Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1
Abstract
1. Introduction
2. Results
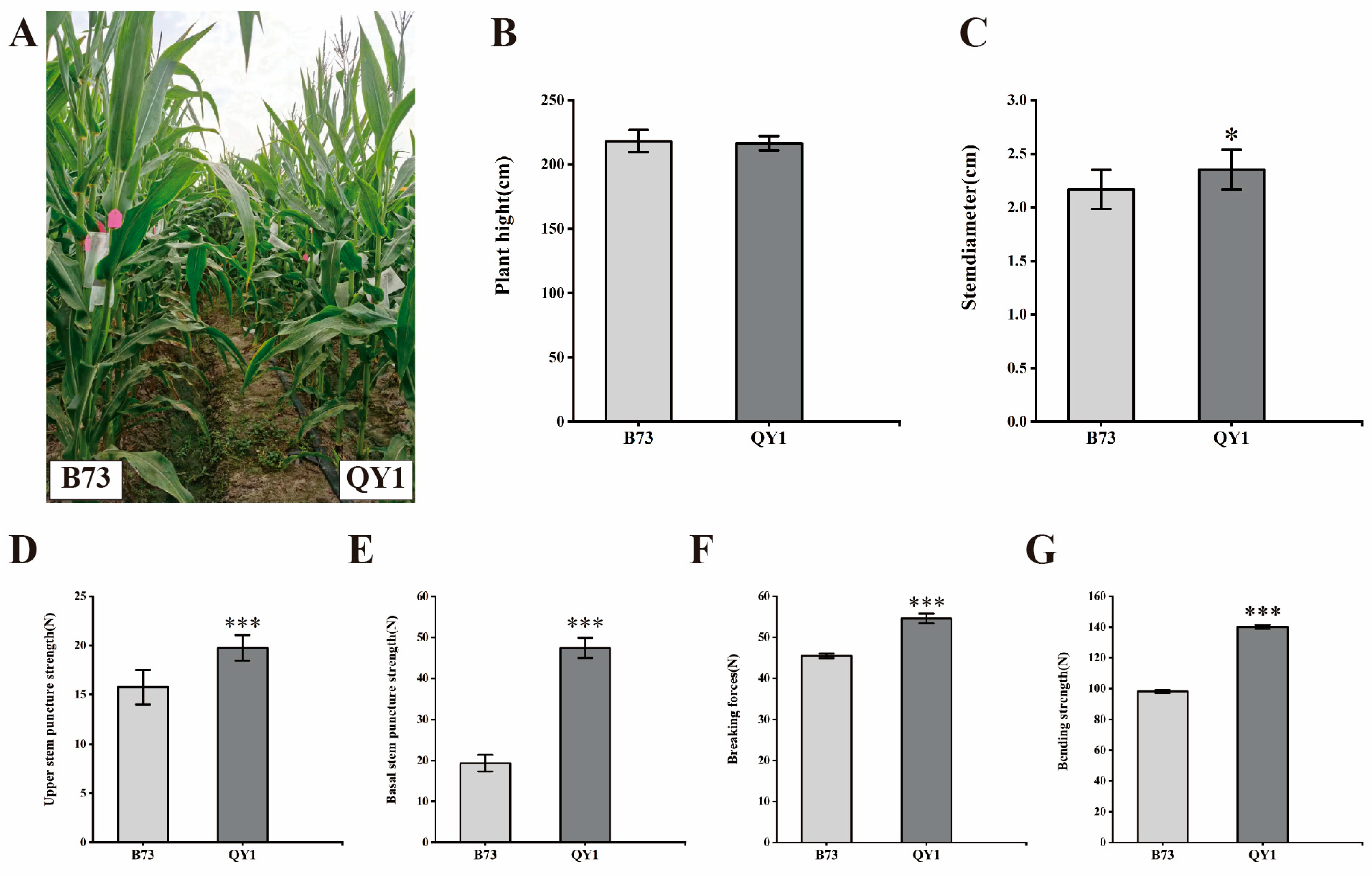
2.1. Enhanced Mechanical Strength in Maize Inbred Line QY1
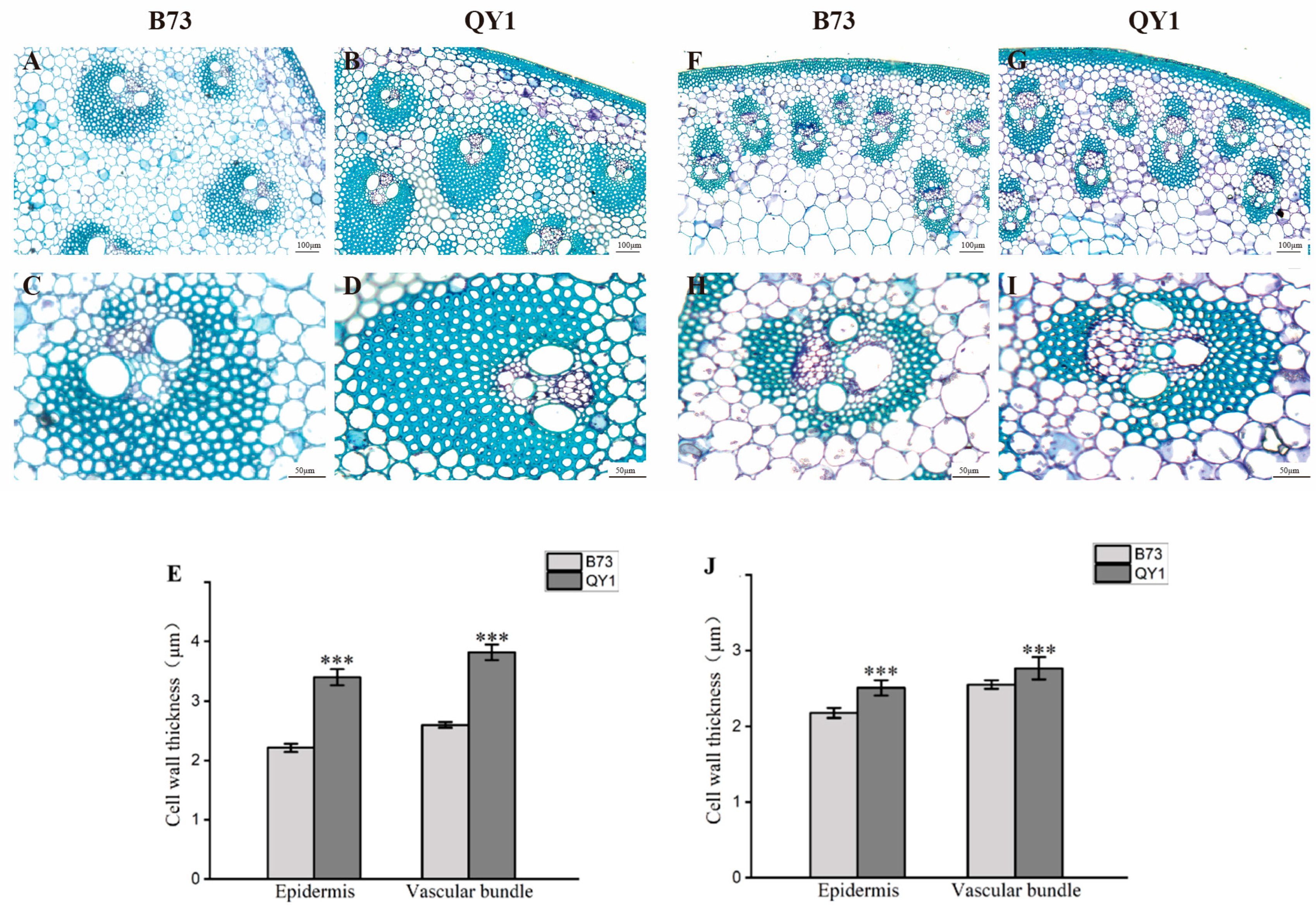
2.2. Increased Cell Wall Thickness in QY1 Stem Vascular Bundles
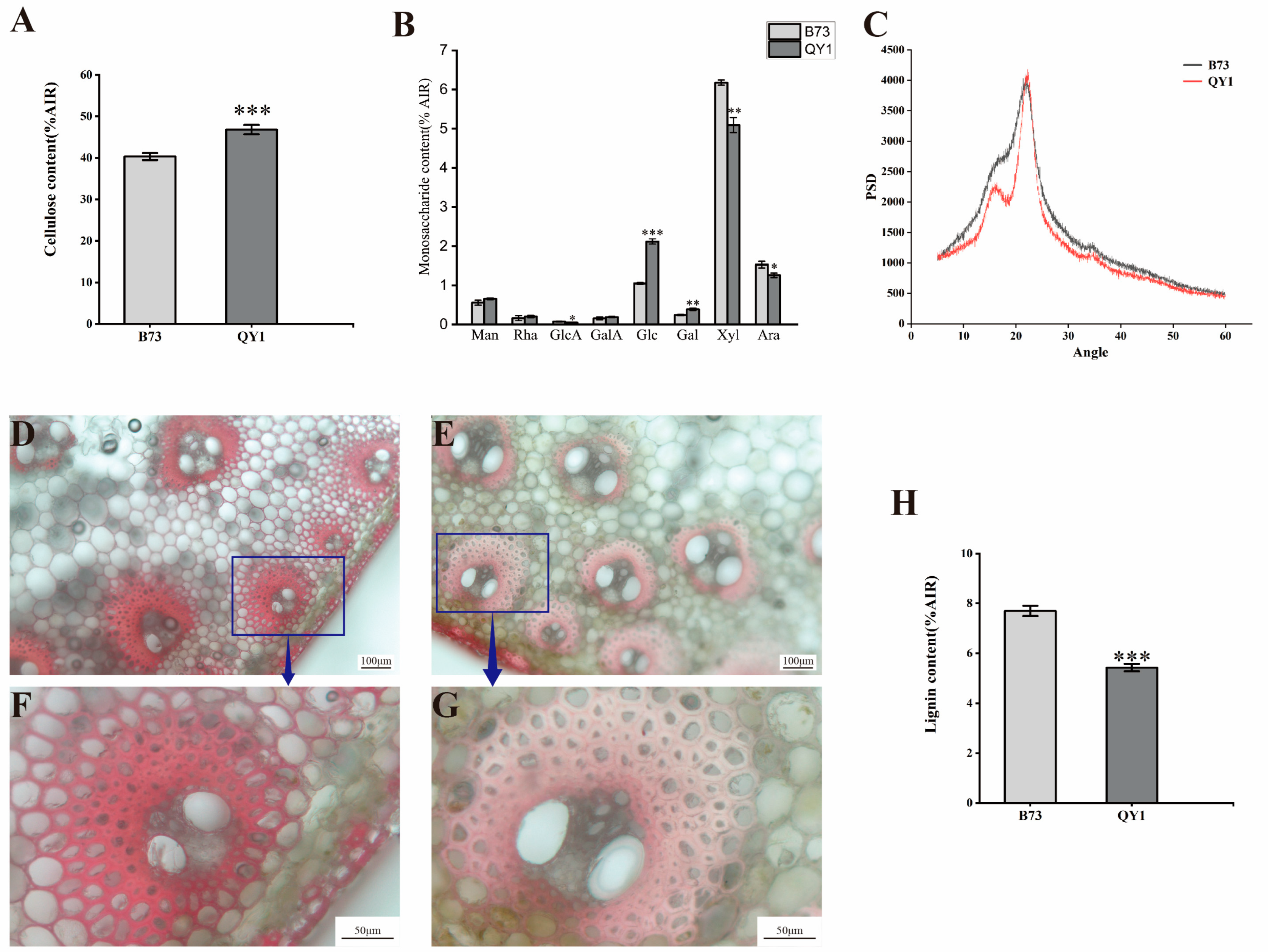
2.3. Increased Cellulose Content and Decreased Lignin Content in QY1 Stalks
2.4. Increased Saccharification Efficiency in Inbred Line QY1
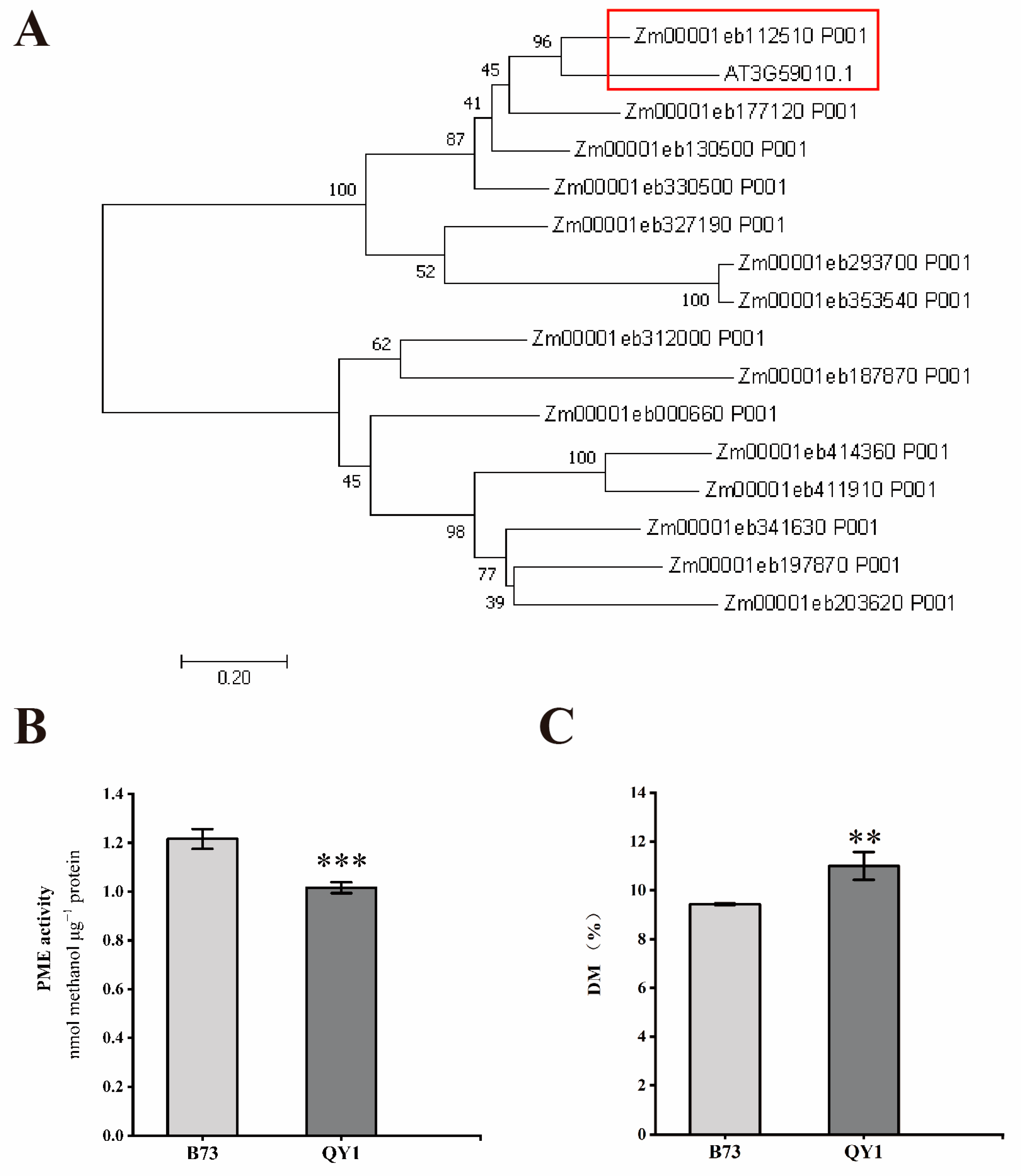
2.5. QY1 Influences Stem Strength through Gene Regulation
2.6. Changes in Pectin Methylation and Methylesterase Activity in QY1
3. Discussion
3.1. Increased Cell Wall Thickness in QY1 Enhances Mechanical Strength of Stalks
3.2. Significant Changes in QY1 Cell Wall Components
3.3. Application of QY1 to Agricultural Production
4. Materials and Methods
4.1. Experimental Material
4.2. Determination of Plant Morphology and Mechanics
4.3. Microscopy Analysis
4.4. Phloroglucinol–HCl Stain
4.5. Extraction of Alcohol-Insoluble Residue
4.6. Determination of Cellulose and Lignin Content
4.7. Determination of Pectin Methylesterase (PME) Activity
4.8. Determination of the Degree of Pectin Methylesterification (DME)
4.9. Matrix Polysaccharide Composition Analysis
4.10. Determination of Cell Wall Saccharification Efficiency
4.11. Transcriptome Analysis
4.12. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, J.; Gao, S.; Fan, Y.; Li, L.; Ming, B.; Wang, K.; Xie, R.; Hou, P.; Li, S. Traits of plant morphology, stalk mechanical strength, and biomass accumulation in the selection of lodging-resistant maize cultivars. Eur. J. Agron. 2020, 117, 126073. [Google Scholar] [CrossRef]
- Coen, E.; Cosgrove, D.J. The mechanics of plant morphogenesis. Science 2023, 379, eade8055. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Lamalakshmi Devi, E.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Wani Adaptation strategies and defence mechanisms of plants during environmental stress. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–413. [Google Scholar]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Wang, X.; Durachko, D.M.; Zhang, S.; Cosgrove, D.J. Molecular insights into the complex mechanics of plant epidermal cell walls. Science 2021, 372, 706–711. [Google Scholar] [CrossRef]
- Hu, Y.; Javed, H.H.; Du, Y.L.; Liao, Q.-W.; Ye, W.; Zhou, J.; Peng, X.; Arslan, M.; Raza, A.; Wu, Y.-C. Improving lignin metabolism, lodging resistance, and yield of rapeseed (Brassica napus, L.) by applying straw-fermented fertilizer. J. Soil Sci. Plant Nutr. 2023, 23, 2832–2848. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Li, J.; Gao, T.; Wu, S.; Wu, L.; Lai, X.; Xu, H.; Hu, H.; Ma, Y. Effects of Straw Returning and New Fertilizer Substitution on Rice Growth, Yield, and Soil Properties in the Chaohu Lake Region of China. Plants 2024, 13, 444. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-generation bioethanol production from corncob–A comprehensive review on pretreatment and bioconversion strategies, including techno-economic and lifecycle perspective. Ind. Crop. Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Eudes, A.; Liang, Y.; Mitra, P.; Loqué, D. Lignin bioengineering. Curr. Opin. Biotechnol. 2014, 26, 189–198. [Google Scholar] [CrossRef]
- Qi, G.; Wang, D.; Yu, L.; Tang, X.; Chai, G.; He, G.; Ma, W.; Li, S.; Kong, Y.; Fu, C.; et al. Metabolic engineering of 2-phenylethanol pathway producing fragrance chemical and reducing lignin in Arabidopsis. Plant Cell Rep. 2015, 34, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Dalton, S.; Roberts, L.A.; Moron-Garcia, O.M.; Iacono, R.; Kosik, O.; Gallagher, J.A.; Bosch, M. Modified expression of ZmMYB167 in Brachypodium distachyon and Zea mays leads to increased cell wall lignin and phenolic content. Sci. Rep. 2019, 9, 8800. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Verma, K.K.; Rajput, V.D.; Minkina, T.; Arora, J. Recent advances in metabolic engineering of microorganisms for advancing lignocellulose-derived biofuels. Bioengineered 2022, 13, 8135–8163. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, Y.; Deng, L.; Li, F.; Wang, Z.; Zhu, Y.; Wu, Y.; Qu, H.; Zhang, S.; Liu, Y.; et al. The transcription factor MYB110 regulates plant height, lodging resistance, and grain yield in rice. Plant Cell 2024, 36, 298–323. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Li, R.; Qian, Q.; Song, X.; Liu, X.; Yu, Y.; Zeng, D.; Wan, J.; Li, J.; Zhou, Y. The rice dynamin-related protein DRP2B mediates membrane trafficking, and thereby plays a critical role in secondary cell wall cellulose biosynthesis. Plant J. 2010, 64, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Hazebroek, J.P.; Chamberlin, M.A.; Perkins, M.; Sandhu, A.S.; Gupta, R.; Simcox, K.D.; Yinghong, L.; Prall, A.; Heetland, L.; et al. Chitinase-like1 plays a role in stalk tensile strength in maize. Plant Physiol. 2019, 181, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, A.; Langewisch, T.; Olek, A.; Multani, D.S.; McCann, M.C.; Vermerris, W.; Carpita, N.C.; Johal, G. Maize Brittle stalk2 encodes a COBRA-like protein expressed in early organ development but required for tissue flexibility at maturity. Plant Physiol. 2007, 145, 1444–1459. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Sakamoto, S.; Mitsuda, N.; Ren, A.; Persson, S.; Zhang, D. ETHYLENE RESPONSE FACTOR 34 promotes secondary cell wall thickening and strength of rice peduncles. Plant Physiol. 2022, 190, 1806–1820. [Google Scholar] [CrossRef]
- Taylor, N.G.; Laurie, S.; Turner, S.R. Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell 2000, 12, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.G.; Howells, R.M.; Huttly, A.K.; Vickers, K.; Turner, S.R. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, B.; Dai, Y.; Zhang, L.; Shang-Guan, K.; Peng, Y.; Zhou, Y.; Zhu, Z. Brittle culm15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice. Plant Physiol. 2012, 159, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Wang, Y.; Wang, M.; Cao, L.; Ruan, N.; Huang, Y.; Li, F.; Xu, Q.; Chen, W. The Fragile culm19 (FC19) mutation largely improves plant lodging resistance, biomass saccharification, and cadmium resistance by remodeling cell walls in rice. J. Hazard. Mater. 2023, 458, 132020. [Google Scholar] [CrossRef] [PubMed]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Höfte, H.; Gonneau, M.; Vernhettes, S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar] [CrossRef] [PubMed]
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell 2012, 24, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Liu, Y.; Wang, T.J.; Meng, Q.; Yin, H.; Wang, X.; Wu, Y.; Nan, N.; Liu, B.; Xu, Z.-Y. GOLDEN2-LIKE transcription factors regulate WRKY40 expression in response to abscisic acid. Plant Physiol. 2019, 179, 1844–1860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L.; Tian, X.; Liu, S.; Xu, D.; Jin, H.; Song, J.; Dong, Y.; Zhao, D.; Li, G.; et al. TaNAC100 acts as an integrator of seed protein and starch synthesis exerting pleiotropic effects on agronomic traits in wheat. Plant J. 2021, 108, 829–840. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Y.; Yang, X.; Yu, Y.; Li, J.; Zhao, C.; Yuan, M.; Huang, W.; Yin, Y.; Liu, G.; et al. Knockout of ZmNST2 promotes bioethanol production from corn stover. Plant Biotechnol. J. 2024; Online ahead of print. [Google Scholar]
- Pomar, F.; Merino, F.; Barceló, A.R. O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma 2002, 220, 17–28. [Google Scholar] [CrossRef]
- Foster, C.E.; Martin, T.M.; Pauly, M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part I: Lignin. JoVE (J. Vis. Exp.) 2010, 37, e1745. [Google Scholar]
- Peng, X.; Qiao, W.; Mi, S.; Jia, X.; Su, H.; Han, Y. Characterization of hemicellulase and cellulase from the extremely thermophilic bacterium Caldicellulosiruptor owensensis and their potential application for bioconversion of lignocellulosic biomass without pretreatment. Biotechnol. Biofuels 2015, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.; Piro, G.; Bellincampi, D. Three pectin methylesterase inhibitors protect cell wall integrity for Arabidopsis immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Yu, L.; Zhang, X.; Li, S.; Liu, X.; Sun, L.; Liu, H.; Iteku, J.; Zhou, Y.; Tai, G. Rhamnogalacturonan I domains from ginseng pectin. Carbohydr. Polym. 2010, 79, 811–817. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, W.; He, F.; Qi, T.; Sun, Z.; Liu, Y.; Bai, S.; Wang, H.; Wu, Z.; Fu, C. Down-regulation of PvSAMS impairs S-adenosyl-L-methionine and lignin biosynthesis, and improves cell wall digestibility in switchgrass. J. Exp. Bot. 2022, 73, 4157–4169. [Google Scholar] [CrossRef]


| Gene Number | Comment | log2 Fold Change |
|---|---|---|
| Zm00001eb293700 | pectin methylesterase37 | 4.381 |
| Zm00001eb130500 | pectin methylesterase19 | 3.458 |
| Zm00001eb187870 | xyloglucan:xyloglucosyl transferase activity | 3.421 |
| Zm00001eb330500 | pectin methylesterase20 | 3.294 |
| Zm00001eb226490 | xyloglucan endo-transglycosylase/hydrolase4 | 2.558 |
| Zm00001eb112510 | pectin methylesterase12 | 1.612 |
| Zm00001eb353540 | pectin methylesterase38 | 1.591 |
| Zm00001eb197870 | xyloglucan endo-transglycosylase/hydrolase6 | 1.348 |
| Zm00001eb177120 | pectin methylesterase15 | 1.278 |
| Zm00001eb411910 | xyloglucan endo-transglycosylase/hydrolase2 | 1.238 |
| Zm00001eb341630 | xyloglucan:xyloglucosyl transferase activity | 1.071 |
| Zm00001eb190890 | −2.232 | |
| Zm00001eb312000 | xyloglucan:xyloglucosyl transferase activity | −2.363 |
| Zm00001eb414360 | xyloglucan endo-transglycosylase/hydrolase7 | −2.501 |
| Zm00001eb000660 | ring finger and WD40 repeat3 | −2.699 |
| Zm00001eb327190 | pectin methylesterase41 | −4.286 |
| Zm00001eb203620 | xyloglucan:xyloglucosyl transferase activity | −5.282 |
| Zm00001eb414390 | xyloglucan endo-transglycosylase/hydrolase1 | −6.566 |
| Gene Number | Comment | log2 Fold Change |
|---|---|---|
| Zm00001eb097410 | CesA3 | 7.88167584 |
| Zm00001eb349960 | CesA3 | 7.74899559 |
| Zm00001eb248410 | CesA2 | 4.276134325 |
| Zm00001eb046440 | CesA10 | −1.139029969 |
| Zm00001eb097100 | CesA6 | −1.99327018 |
| Zm00001eb370930 | CesA14 | −2.746385239 |
| Zm00001eb370940 | CesA8 | −3.092633023 |
| Zm00001eb043490 | cellulose synthase (UDP-forming) activity | −5.400036017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Mu, J.; Hao, X.; Yang, K.; Cao, Z.; Feng, J.; Li, R.; Zhang, N.; Zhou, G.; Kong, Y.; et al. Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1. Int. J. Mol. Sci. 2024, 25, 8195. https://doi.org/10.3390/ijms25158195
Yang Y, Mu J, Hao X, Yang K, Cao Z, Feng J, Li R, Zhang N, Zhou G, Kong Y, et al. Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1. International Journal of Molecular Sciences. 2024; 25(15):8195. https://doi.org/10.3390/ijms25158195
Chicago/Turabian StyleYang, Yumeng, Jianing Mu, Xiaoning Hao, Kangkang Yang, Ziyu Cao, Jiping Feng, Runhao Li, Ning Zhang, Gongke Zhou, Yingzhen Kong, and et al. 2024. "Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1" International Journal of Molecular Sciences 25, no. 15: 8195. https://doi.org/10.3390/ijms25158195
APA StyleYang, Y., Mu, J., Hao, X., Yang, K., Cao, Z., Feng, J., Li, R., Zhang, N., Zhou, G., Kong, Y., & Wang, D. (2024). Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1. International Journal of Molecular Sciences, 25(15), 8195. https://doi.org/10.3390/ijms25158195








