, J.; Tamimi, R.M.; Lindström, S.; Hunter, D.J.; Vachon, C.M.; Couch, F.J.; et al. Genetic variation in the estrogen metabolic pathway and mammographic density as an intermediate phenotype of breast cancer. Breast Cancer Res. 2010, 12, R19. [Google Scholar] [CrossRef] [PubMed]
- Casamassimi, A.; Federico, A.; Rienzo, M.; Esposito, S.; Ciccodicola, A. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017, 18, 1652. [Google Scholar] [CrossRef]
- Cunliffe, H.E.; Ringner, M.; Bilke, S.; Walker, R.L.; Cheung, J.M.; Chen, Y.; Meltzer, P.S. The gene expression response {alpha}-binding program on human gene promoters. Proc. Natl. Acad. Sci. USA 2003, 104, 4852–4857. [Google Scholar]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Identification of estrogen-responsive genes based on the DNA binding properties of estrogen receptors using high-throughput sequencing technology. Acta. Pharmacol. Sin. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Lazúrová, Z.; Lazúrová, I. Environmentálny estrogén bisfenol A a jeho účinky na organizmus človeka. Vnitr. Lek. 2013, 59, 466–471. [Google Scholar] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Söngen, M. Molecular Mechanisms of Estrogenic Xenobiotica. Doctoral Dissertation, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2021. [Google Scholar]
- Böckers, M.; Paul, N.W.; Efferth, T. Bisphenolic compounds alter gene expression in MCF-7 cells through interaction with estrogen receptor α. Toxicol. Appl. Pharmacol. 2020, 399, 115030. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Tan, M.; Zhou, W.; Chen, C.; Xi, Y.; Gao, P.; Ma, Q.; Liang, Y.; Chen, M.; Tian, L.; et al. Bisphenol A promotes breast cancer cell proliferation by driving miR-381-3p-PTTG1-dependent cell cycle progression. Chemosphere 2021, 268, 129221. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Steinmetz, G.; Montillet, G.; Perrard, M.H.; Loundou, A.; Durand, P.; Guichaoua, M.R.; Prat, O. Exposure to low-dose bisphenol A impairs meiosis in the rat seminiferous tubule culture model: A physiotoxicogenomic approach. PLoS ONE 2014, 9, e106245. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Goodwin, B.; Shockley, K.; Xia, M.; Huang, R.; Norris, J.; Merrick, B.A.; Jetten, A.M.; Austin, C.P.; Tice, R.R. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem. Biol. Interact. 2013, 203, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, Y.; Lv, L.; Li, X.; Qin, Z. Effects of low-dose bisphenol AF on mammal testis development via complex mechanisms: Alterations are detectable in both infancy and adulthood. Arch. Toxicol. 2022, 96, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Hu, C.; Yu, X.J. High-content analysis of testicular toxicity of BPA and its selected analogs in mouse spermatogonial, Sertoli cells, and Leydig cells revealed BPAF induced unique multinucleation phenotype associated with the increased DNA synthesis. Toxicol. Vitro 2023, 89, 105589. [Google Scholar] [CrossRef]
- Yu, Y.; Xin, X.; Ma, F.; Li, X.; Wang, Y.; Zhu, Q.; Chen, H.; Li, H.; Ge, R.S. Bisphenol AF blocks Leydig cell regeneration from stem cells in male rats. Environ. Pollut. 2022, 298, 118825. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef]
- Xue, S.; Li, X.; Zhou, S.; Zhang, J.; Sun, K.; Peng, X.; Chen, N.; Dong, M.; Jiang, T.; Chen, Y.; et al. Effects and mechanisms of endocrine disruptor bisphenol AF on male reproductive health: A mini review. Ecotoxicol. Environ. Saf. 2024, 276, 116300. [Google Scholar] [CrossRef] [PubMed]
- Mentor, A.; Wänn, M.; Brunström, B.; Jönsson, M.; Mattsson, A. Bisphenol AF and Bisphenol F Induce Similar Feminizing Effects in Chicken Embryo Testis as Bisphenol, A. Toxicol. Sci. 2020, 178, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Andersson, A.M.; Skakkebæk, N.E. Bisphenol A Diglycidyl Ether (BADGE) and Bisphenol Analogs, but Not Bisphenol A (BPA), Activate the CatSper Ca2+ Channel in Human Sperm. Front. Endocrinol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Kelley, K.E.; Hernández-Díaz, S.; Chaplin, E.L.; Hauser, R.; Mitchell, A.A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2012, 120, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, S.; Mitchell, A.A.; Kelley, K.E.; Calafat, A.M.; Hauser, R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ. Health Perspect. 2009, 117, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.H.; Waxman, D.J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 74, 297–308. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Tong, J.; Lv, H.; Zhang, C.; Chen, Z.J. Dysfunction of DNA damage-inducible transcript 4 in the decidua is relevant to the pathogenesis of preeclampsia. Biol. Reprod. 2018, 98, 821–833. [Google Scholar] [CrossRef]
- Böckers, M.; Paul, N.W.; Efferth, T. Butyl octyl phthalate interacts with estrogen receptor α in MCF-7 breast cancer cells to promote cancer development. World Acad. Sci. J. 2021, 3, 1. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tsai, C.F.; Chuang, H.L.; Chang, Y.C.; Chen, H.S.; Lee, J.N.; Tsai, E.M. Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget 2016, 7, 29563–29576. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhang, C.; Yao, Y.; Wang, L.; Sun, H. A review of organophosphate esters in soil: Implications for the potential source, transfer, and transformation mechanism. Environ. Res. 2022, 204, 112122. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.L. A review on organophosphate esters: Physiochemical properties, applications, and toxicities as well as occurrence and human exposure in dust environment. J. Environ. Manag. 2023, 325 Pt B, 116601. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Mu, Y.; Qu, G.; Zhang, A.; Fu, J.; Jiang, G. Structure-Oriented Research on the Antiestrogenic Effect of Organophosphate Esters and the Potential Mechanism. Environ. Sci. Technol. 2020, 54, 14525–14534. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yin, P.; Reierstad, S.; O’Halloran, M.; Coon, V.J.; Pearson, E.K.; Mutlu, G.M.; Bulun, S.E. Adenosine A1 receptor, a target and regulator of estrogen receptoralpha action, mediates the proliferative effects of estradiol in breast cancer. Oncogene 2010, 29, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Jiang, Y.; Wang, L.; Wang, L.; Jiang, J.; Zhang, J. Mutations in KIAA1109, CACNA1C, BSN, AKAP13, CELSR2, and HELZ2 Are Associated with the Prognosis in Endometrial Cancer. Front. Genet. 2019, 10, 909. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Milde-Langosch, K.; Röder, H.; Andritzky, B.; Aslan, B.; Hemminger, G.; Brinkmann, A.; Bamberger, C.M.T.; Bamberger, A.M. The role of the AP-1 transcription factors c-Fos, FosB, Fra-1 and Fra-2 in the invasion process of mammary carcinomas. Breast Cancer Res. Treat. 2004, 86, 139–152. [Google Scholar] [CrossRef]
- Cen, X.; Lu, Y.; Lu, J.; Luo, C.; Zhan, P.; Cheng, Y.; Yang, F.; Xie, C.; Yin, Z.; Wang, F. Heat shock protein HSPA13 promotes hepatocellular carcinoma progression by stabilizing TANK. Cell Death Discov. 2023, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hahn, J.K.; Neupane, B.; Aidery, P.; Labeit, S.; Gawaz, M.; Gramlich, M. Dysregulated IER3 Expression is Associated with Enhanced Apoptosis in Titin-Based Dilated Cardiomyopathy. Int. J. Mol. Sci. 2017, 18, 723. [Google Scholar] [CrossRef]
- Fagan, D.H.; Yee, D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J. Mammary Gland Biol. Neoplasia 2008, 13, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.V.; Jackson, J.G.; Gooch, J.L.; Hilsenbeck, S.G.; Coronado-Heinsohn, E.; Osborne, C.K.; Yee, D. Enhancement of insulin-like growth factor signaling in human breast cancer: Estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol. Endocrinol. 1999, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, T.L.; Chang, W.Z.; Liu, Y.; Centrella, M. Runx2 integrates estrogen activity in osteoblasts. J. Biol. Chem. 2003, 278, 43121–43129. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflug. Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Bi, F.; Liu, Z.; Yang, Q. SLC7A2 serves as a potential biomarker and therapeutic target for ovarian cancer. Aging 2020, 12, 13281–13296. [Google Scholar] [CrossRef]
- Törnroos, R.; Tina, E.; Göthlin Eremo, A. SLC7A5 is linked to increased expression of genes related to proliferation and hypoxia in estrogen-receptor-positive breast cancer. Oncol. Rep. 2022, 47, 17. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Cheng, H.; Yang, G.; Tan, W. Stanniocalcin 2 promotes cell proliferation and cisplatin resistance in cervical cancer. Biochem. Biophys. Res. Commun. 2015, 466, 362–368. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, R.; Liu, W.; Yang, T.; Li, G.; Huang, W.; Teng, X.; Yang, Y.; Yu, H.; Yang, Y.; et al. BCAS3 exhibits oncogenic properties by promoting CRL4A-mediated ubiquitination of p53 in breast cancer. Cell Prolif. 2021, 54, e13088. [Google Scholar] [CrossRef]
- Tao, F.; Tian, X.; Ruan, S.; Shen, M.; Zhang, Z. miR-211 sponges lncRNA MALAT1 to suppress tumor growth and progression through inhibiting PHF19 in ovarian carcinoma. FASEB J. 2018, 32, 6330–6343. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Hou, J.; Feng, L.; Lv, A.; Ke, X.; Liang, H.; Wang, F.; Zhang, K.; Chen, K.; Cui, H. PHF19 promotes the proliferation, migration, and chemosensitivity of glioblastoma to doxorubicin through modulation of the SIAH1/β-catenin axis. Cell Death Dis. 2018, 9, 1049. [Google Scholar] [CrossRef]
- Isakov, N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018, 48, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Potter, D.A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 2013, 11, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, Y.; Li, T.; Shu, G.; Yin, G. CCNA2 acts as a novel biomarker in regulating the growth and apoptosis of colorectal cancer. Cancer Manag. Res. 2018, 10, 5113–5124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, K.; Zhang, J.; Wang, L.; Sheng, L.; Yan, L. Inhibition of CDK1 Reverses the Resistance of 5-Fu in Colorectal Cancer. Cancer Manag. Res. 2020, 12, 11271–11283. [Google Scholar] [CrossRef] [PubMed]
- Mangé, A.; Coyaud, E.; Desmetz, C.; Laurent, E.; Béganton, B.; Coopman, P.; Raught, B.; Solassol, J. FKBP4 connects mTORC2 and PI3K to activate the PDK1/Akt-dependent cell proliferation signaling in breast cancer. Theranostics 2019, 9, 7003–7015. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.R.; Ryan, É.J.; Dunne, E.; Aherne, T.M.; Bhatt, N.R.; Lowery, A.J. Meta-analysis of the impact of progesterone receptor status on oncological outcomes in oestrogen receptor-positive breast cancer. Br. J. Surg. 2020, 107, 33–43. [Google Scholar] [CrossRef]
- Yang, S.; Jia, Y.; Liu, X.; Winters, C.; Wang, X.; Zhang, Y.; Devor, E.J.; Hovey, A.M.; Reyes, H.D.; Xiao, X.; et al. Systematic dissection of the mechanisms underlying progesterone receptor downregulation in endometrial cancer. Oncotarget 2014, 5, 9783–9797. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Y.; Zhang, G.; Garen, A.; Song, X. Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA. Proc. Natl. Acad. Sci. USA 2009, 106, 16794–16798. [Google Scholar] [CrossRef] [PubMed]
- Pellikainen, J.M.; Kosma, V.M. Activator protein-2 in carcinogenesis with a special reference to breast cancer—A mini review. Int. J. Cancer 2007, 120, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Dey, G.; Lin, F.; Lathia, J.; Reizes, O. CD55 in cancer: Complementing functions in a non-canonical manner. Cancer Lett. 2022, 551, 215935. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, J.W.; Jeong, M.H.; An, J.H.; Jang, S.M.; Song, K.H.; Choi, K.H. Microtubule-associated protein 1B light chain (MAP1B-LC1) negatively regulates the activity of tumor suppressor p53 in neuroblastoma cells. FEBS Lett. 2008, 582, 2826–2832. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Q.; Gu, J.; Chen, S.; Li, Q.; Ying, L. Down-regulation of CCNE1 expression suppresses cell proliferation and sensitizes gastric carcinoma cells to Cisplatin. Biosci. Rep. 2019, 39, BSR20190381. [Google Scholar] [CrossRef]
- Pabst, T.; Mueller, B.U. Complexity of CEBPA dysregulation in human acute myeloid leukemia. Clin. Cancer Res. 2009, 15, 5303–5307. [Google Scholar] [CrossRef]
- Saha, S.K.; Choi, H.Y.; Kim, B.W.; Dayem, A.A.; Yang, G.M.; Kim, K.S.; Yin, Y.F.; Cho, S.G. KRT19 directly interacts with β-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene 2017, 36, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shi, Y.; Wen, Y.; Li, W.; Feng, J.; Chen, C. The roles of TNFAIP2 in cancers and infectious diseases. J. Cell Mol. Med. 2018, 22, 5188–5195. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.C.; Bennetau-Pelissero, C. Phytoestrogens and Health Effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef]
- Zhu, B.T.; Han, G.Z.; Shim, J.Y.; Wen, Y.; Jiang, X.R. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 2006, 147, 4132–4150. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.; et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 2002, 123, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Nasri, A. The Potent Phytoestrogen 8-Prenylnaringenin: A Friend or a Foe? Int. J. Mol. Sci. 2022, 23, 3168. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, C.; Bertoni, A.; Nalin, M.; Sampietro, S.; Zanfa, M.; Sinigaglia, F. The phytoestrogen 8-prenylnaringenin inhibits agonist-dependent activation of human platelets. Biochim. Biophys. Acta 2012, 1820, 1724–1733. [Google Scholar] [CrossRef]
- Izzo, G.; Söder, O.; Svechnikov, K. The prenylflavonoid phytoestrogens 8-prenylnaringenin and isoxanthohumol diferentially suppress steroidogenesis in rat Leydig cells in ontogenesis. J. Appl. Toxicol. 2011, 31, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Inoue, M.; Tsugane, S. Japan Public Health Center-Based Prospective Study Group. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol. Biomark. Prev. 2007, 16, 538–545. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Labrie, F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015, 145, 133–138. [Google Scholar] [CrossRef]
- Buendía-González, F.O.; Legorreta-Herrera, M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A. Catechol estrogen 4-hydroxyestradiol is an ultimate carcinogen in breast cancer. Biomed. Sci. Lett. 2018, 24, 143–149. [Google Scholar] [CrossRef][Green Version]
- Traish, A.M.; Kang, H.P.; Saad, F.; Guay, A.T. Dehydroepiandrosterone (DHEA)--a precursor steroid or an active hormone in human physiology. J. Sex Med. 2011, 8, 2960–2982. [Google Scholar] [CrossRef]
- Rabkin, J.G.; McElhiney, M.C.; Rabkin, R.; McGrath, P.J.; Ferrando, S.J. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am. J. Psychiatry 2006, 163, 59–66. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Daly, R.C.; Bloch, M.; Smith, M.J.; Danaceau, M.A.; St Clair, L.S.; Murphy, J.H.; Haq, N.; Rubinow, D.R. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch. Gen. Psychiatry 2005, 62, 154–162. [Google Scholar] [CrossRef]
- Veronese, N.; Trevisan, C.; De Rui, M.; Bolzetta, F.; Maggi, S.; Zambon, S.; Corti, M.C.; Baggio, G.; Perissinotto, E.; Crepaldi, G.; et al. Serum Dehydroepiandrosterone Sulfate and Risk for Type 2 Diabetes in Older Men and Women: The Pro.V.A Study. Can. J. Diabetes 2016, 40, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Papierska, L.; Rabijewski, M.; Kasperlik-Załuska, A.; Zgliczyński, W. Effect of DHEA supplementation on serum IGF-1, osteocalcin, and bone mineral density in postmenopausal, glucocorticoid-treated women. Adv. Med. Sci. 2012, 57, 51–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwartz, A.G. Dehydroepiandrosterone, Cancer, and Aging. Aging Dis. 2022, 13, 423–432. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Bradley, M.; McElhiney, M.J. DHEA and cognition in HIV-positive patients with non-major depression. Psychosomatics 2012, 53, 244–249. [Google Scholar] [CrossRef]
- Hartkamp, A.; Geenen, R.; Godaert, G.L.; Bijl, M.; Bijlsma, J.W.; Derksen, R.H. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: A randomised controlled trial. Ann. Rheum. Dis. 2010, 69, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.M.; Al-Sharif, S.; Ala’ Bereshy, R.; Al-Eitan, S.F.; Banikhaled, S.H.; Al-Qudimat, A.R.; Al-Zoubi, R.M.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The necessary evil for human health, and ways to tame it. Biomed Pharmacother. 2018, 102, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Cuhaci, N.; Polat, S.B.; Evranos, B.; Ersoy, R.; Cakir, B. Gynecomastia: Clinical evaluation and management. Indian J. Endocrinol. Metab. 2014, 18, 150–158. [Google Scholar] [PubMed]
- Bukato, K.; Kostrzewa, T.; Gammazza, A.M.; Gorska-Ponikowska, M.; Sawicki, S. Endogenous estrogen metabolites as oxidative stress mediators and endometrial cancer biomarkers. Cell Commun. Signal 2024, 22, 205. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.J.; McNeil, C.M.; Musgrove, E.A.; Sutherland, R.L. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: The potential roles of c-Myc, cyclin D1 and cyclin E. Endocr. Relat. Cancer 2005, 12 (Suppl. S1), S47–S59. [Google Scholar] [CrossRef] [PubMed]
- Gompel, A.; Somaï, S.; Chaouat, M.; Kazem, A.; Kloosterboer, H.J.; Beusman, I.; Forgez, P.; Mimoun, M.; Rostène, W. Hormonal regulation of apoptosis in breast cells and tissues. Steroids 2000, 65, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef]
- Bendrik, C.; Dabrosin, C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J. Immunol. 2009, 182, 371–378. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Mohammed, K.; Abu Dabrh, A.M.; Benkhadra, K.; Al Nofal, A.; Carranza Leon, B.G.; Prokop, L.J.; Montori, V.M.; Faubion, S.S.; Murad, M.H. Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 4012–4020. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [PubMed]
- Zdrojewicz, Z.; Dubinski, A.; Dubinska, K. The Role of Estrogen Receptors and Their Polymorphism in Endothelial Dysfunction and Atherosclerosis. Adv. Clin. Exp. Med. 2005, 14, 1289. [Google Scholar]
- Xing, D.; Nozell, S.; Chen, Y.F.; Hage, F.; Oparil, S. Estrogen and mechanisms of vascular protection. Arter. Thromb. Vasc. Biol. 2009, 29, 289–295. [Google Scholar] [CrossRef]
- Ruszkowska, B.; Manysiak, S.; Małecka, B.; Dymek, G.; Rość, D.; Odrowąż-Sypniewska, G. Parameters of fibrinolysis in postmenopausal women taking oral and transdermal hormone replacement therapy. Adv. Clin. Exp. Med. 2010, 19, 203–210. [Google Scholar]
- Roy, D.; Cai, Q.; Felty, Q.; Narayan, S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 235–257. [Google Scholar] [CrossRef]
- Walker, S.E. Estrogen and autoimmune disease. Clin. Rev. Allergy Immunol. 2011, 40, 60–65. [Google Scholar] [CrossRef]
- McCarty, D.J.; Manzi, S.; Medsger, T.A., Jr.; Ramsey-Goldman, R.; LaPorte, R.E.; Kwoh, C.K. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995, 38, 1260–1270. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Feskanich, D.; Stampfer, M.J.; Karlson, E.W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007, 56, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Ostensen, M. Sex hormones and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1999, 876, 131–143. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, J.; González-Pérez, M.; Durand-Carbajal, M.; Lara-Reyes, P.; Jiménez-Santana, L.; Romero-Díaz, J.; Cravioto, M.D. Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Petri, M.A.; Kim, M.Y.; Kalunian, K.C.; Grossman, J.; Hahn, B.H.; Merrill, J.T.; Sammaritano, L.; Lockshin, M.; Alarcón, G.S.; et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann. Intern. Med. 2005, 142 Pt 1, 953–962. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S.; et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Germain, D. Estrogen carcinogenesis in breast cancer. Endocrinol. Metab. Clin. N. Am. 2011, 40, 473–484. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Clemons, M.; Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001, 344, 276–285. [Google Scholar] [CrossRef]
- Joo, B.S.; Park, S.H.; An, B.M.; Kim, K.S.; Moon, S.E.; Moon, H.S. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil. Steril. 2010, 93, 442–446. [Google Scholar] [CrossRef]
- de Blok, C.J.M.; Wiepjes, C.M.; Nota, N.M.; van Engelen, K.; Adank, M.A.; Dreijerink, K.M.A.; Barbé, E.; Konings, I.R.H.M.; den Heijer, M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. BMJ 2019, 365, l1652. [Google Scholar] [CrossRef]
- Yoo, T.K.; Han, K.D.; Kim, D.; Ahn, J.; Park, W.C.; Chae, B.J. Hormone Replacement Therapy, Breast Cancer Risk Factors, and Breast Cancer Risk: A Nationwide Population-Based Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ 2020, 371, m3873. [Google Scholar] [CrossRef] [PubMed]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N. Engl. J. Med. 2017, 377, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.M.; Holman, C.D.; Hart, R.; Bulsara, M.K.; Preen, D.B.; Finn, J.C. In vitro fertilization and breast cancer: Is there cause for concern? Fertil. Steril. 2012, 98, 334–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katz, D.; Paltiel, O.; Peretz, T.; Revel, A.; Sharon, N.; Maly, B.; Michan, N.; Sklair-Levy, M.; Allweis, T. Beginning IVF treatments after age 30 increases the risk of breast cancer: Results of a case-control study. Breast J. 2008, 14, 517–522. [Google Scholar] [CrossRef]
- van den Belt-Dusebout, A.W.; Spaan, M.; Lambalk, C.B.; Kortman, M.; Laven, J.S.; van Santbrink, E.J.; van der Westerlaken, L.A.; Cohlen, B.J.; Braat, D.D.; Smeenk, J.M.; et al. Ovarian Stimulation for In Vitro Fertilization and Long-term Risk of Breast Cancer. JAMA 2016, 316, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Brish, M.; Serr, D.M.; Insler, V.; Salomy, M.; Lunenfeld, B. Outcome of pregnancy after induced ovulation. Follow-up of pregnancies and children born after clomiphene therapy. JAMA 1972, 220, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Robabeh, T.; Firoozeh, B.; Robab, A.; Mohammedreza, M.; Azadeh, A.S. Breast Cancer and Ovulation Induction Treatments. Clin. Breast Cancer 2018, 18, 395–399. [Google Scholar]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef]
- Siiteri, P.K.; MacDonald, P.C. Placental estrogen biosynthesis during human pregnancy. J. Clin. Endocrinol. Metab. 1966, 26, 751–761. [Google Scholar] [CrossRef]
- Minors, D.S.; Waterhouse, J.M. Anchor sleep as a synchronizer of rhythms on abnormal routines. Int. J. Chronobiol. 1981, 7, 165–188. [Google Scholar]
- Avery, D.H.; Dahl, K.; Savage, M.V.; Brengelmann, G.L.; Larsen, L.H.; Kenny, M.A.; Prinz, P.N. Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol. Psychiatry 1997, 41, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, H.W.; Teicher, M.H.; Mitropoulou, V.; Navalta, C.; New, A.S.; Trestman, R.; Siever, L.J. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J. Psychiatr. Res. 2004, 38, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Alesci, S.; Martinez, P.E.; Kelkar, S.; Kelkar, S.; Ilias, I.; Ronsaville, D.S.; Listwak, S.J.; Gold, P.W. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: Clinical implications. J. Clin. Endocrinol. Metab. 2005, 90, 2522–2530. [Google Scholar] [CrossRef]
- Jarrett, D.B.; Colble, P.A.; Kupfer, D.J. Reduced cortisol latency in depressive illness. Arch. Gen. Psychiatry 1983, 40, 506–511. [Google Scholar] [CrossRef]
- Bao, A.-M.; Liu, R.-Y.; van Someren, E.J.W.; Hofman, M.A.; Cao, Y.-X.; Zhou, J.-N.K. Diurnal rhythm of free estradiol during the menstrual cycle. Eur. J. Endocrinol. 2003, 148, 227–232. [Google Scholar] [CrossRef]
- Taleb, J.; Krause, B.; Göretzlehner, G. Twenty-four hours hormone secretion profiles of cortisol and estradiol in preterm labour. Horm. Metab. Res. 1993, 25, 442–443. [Google Scholar] [CrossRef]
- Andreano, J.M.; Arjomandi, H.; Cahill, L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology 2008, 33, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Symonds, C.S.; Gallagher, P.; Thompson, J.M.; Young, A.H. Effects of the menstrual cycle on mood, neurocognitive and neuroendocrine function in healthy premenopausal women. Psychol. Med. 2004, 34, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Koukkari, W.L.; Sothern, R.B. Chronobiometry: Analyzing for rhythms. In Introducing Biological Rhythms; Springer: New York, NY, USA, 2006. [Google Scholar]
- Klipping, C.; Duijkers, I.; Mawet, M.; Maillard, C.; Bastidas, A.; Jost, M.; Foidart, J.M. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception 2021, 103, 213–221. [Google Scholar] [CrossRef]
- Wang, F.; Giskeødegård, G.F.; Skarra, S.; Engstrøm, M.J.; Hagen, L.; Geisler, J.; Mikkola, T.S.; Tikkanen, M.J.; Debik, J.; Reidunsdatter, R.J.; et al. Association of serum cortisol and cortisone levels and risk of recurrence after endocrine treatment in breast cancer. Clin. Exp. Med. 2023, 23, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Biason-Lauber, A.; Lang-Muritano, M. Estrogens: Two nuclear receptors, multiple possibilities. Mol. Cell Endocrinol. 2022, 554, 111710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Demura, M.; Martin, R.M.; Shozu, M.; Sebastian, S.; Takayama, K.; Hsu, W.T.; Schultz, R.A.; Neely, K.; Bryant, M.; Mendonca, B.B.; et al. Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum. Mol. Genet. 2007, 16, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Kangasniemi, M.H.; Arffman, R.K.; Haverinen, A.; Luiro, K.; Hustad, S.; Heikinheimo, O.; Tapanainen, J.S.; Piltonen, T.T. Effects of estradiol- and ethinylestradiol-based contraceptives on adrenal steroids: A randomized trial. Contraception 2022, 116, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, Estrogens and Male Fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Pietro, P.; D’Auria, R.; Viggiano, A.; Ciaglia, E.; Meccariello, R.; Russo, R.D.; Puca, A.A.; Vecchione, C.; Nori, S.L.; Santoro, A. Bisphenol A induces DNA damage in cells exerting immune surveillance functions at peripheral and central level. Chemosphere 2020, 254, 126819. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Scafuro, M.; Troisi, J.; Piegari, G.; Di Pietro, P.; Mele, E.; Cappetta, D.; Marino, M.; De Angelis, A.; Vecchione, C.; et al. Multi-Systemic Alterations by Chronic Exposure to a Low Dose of Bisphenol A in Drinking Water: Effects on Inflammation and NAD+-Dependent Deacetylase Sirtuin1 in Lactating and Weaned Rats. Int. J. Mol. Sci. 2021, 22, 9666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
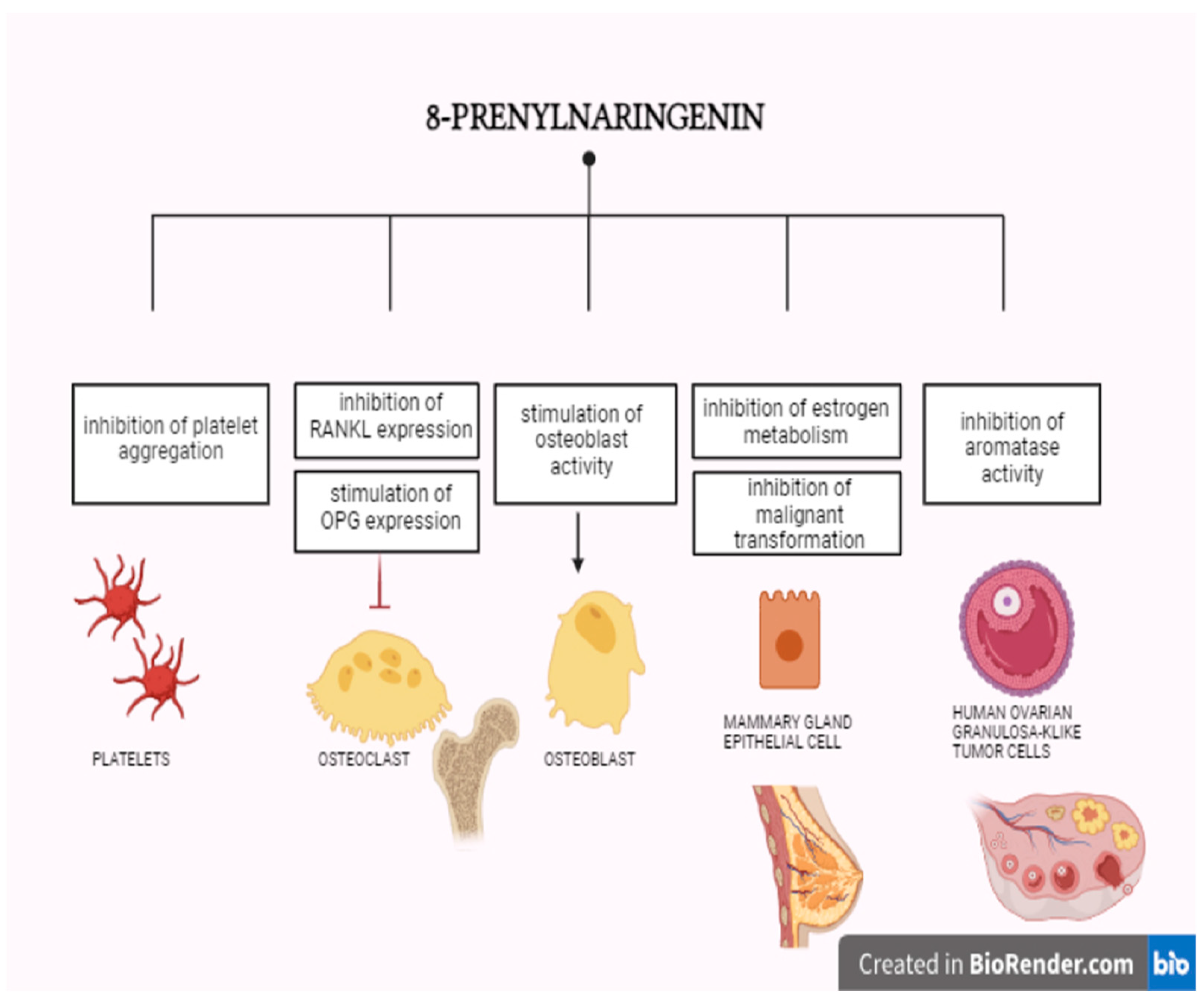
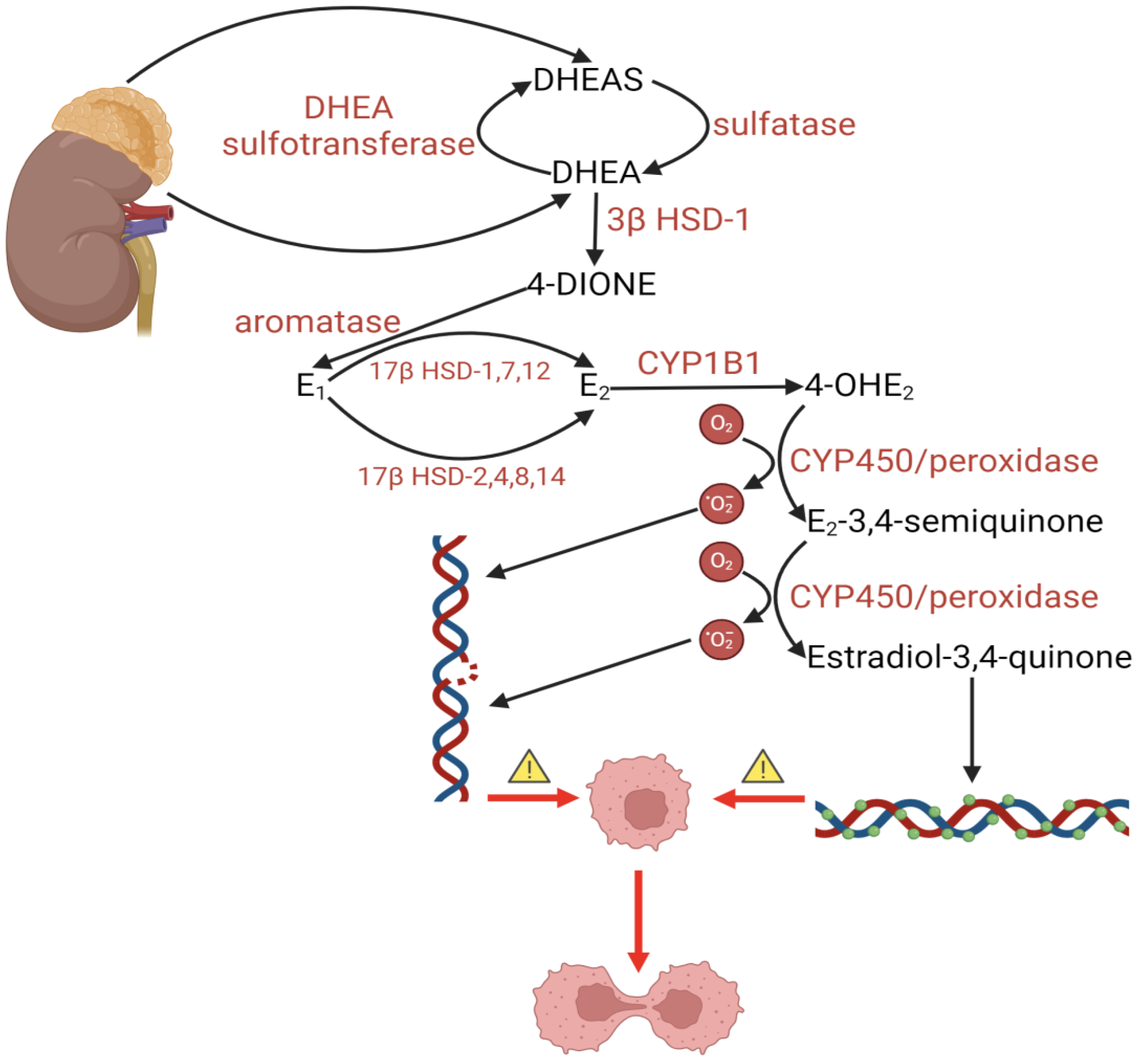
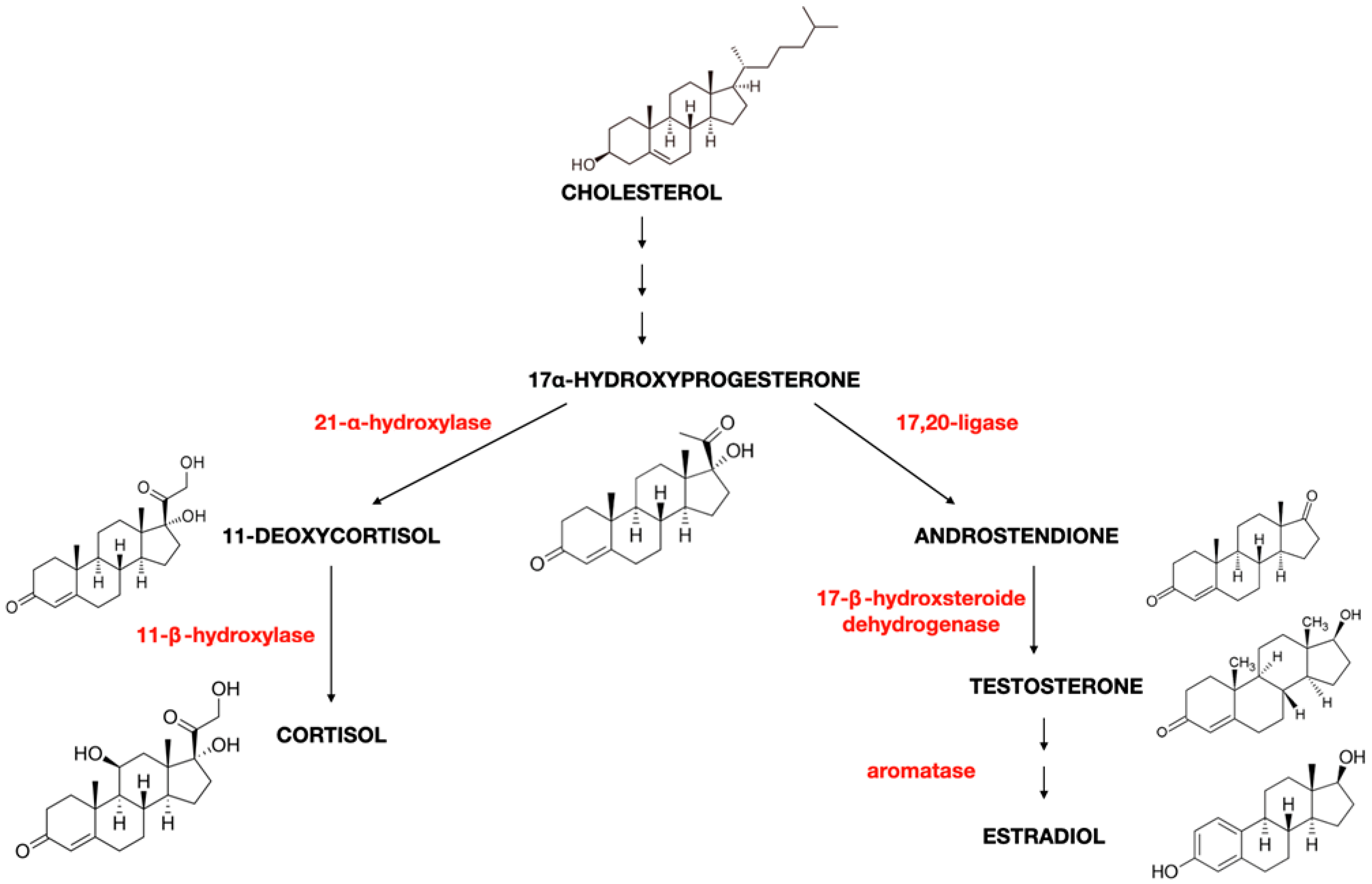
| Process | Description | Tissue | Function of Aromatase | Regulation of Expression |
|---|---|---|---|---|
| Conversion of androstenedione to oestrone | Aromatase converts androstenedione to oestrone through hydroxylation and dehydration. | Ovaries | Main site of oestrogen synthesis in premenopausal women. In ovarian follicle granulosa cells, aromatase converts androgens from theca cells to oestrogens. | Expression stimulated by LH 1 |
| Conversion of testosterone to oestradiol | Testosterone is converted to oestradiol also through hydroxylation and dehydration. | Adipose tissue | Main site of oestrogen production in postmenopausal women. In adipose tissue, aromatase converts excess androgens to oestrogens, crucial for maintaining oestrogen levels post menopause. | Expression regulated by insulin and cytokines |
| Reduction of oestrone to oestradiol | Estrone can be reduced to oestradiol by the enzyme 17β-HSD. 2 | Placenta | During pregnancy, placental aromatase synthesises oestrogens necessary for maintaining pregnancy and proper foetal development. | Regulated by various hormones and growth factors |
| Aromatase is also present in other tissues where locally synthesised oestrogens may have paracrine and autocrine functions. | Brain, bones, muscles, skin | Regulated by local signals |
| Aspect | Women | Men |
|---|---|---|
| Bone health and growth | Oestrogen is crucial for bone maturation and density. Deficiency leads to delayed skeletal maturation and osteoporosis. | Similar effects as in women: tall stature without a pubertal growth spurt, delayed skeletal maturation, and severe osteopenia. Bone density improves with oestrogen replacement. |
| Reproductive system | Essential for reproductive function; influences ovarian and uterine health. Oestrogen receptor α (ERα) plays a significant role in fertility and uterine function. | Leads to hypergonadotropism, macro-orchidism, and increased serum testosterone when deficient. Oestrogen is crucial for proper testicular function and spermatogenesis. |
| Cardiovascular health | Protective against cardiovascular diseases; affects lipid and carbohydrate metabolism. | Deficiency can lead to premature coronary atherosclerosis due to its impact on lipid and carbohydrate metabolism. |
| Skeletal system | Important for growth plate chondrocytes; accrual and the maintenance of bone mass and density. | Oestrogen deficiency in men has a significant impact on skeletal growth and bone mass, similar to women. |
| Metabolism | Influences carbohydrate and lipid metabolism, contributing to the regulation of body weight and composition. | Oestrogen plays a role in metabolic processes, and its deficiency can negatively impact carbohydrate and lipid metabolism. |
| Immune system | Oestrogen impacts the immune system, contributing to immune response regulation. | Similar effects on the immune system as in women, though specific impacts might vary. |
| Endocrine function | Affects neuroendocrine system and hormonal balance. Plays a role in the timing of pubertal onset. | Oestrogen influences neuroendocrine function and hormonal regulation. |
| Developmental effects | Critical for normal development and function of the reproductive organs and secondary sexual characteristics. | Essential for normal sexual development and reproductive function; deficiency can lead to eunuchoid proportions and other developmental issues. |
| Gene: | Regulation: Up/Down ↑/↓ | Cancerogenesis Process |
|---|---|---|
| CEACAM5 | ↑ | Protection from apoptosis, adhesion, and inhibition of differentiation [159] |
| CYP1A1 | ↑ | AMPK inhibition: excessive proliferation and prolonged viability of cancer cells [160] |
| DDIT4 | ↑ | mTOR activity suppression: excessive proliferation and apoptosis disorders [134,142] |
| IER3 | ↑ | Apoptosis disorders [145] |
| KLHL24 | ↑ | Overexpression of KLHL24 [136] |
| SLC7A5 | ↑ | Provides the tumour with access to amino acid development and proliferation and activates the mTORC1 pathway [152] |
| SLC7A11 | ↑ | Protection of cancer cells from oxygen radicals; Delivery of glucose and glutamine to cancer cells [15] |
| STC2 | ↑ | Increased proliferation and viability; Regulation of the MAPK pathway [154] |
| ADORA1 | ↓ | Proliferation inhibition (positive effect) and inhibition of ERalpha transcriptional activity [140] |
| CCNA2 | ↓ | Cell cycle arrest and induction of apoptosis [161] |
| CDK1 | ↓ | Cell cycle arrest [162] |
| FKBP4 | ↓ | Proliferation and growth inhibition (positive effect) [163] |
| PGR | ↓ | Increased risk of recurrence [164,165] |
| SFPQ | ↓ | Excessive proliferation, tumour growth [166] |
| TFAP2C | ↓ | Excessive proliferation, tumour growth [167] |
| Gene: | Regulation: Up/Down ↑/↓ | Cancerogenesis Process |
|---|---|---|
| CCNG2 | ↑ | Degradation of CDK2, inhibition of proliferation, and cell cycle arrest [136] |
| CD55 | ↑ | More durable and regenerable cancer cells, proliferation, angiogenesis, and immune system evasion [168] |
| CEACAM5 | ↑ | Avoidance of apoptosis, adhesion, and inhibition of differentiation [159] |
| CYP1A1 | ↑ | AMPK inhibition: excessive proliferation and prolonged viability of cancer cells [160] |
| DDIT4 | ↑ | mTOR activity suppression: excessive proliferation and apoptosis disorders [134,142] |
| FOSL2 | ↑ | Participates in activating the metastasis cascade [143] |
| HSPA13 | ↑ | TANK stabilisation, proliferation, and migration [144] |
| IGF1R | ↑ | Inhibition of apoptosis, proliferation, and enhanced ER activation [146,147] |
| KLHL24 | ↑ | Cancer progression via influencing cellular proliferation and survival mechanisms [136] |
| MAP1B | ↑ | Inhibition of apoptosis (via p53) [169] |
| RUNX2 | ↑ | Bone metastasis and impaired function and development of osteoblasts [149] |
| SLC7A2 | ↑ | Inflammation and oxidative stress caused by increased NO synthesis, but inhibited invasion and migration [150,151] |
| SLC7A5 | ↑ | Provides the tumour with access to amino acid development and proliferation and activates the mTORC1 pathway [152] |
| SLC7A11 | ↑ | Protection of cancer cells from oxygen radicals and delivery of glucose and glutamine to cancer cells [153] |
| STC2 | ↑ | Increased proliferation and viability, and regulation of the MAPK pathway [154] |
| VEGFA | ↑ | Angiogenesis and increased vascular permeability [170] |
| CCNE1 | ↓ | Cell cycle and proliferation inhibition, and reduced cell viability [171] |
| CEBPA | ↓ | Suppressor gene silencing: accelerated proliferation [172] |
| KRT19 | ↓ | Increased invasiveness, migration, and proliferation of cancer cells [173] |
| PRKCD | ↓ | Accelerated development of cancer cells [158] |
| SFPQ | ↓ | Excessive proliferation and tumour growth [166] |
| TNFAIP2 | ↓ | Migration and invasion, proliferation, and angiogenesis [174] |
| Chemical Compound | EC50 | RBAβ [%] | RBAα [%] | Occurrence |
|---|---|---|---|---|
| 8-Prenylnaringenin | 4 | 6.5 | 19.5 | Common hop |
| Genistein | 200 | 79 | 6 | Soybean |
| Coumestrol | 30 | 35 | 22 | Red clover |
| E2 | 0.8 | 100 | 100 | Endogenous oestrogen |
| Type of Therapy | (n) | Breast Cancer Risk (Compared to Control Group) | Breast Cancer Risk (Related to Specific Conditions of the Therapy) | Breast Cancer Risk (Related to Substance Used during Treatment) |
|---|---|---|---|---|
| Hormonal contraception | 1,797,932 | 1.2 (RR) | ≥5 years of usage: 1.24 >10 years of usage: 1.38 | Oestradiol valerate and dienogest: 1.62 Levonogestrel: 1.93 [228] |
| Hormonal replacement therapy | 696,084 | 1.25 (HR) | <2 years of treatment: 1.08 2–5 years of treatment: 1.33 >5 years of treatment: 1.72 | No data [226] |
| 98,611 | 1.21 (OR) | Oestrogen–progesterone: <1 year of treatment: 1.05 1–4 years of treatment: 1.34 ≥5 years of treatment: 1.57 | Oestrogen– progesterone: 1.26 Oestrogen: 1.06 [227] | |
| In vitro fertilisation (IVF) | 25,108 | 1.01 (SIR) | ≥7 IVF cycles: 0.55 (compared with 1–2 IVF cycle group) <4 oocytes collected: 0.77 (compared with group with ≥4 oocytes collected) [231] | - |
| 21,025 | 1.1 (HR) | Age at start of IVF: 24 years old: 1.56 32 years old: 1.16 36 years old: 1.00 [229] | ||
| 7162 | - | Age at start of IVF: >30 years old: 1.24 (RR) [230] | ||
| Pharmalogical infertility treatment | 1856 | 1.13 (OR) | <6 months of treatment: 0.4 ≥6 months of treatment: 1.7 | Human menopausal gonadotropin: 2.25 Clomiphene citrate: 0.8 hCG and clomiphene citrate: 1.0 [233] |
| Gender-affirming hormone treatment | 2260 trans women, 1229 trans men | Trans women: 0.3 (SIR, compared with cisgender women) 46.7 (SIR, compared with cisgender men) Trans men: 0.2 (SIR, compared with cisgender women) 58.9 (SIR, compared with cisgender men) | No data | No data [225] |
| Characteristics/Properties | Cortisol | Oestrogen (Oestradiol) |
|---|---|---|
| Circadian rhythm | Exhibits a circadian rhythm with a peak within 45 min of waking, declining throughout the day and rising at night [236]. | Exhibits a circadian rhythm with a morning peak and several ultradian harmonics throughout the 24 h period [241]. |
| Differences between healthy and ill | The rhythm can differ between healthy and ill individuals; e.g., in SAD, the rhythm is delayed [237]. | The rhythm remains relatively unchanged across menstrual cycle phases, except for the acrophase during the menstrual phase [241]. |
| Phase studies | Numerous studies have examined the relationship of the cortisol circadian rhythm to other physiological processes [237,238,239]. | Few studies on the circadian rhythm of oestradiol alone; some studies compare oestradiol and cortisol rhythms [241,242]. |
| Phases in diseases | In diseases such as depression, the cortisol rhythm may be advanced or delayed [238]. | The oestradiol rhythm does not phase-shift in preterm labour compared to term labour [242]. |
| Relationship with other hormones | Exhibits various phase differences in relation to hormones such as TSH, prolactin, and growth hormones [237,238,239]. | Studies suggest possible decoupling of oestradiol and cortisol rhythms in depression [241]. |
| Influence of the menstrual cycle | May vary depending on the phase of the menstrual cycle [243,244]. | The character of the oestradiol rhythm is relatively unaffected by the menstrual cycle, except for the acrophase [241]. |
| Other | Phase changes in cortisol may indicate differences in health and disease states [237,238,239,240]. | Few studies on optimal phase differences between oestradiol and other hormonal rhythms [245]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowiak-Wieczorek, J.; Jaros, A.; Gajdzińska, A.; Wojtyła-Buciora, P.; Szymański, I.; Szymaniak, J.; Janusz, W.; Walczak, I.; Jonaszka, G.; Bienert, A. The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs. Int. J. Mol. Sci. 2024, 25, 8167. https://doi.org/10.3390/ijms25158167
Bartkowiak-Wieczorek J, Jaros A, Gajdzińska A, Wojtyła-Buciora P, Szymański I, Szymaniak J, Janusz W, Walczak I, Jonaszka G, Bienert A. The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs. International Journal of Molecular Sciences. 2024; 25(15):8167. https://doi.org/10.3390/ijms25158167
Chicago/Turabian StyleBartkowiak-Wieczorek, Joanna, Agnieszka Jaros, Anna Gajdzińska, Paulina Wojtyła-Buciora, Igor Szymański, Julian Szymaniak, Wojciech Janusz, Iga Walczak, Gabriela Jonaszka, and Agnieszka Bienert. 2024. "The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs" International Journal of Molecular Sciences 25, no. 15: 8167. https://doi.org/10.3390/ijms25158167
APA StyleBartkowiak-Wieczorek, J., Jaros, A., Gajdzińska, A., Wojtyła-Buciora, P., Szymański, I., Szymaniak, J., Janusz, W., Walczak, I., Jonaszka, G., & Bienert, A. (2024). The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs. International Journal of Molecular Sciences, 25(15), 8167. https://doi.org/10.3390/ijms25158167








