Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value
Abstract
1. Introduction
2. Results
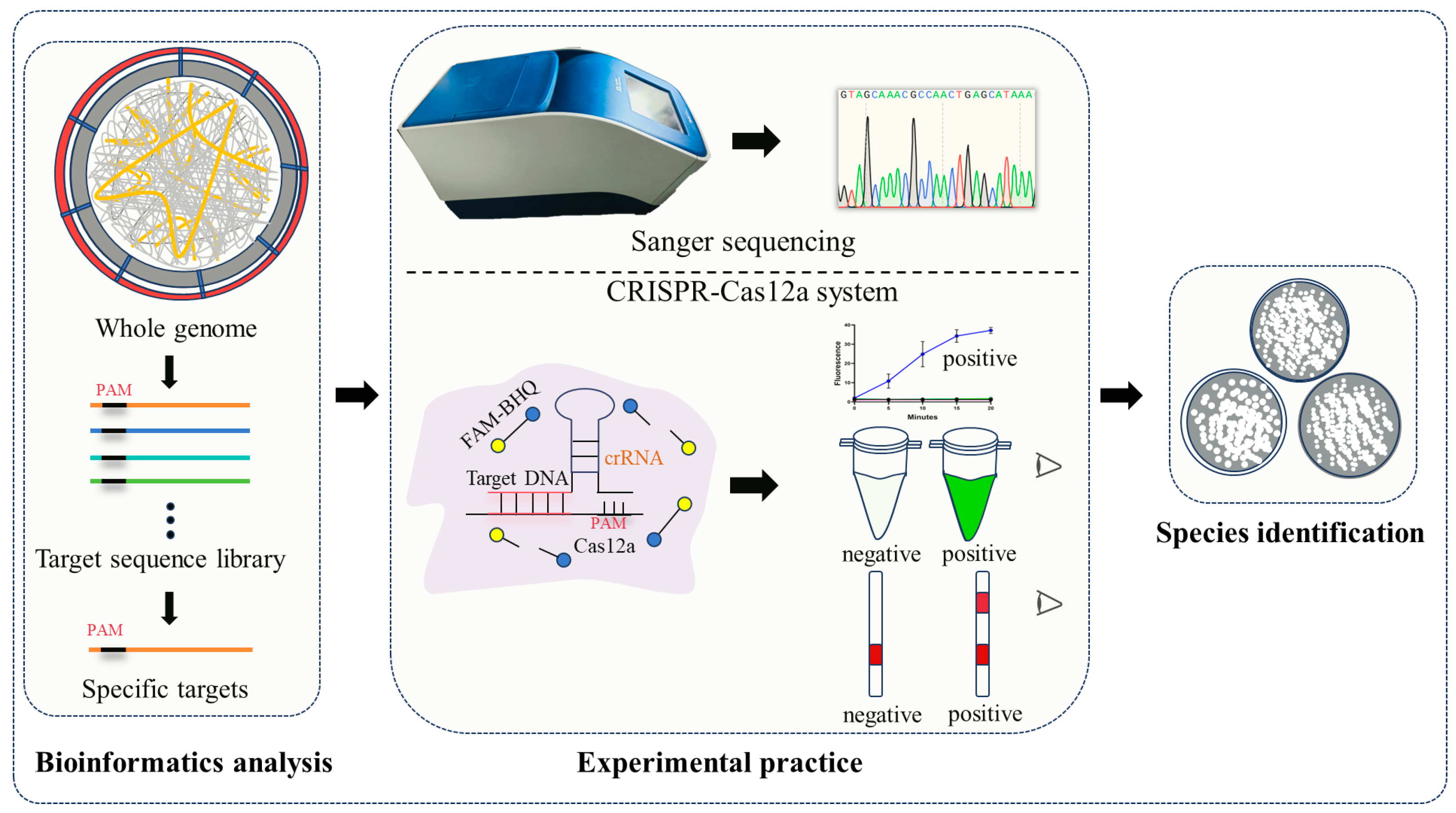
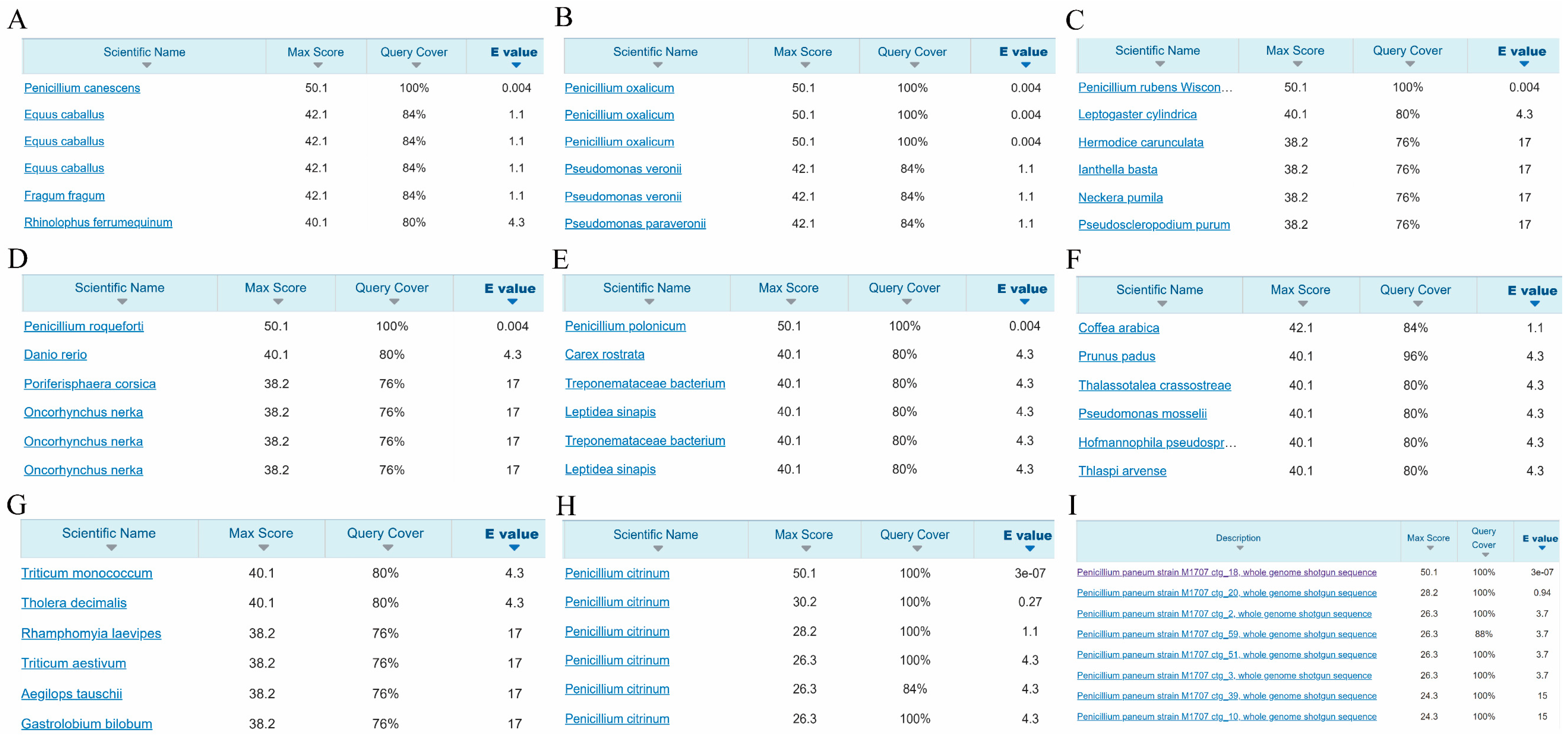
2.1. Bioinformatics Analysis Screening of Species-Specific Targets for Seven Penicillium Species Identification
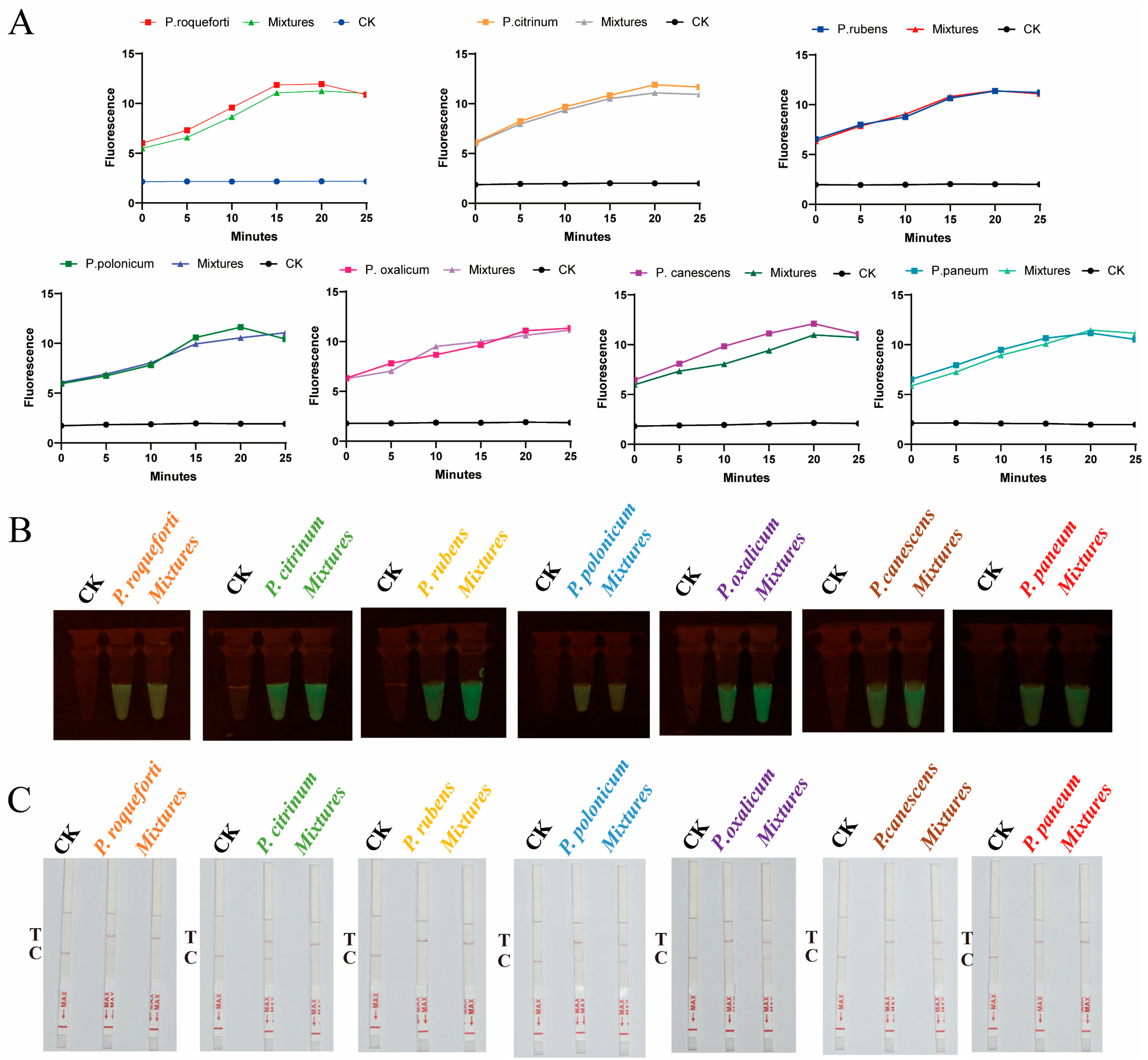
2.2. Sequencing Successfully Identified Target Species
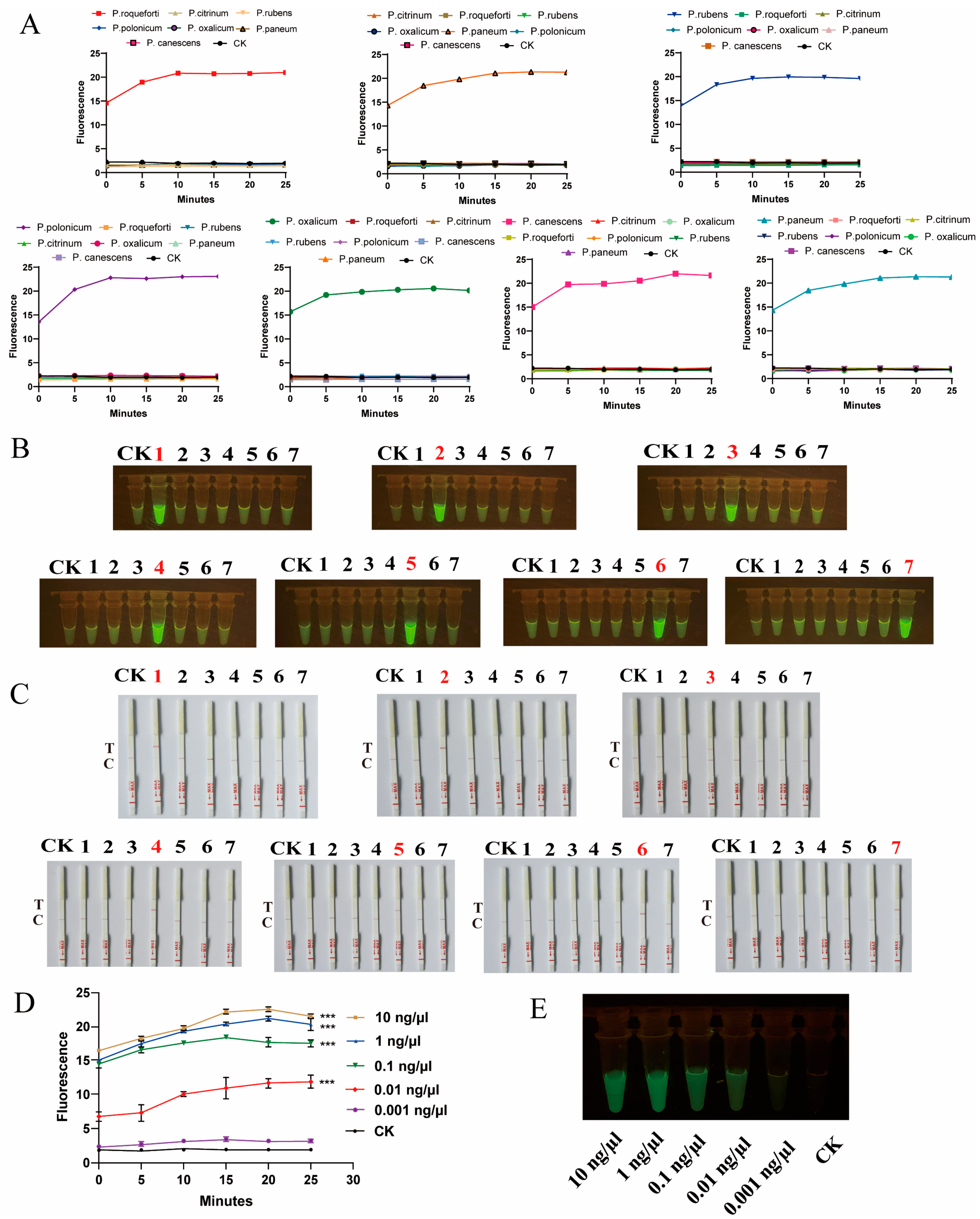
2.3. CRISPR-Cas12a Technology Rapidly Detected Target Species
3. Discussion
3.1. AGE Enables Construct-Specific Target Libraries for Seven Penicillium Species Identification
3.2. AGE-Integrated CRISPR-Cas12a Technology for Rapid and Accurate Species On-Site Detection
3.3. Challenge and Development of AGE
4. Materials and Methods
4.1. Strain Preparation and DNA Extraction
4.2. Screening Species-Specific Targets with Bioinformatics Analysis
4.3. Identification of Species-Specific Targets
4.3.1. Recognition of Specific Targets via Sanger Sequencing
4.3.2. CRISPR-Cas12a-Based Detection of Specific Targets
4.4. Detection of Penicillium to Assess Specificity and Sensitivity
4.5. Mixture Sample Detection for Penicillium Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [PubMed]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium . Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, T.C.; Ferreira, M.C.; Rosa, L.H.; de Oliveira, A.M.; Oliveira Júnior, E.N. Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydr. Polym. 2020, 234, 115918. [Google Scholar] [CrossRef]
- Yang, L.; Xie, J.; Jiang, D.; Fu, Y.; Li, G.; Lin, F. Antifungal substances produced by Penicillium oxalicum strain PY-1—Potential antibiotics against plant pathogenic fungi. World J. Microbiol. Biotechnol. 2008, 24, 909–915. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, H.; Bai, Z.; Li, B.J.P. Induced resistance to Cladosporium cucumerinum in cucumber by pectinases extracted from Penicillium oxalicum. Phytoparasitica 2004, 32, 377–387. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin-producing fungi. Saudi J. Biol. Sci. 2010, 17, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Jiao, X.; Hu, Y.; Lu, X.; Gao, W. Mycobiota and mycotoxins in traditional medicinal seeds from China. Toxins 2015, 7, 3858–3875. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Tang, D.; Zhou, Y.Q.; Sun, B.D.; Li, X.J.; Wang, L.Z.; Gao, W.W. Identification of ochratoxin A producing fungi associated with fresh and dry liquorice. PLoS ONE 2013, 8, e78285. [Google Scholar] [CrossRef]
- Keromnes, J.; Thouvenot, D. Role of Penicillic Acid in the phytotoxicity of Penicillium cyclopium and Penicillium canescens to the Germination of corn seeds. Appl. Environ. Microbiol. 1985, 49, 660–663. [Google Scholar] [CrossRef]
- O’Brien, M.; Egan, D.; O’Kiely, P.; Forristal, P.D.; Doohan, F.M.; Fuller, H.T. Morphological and molecular characterisation of Penicillium roqueforti and P. paneum isolated from baled grass silage. Mycol. Res. 2008, 112, 921–932. [Google Scholar] [CrossRef]
- Rundberget, T.; Skaar, I.; Flåøyen, A. The presence of Penicillium and Penicillium mycotoxins in food wastes. Int. J. Food Microbiol. 2004, 90, 181–188. [Google Scholar] [CrossRef]
- Malekinejad, H.; Aghazadeh-Attari, J.; Rezabakhsh, A.; Sattari, M.; Ghasemsoltani-Momtaz, B. Neurotoxicity of mycotoxins produced in vitro by Penicillium roqueforti isolated from maize and grass silage. Hum. Exp. Toxicol. 2015, 34, 997–1005. [Google Scholar] [CrossRef]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- Storm, I.M.; Kristensen, N.B.; Raun, B.M.; Smedsgaard, J.; Thrane, U. Dynamics in the microbiology of maize silage during whole-season storage. J. Appl. Microbiol. 2010, 109, 1017–1026. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef]
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.L.; Barbier, G.; Coton, E. Filamentous fungi and mycotoxins in Cheese: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456. [Google Scholar] [CrossRef]
- Punt, M.; van den Brule, T.; Teertstra, W.R.; Dijksterhuis, J.; den Besten, H.M.W.; Ohm, R.A.; Wösten, H.A.B. Impact of maturation and growth temperature on cell-size distribution, heat-resistance, compatible solute composition and transcription profiles of Penicillium roqueforti conidia. Food Res. Int. 2020, 136, 109287. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef]
- García-Estrada, C.; Martín, J.F. Biosynthetic gene clusters for relevant secondary metabolites produced by Penicillium roqueforti in blue cheeses. Appl. Microbiol. Biotechnol. 2016, 100, 8303–8313. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef]
- Peng, B.; Dai, L.; Iacovelli, R.; Driessen, A.J.M.; Haslinger, K. Heterologous naringenin production in the filamentous Fungus Penicillium rubens . J. Agric. Food Chem. 2023, 71, 20782–20792. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Mok, T.; Koehler, A.P.; Yu, M.Y.; Ellis, D.H.; Johnson, P.J.; Wickham, N.W. Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. J. Clin. Microbiol. 1997, 35, 2654–2656. [Google Scholar] [CrossRef]
- Gugnani, H.C.; Gupta, S.; Talwar, R.S. Role of opportunistic fungi in ocular infections in Nigeria. Mycopathologia 1978, 65, 155–166. [Google Scholar] [CrossRef]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Pan, Z.N.; Sun, S.C. Citrinin exposure disrupts organelle distribution and functions in mouse oocytes. Environ. Res. 2020, 185, 109476. [Google Scholar] [CrossRef]
- Gao, L.; Xu, W.; Xin, T.; Song, J. Application of third-generation sequencing to herbal genomics. Front. Plant Sci. 2023, 14, 1124536. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, Y.; Pu, X.; Gao, R.; Xu, Z.; Song, J. Trends in herbgenomics. Sci. China Life Sci. 2019, 62, 288–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gilchrist, C.L.M.; Chun, J.; Steinegger, M. UFCG: Database of universal fungal core genes and pipeline for genome-wide phylogenetic analysis of fungi. Nucleic Acids Res. 2023, 51, D777–D784. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, D785–D794. [Google Scholar] [CrossRef]
- Alanjary, M.; Steinke, K.; Ziemert, N. AutoMLST: An automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 2019, 47, W276–W282. [Google Scholar] [CrossRef]
- Kim, J.; Na, S.I.; Kim, D.; Chun, J. UBCG2: Up-to-date bacterial core genes and pipeline for phylogenomic analysis. J. Microbiol. 2021, 59, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Xin, T.; Xu, W.; Hao, L.; Qi, G.; Lou, Q.; Song, J. Principles and strategies for species identification based on analysis of whole-genome. Acta Pharm. Sin. 2023, 58, 2364–2374. [Google Scholar]
- Hao, L.; Xu, W.; Qi, G.; Xin, T.; Xu, Z.; Lei, H.; Song, J. GAGE is a method for identification of plant species based on whole genome analysis and genome editing. Commun. Biol. 2022, 5, 947. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Qi, G.; Hao, L.; Xin, T.; Lou, Q.; Xu, W.; Song, J. Analysis of Whole-Genome as a novel strategy for animal species identification. Int. J. Mol. Sci. 2024, 25, 2955. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Hao, L.; Xin, T.; Gan, Y.; Lou, Q.; Xu, W.; Song, J. Analysis of Whole-Genome facilitates rapid and precise identification of fungal species. Front. Microbiol. 2024, 15, 1336143. [Google Scholar] [CrossRef]
- Qi, G.; Hao, L.; Gan, Y.; Xin, T.; Lou, Q.; Xu, W.; Song, J. Identification of closely related species in Aspergillus through Analysis of Whole-Genome. Front. Microbiol. 2024, 15, 1323572. [Google Scholar] [CrossRef]
- Redondo, C.; Cubero, J.; Melgarejo, P. Characterization of Penicillium species by ribosomal DNA sequencing and BOX, ERIC and REP-PCR analysis. Mycopathologia 2009, 168, 11–22. [Google Scholar] [CrossRef]
- Guevara-Suarez, M.; Sutton, D.A.; Cano-Lira, J.F.; García, D.; Martin-Vicente, A.; Wiederhold, N.; Guarro, J.; Gené, J. Identification and antifungal susceptibility of Penicillium-like fungi from clinical samples in the United States. J. Clin. Microbiol. 2016, 54, 2155–2161. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium . In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 651–667. [Google Scholar]
- Kõljalg, U.; Tedersoo, L.; Nilsson, R.H.; Abarenkov, K. Digital identifiers for fungal species. Science 2016, 352, 1182–1183. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar] [PubMed]
- Sivashankari, S.; Shanmughavel, P. Functional annotation of hypothetical proteins—A review. Bioinformation 2006, 1, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sathianathan, A.; Ravichandran, P.; Lippi, J.M.; Cohen, L.; Messina, A.; Shaju, S.; Swede, M.J.; Ginsburg, D.S. The Eaf3/5/7 subcomplex stimulates NuA4 Interaction with methylated histone H3 Lys-36 and RNA polymerase II. J. Biol. Chem. 2016, 291, 21195–21207. [Google Scholar] [CrossRef]
- Leung, C.S.; Douglass, S.M.; Morselli, M.; Obusan, M.B.; Pavlyukov, M.S.; Pellegrini, M.; Johnson, T.L. H3K36 methylation and the chromodomain protein Eaf3 are required for proper cotranscriptional spliceosome assembly. Cell Rep. 2019, 27, 3760–3769.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, J.; He, J.S.; Zhang, F.; Ping, J.; Qian, C.; Wu, J. Visual detection for nucleic acid-based techniques as potential on-site detection methods. A review. Anal. Chim. Acta 2020, 1099, 1–15. [Google Scholar] [CrossRef]
- Luo, M.; Meng, F.Z.; Tan, Q.; Yin, W.X.; Luo, C.X. Recombinase Polymerase Amplification/Cas12a-based identification of xanthomonas arboricola pv. pruni on Peach. Front. Plant Sci. 2021, 12, 740177. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020, 11, 4711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J.; von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144, 31–67. [Google Scholar] [CrossRef]
- Randhawa, G.J.; Singh, M.; Morisset, D.; Sood, P.; Zel, J. Loop-mediated isothermal amplification: Rapid visual and real-time methods for detection of genetically modified crops. J. Agric. Food Chem. 2013, 61, 11338–11346. [Google Scholar] [CrossRef]
- Wang, X.; Jin, W.; Yang, Y.; Ma, H.; Liu, H.; Lei, J.; Wu, Y.; Zhang, L. CRISPR/Cas12a-mediated Enzymatic recombinase amplification for rapid visual quantitative authentication of halal food. Anal. Chim. Acta 2023, 1255, 341144. [Google Scholar] [CrossRef]
- Paul, R.; Saville, A.C.; Hansel, J.C.; Ye, Y.; Ball, C.; Williams, A.; Chang, X.; Chen, G.; Gu, Z.; Ristaino, J.B.; et al. Extraction of plant DNA by microneedle patch for rapid detection of plant diseases. ACS Nano 2019, 13, 6540–6549. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, X.; Han, J.; Sun, W.; Yao, H.; Song, J.; Li, X. DNA barcoding in herbal medicine: Retrospective and prospective. J. Pharm. Anal. 2023, 13, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Xu, Z.; Jia, J.; Leon, C.; Hu, S.; Lin, Y.; Ragupathy, S.; Song, J.; Newmaster, S.G. Biomonitoring for traditional herbal medicinal products using DNA metabarcoding and single molecule, real-time sequencing. Acta Pharm. Sin. B 2018, 8, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xu, Z.; Xin, T.; Shi, L.; Song, J. Quality control of the traditional patent medicine Yimu Wan based on SMRT sequencing and DNA barcoding. Front. Plant Sci. 2017, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiang, L.; Shi, L.; Li, G.; Yao, H.; Han, J.; Lin, Y.; Song, J.; Chen, S. Identification of crude drugs in the Japanese pharmacopoeia using a DNA barcoding system. Sci. Rep. 2017, 7, 42325. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Skouboe, P.; Frisvad, J.C.; Taylor, J.W.; Lauritsen, D.; Boysen, M.; Rossen, L. Phylogenetic analysis of nucleotide sequences from the ITS region of terverticillate Penicillium species. Mycol. Res. 1999, 103, 873–881. [Google Scholar] [CrossRef]
- Moreira, F.M.; Pereira, P.A.; Miranda, R.; Reis, C.; Braga, L.; de Andrade, J.M.; do Nascimento, L.G.; Mattoso, J.M.V.; Forsythe, S.J.; da Costa, L.V.; et al. Evaluation of MALDI-TOF MS, sequencing of D2 LSU rRNA and internal transcribed spacer regions (ITS) for the identification of filamentous fungi isolated from a pharmaceutical facility. J. Pharm. Biomed. Anal. 2023, 234, 115531. [Google Scholar] [CrossRef]
- Houbraken, J.; Visagie, C.M.; Frisvad, J.C. Recommendations To prevent taxonomic misidentification of genome-sequenced fungal strains. Microbiol. Resour. Announc. 2021, 10, e01074-20. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, M.A.; Fernandez, J.P.; Rouet, R.; Vejnar, C.E.; Lane, M.A.; Mis, E.; Khokha, M.K.; Doudna, J.A.; Giraldez, A.J. CRISPR-Cpf1 mediates efficient homology-directed repair and temperature-controlled genome editing. Nat. Commun. 2017, 8, 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, X.; Zhang, H.; Ishfaq, S.; Xi, K.; Zhou, X.; Yang, X.; Guo, W. Rapid and sensitive detection of toxigenic Fusarium asiaticum integrating recombinase polymerase amplification, CRISPR/Cas12a, and lateral flow techniques. Int. J. Mol. Sci. 2023, 24, 14134. [Google Scholar] [CrossRef] [PubMed]
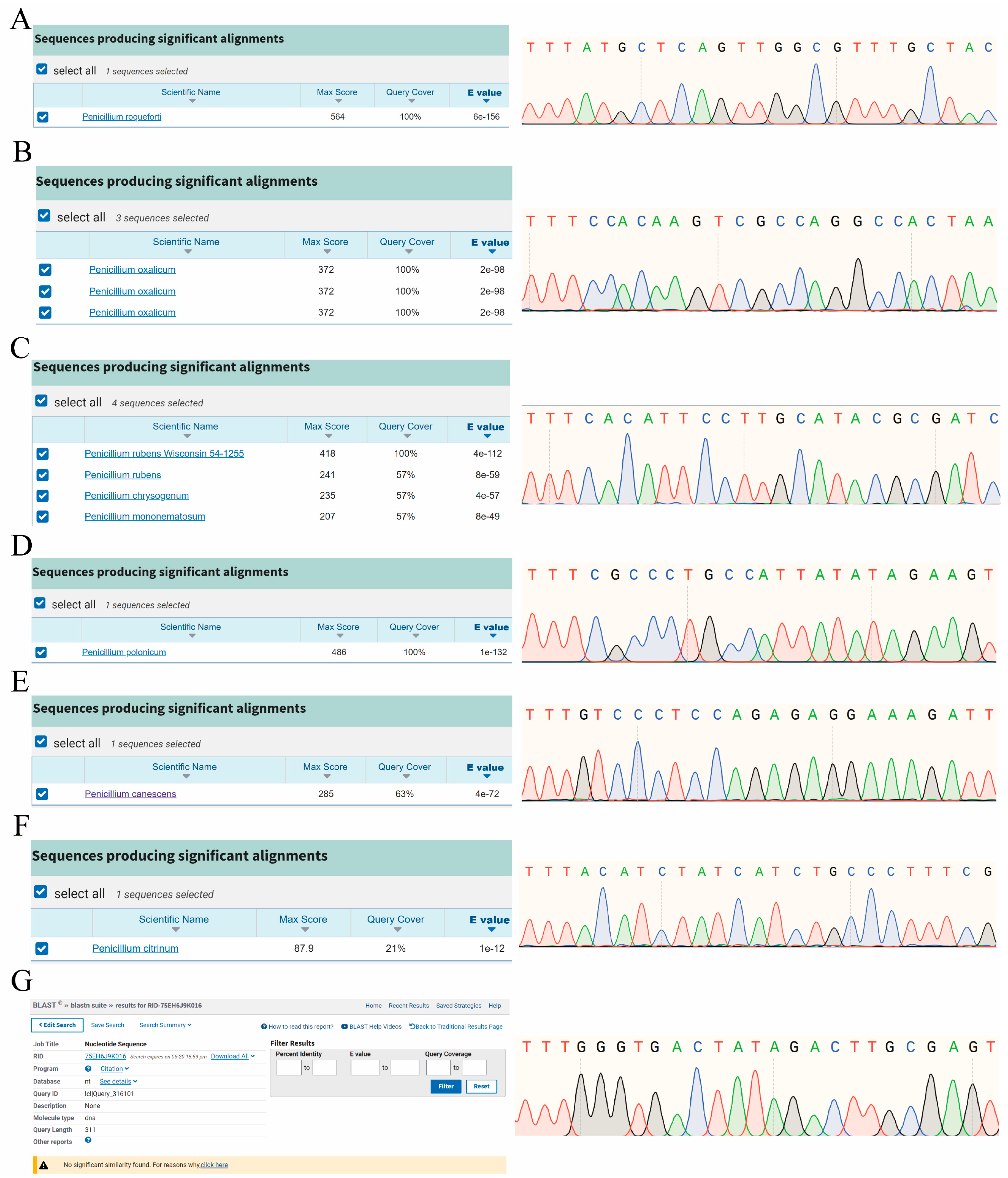
| Species | No. | Specific Target (5′→3′) |
|---|---|---|
| P. canescens | Pcan_target | TTTGTCCCTCCAGAGAGGAAAGATT |
| P. oxalicum | Pox_target | TTTCCACAAGTCGCCAGGCCACTAA |
| P. citrinum | Pcit_target | TTTACATCTATCATCTGCCCTTTCG |
| P. paneum | Ppan_target | TTTGGGTGACTATAGACTTGCGAGT |
| P. roqueforti | Proq_target | TTTATGCTCAGTTGGCGTTTGCTAC |
| P. rubens | Prub_target | TTTCACATTCCTTGCATACGCGATC |
| P. polonicum | Ppol_target | TTTCGCCCTGCCATTATATAGAAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Fu, L.; Gan, Y.; Qi, G.; Hao, L.; Xin, T.; Xu, W.; Song, J. Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value. Int. J. Mol. Sci. 2024, 25, 8172. https://doi.org/10.3390/ijms25158172
Huang Y, Fu L, Gan Y, Qi G, Hao L, Xin T, Xu W, Song J. Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value. International Journal of Molecular Sciences. 2024; 25(15):8172. https://doi.org/10.3390/ijms25158172
Chicago/Turabian StyleHuang, Yuanhao, Lianguo Fu, Yutong Gan, Guihong Qi, Lijun Hao, Tianyi Xin, Wenjie Xu, and Jingyuan Song. 2024. "Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value" International Journal of Molecular Sciences 25, no. 15: 8172. https://doi.org/10.3390/ijms25158172
APA StyleHuang, Y., Fu, L., Gan, Y., Qi, G., Hao, L., Xin, T., Xu, W., & Song, J. (2024). Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value. International Journal of Molecular Sciences, 25(15), 8172. https://doi.org/10.3390/ijms25158172






