Abstract
Worldwide, the incidence of renal cell carcinoma (RCC) is rising, accounting for approximately 2% of all cancer diagnoses and deaths. The etiology of RCC is still obscure. Here, we assessed the presence of HPyVs in paraffin-embedded tissue (FFPE) resected tissue from patients with RCC by using different molecular techniques. Fifty-five FFPE tissues from 11 RCC patients were included in this study. Consensus and HPyV-specific primers were used to screen for HPyVs. Both PCR approaches revealed that HPyV is frequently detected in the tissues of RCC kidney resections. A total of 78% (43/55) of the tissues tested were positive for at least one HPyV (i.e., MCPyV, HPyV6, HPyV7, BKPyV, JCPyV, or WUyV). Additionally, 25 tissues (45%) were positive for only one HPyV, 14 (25%) for two HPyVs, 3 (5%) for three HPyVs, and 1 one (1%) tissue specimen was positive for four HPyVs. Eleven (20%) RCC specimens were completely devoid of HPyV sequences. MCPyV was found in 24/55 RCC tissues, HPyV7 in 19, and HPyV6 in 8. The presence of MCPyV and HPyV6 was confirmed by specific FISH or RNA-ISH. In addition, we aimed to confirm HPyV gene expression by IHC. Our results strongly indicate that these HPyVs infect RCC and nontumor tissues, possibly indicating that kidney tissues serve as a reservoir for HPyV latency. Whether HPyVs possibly contribute to the etiopathogenesis of RCC remains to be elucidated.
Keywords:
Merkel cell polyomavirus; HPyV7; HPyV6; BKPyV; JCPyV; WUPyV; small DNA viruses; RCC; adjacent tissues; tumorigenesis 1. Introduction
Kidney cancer accounts for approximately 2% of all human cancers and causes an estimated 179.000 cancer-related deaths each year. This makes kidney cancer one of the most common causes of cancer-related deaths worldwide [1]. With approximately 70–80%, renal cell carcinoma (RCC) constitutes the most common type of kidney cancer [2,3]. Worldwide, RCC is the 6th most common cancer in men and the 9th most common cancer in women [4]. Clear cell renal cell carcinoma (CCRCC) and papillary renal cell carcinoma (PRCC) are the most common and widespread subtypes of RCC [5]. Among others, hereditary diseases, e.g., von Hippel-Lindau (VHL) disease, smoking, obesity, hypertension, and immunosuppression of solid organ transplant recipients have been identified to be associated with an increased risk to develop RCC [6,7,8,9,10]. Tumor virus-related human malignancies have extensively been studied [11,12,13]. The presence of nucleic acid sequences of Epstein–Barr virus (EBV), human papillomaviruses (HPVs), hepatitis C virus (HCV), human polyoma-viruses BK (BKPyV), and JC (JCPyV) in human neoplastic kidney tissues has been previously reported [14,15,16,17,18,19,20,21]. The latter belong to the family of human polyomaviruses (HPyVs), which are small DNA viruses, both of which [22] were identified in 1971 [23,24]. Since then, they have been suspected to contribute to tumorigenesis in humans, but no convincing role for either has yet been demonstrated [25]. BKPyV and JCPyV enter latency after primary infection in renal tubular epithelial cells and, to a lesser extent in other cell types, can be reactivated, e.g., due to immunodeficiency or immunosuppression [26,27,28]. BKPyV reactivation in immunocompromised individuals is associated with hemorrhagic cystitis, BKPyV nephropathy, and ureteral stenosis, whereas JCPyV is associated with progressive multifocal leukoencephalopathy (PML) [23,24,29]. Little is known about the mechanisms by which these viruses remain latent in their hosts and are reactivated from latency [30]. To date, 12 HPyVs have been identified [22,31,32], of which only Merkel cell polyomavirus (MCPyV) has so far been identified as a human tumor virus [33,34,35].
In this study, our objective was to determine the presence and distribution of HPyVs in RCC and adjacent non-neoplastic tissues. We tested the presence of HPyVs in formalin-fixed and paraffin-embedded (FFPE) RCC tissues, including four differentially spaced (transition, 1, 2, and 3 cm; Figure 1) adjacent non-neoplastic tissues from each of these RCCs, by HPyV consensus PCR. In addition, HPyV-specific PCRs, -FISH, -RISH, and -immunohistochemistry were performed.
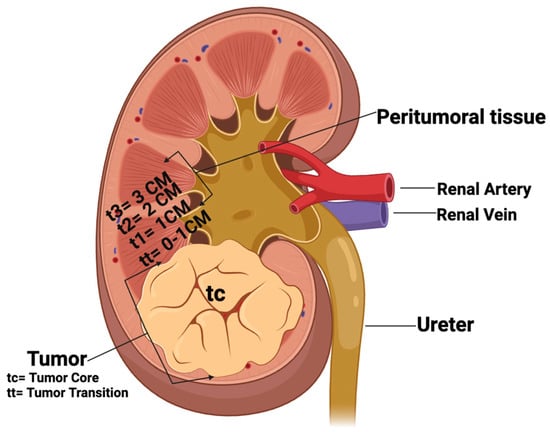
Figure 1.
Schematic representation of tissue sampling of RCC and non-tumoral kidney tissues. tc: tumor core; tt: tumor transition; t1: 1 cm, t2: 2 cm, and t3: 3 cm distance to tumor. (Created by Bio—render.com).
2. Results
2.1. Human Polyomaviruses—DNA PCR
Prior to PCR, all isolated DNAs of the FFPE blocks were tested and showed sufficient DNA quality and integrity (PCR products > 300 bp).
2.1.1. HPyV Consensus PCR
The 55 RCC tissues—sampled according to the scheme shown in Figure 1—were screened for the presence of HPyVs DNA by consensus PCR (Figure 2).
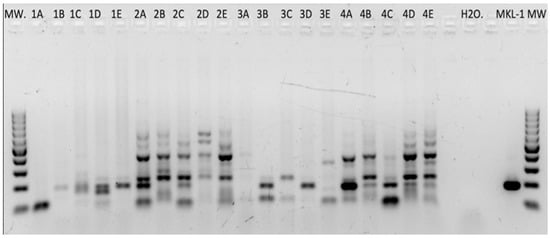
Figure 2.
HPyV consensus DNA PCR. Multiple PCR products after amplifying DNA of RCC tissues with HPyV-consensus primers. On the far right, the MKL-1-positive control reveals a specific PCR product at the expected size of 186 bp. All PCR products in the range of 150 to 220 bp were sequenced and analyzed.
Of these, 34/55 (61%) tissues were positive for one HPyV sequence. In detail, MCPyV DNA was found in 21 tissues (38%), HPyV7 DNA in 9 (16%), and HPyV6 in 3 (5%), followed by WUPyV DNA in 1 (1%) (Table 1 and Table 2, Figure 3A). No BKPyV and JCPyV was detected by HPyV consensus PCR.

Table 1.
HPyV-PCR primers used in this study; HPyV6, human polyomavirus 6; HPyV7, human polyoma virus 7; MCPyV, Merkel cell polyomavirus; sTAg, small tumor antigen; LTAg, large tumor antigen; LT, large antigen; VP, viral protein; M1/M2, the common region between sTAg and LTAg; JCPyV, JC polyomavirus; BKPyV, BK polyomavirus; bp, base pairs.

Table 2.
Summary of the clinicopathological data and DNA PCR results of all RCC tissues used in this study; M, male; F, female; CCRCC, clear cell renal cell carcinoma; PRCC, papillary renal cell carcinoma; bp, base pairs; N.D., not determined; +, positive immunoreactivity; (), weak; −, negative immunoreactivity; *, samples were blind tested to perform experiment.
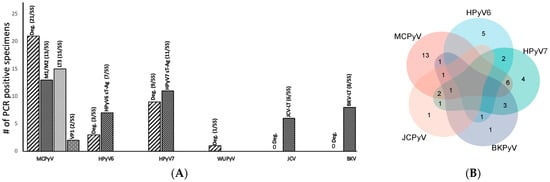
Figure 3.
(A) Summary of the total HPyV PCR results for both consensus and HPyV-specific DNA PCR in 55 tissues. (B) Venn diagram showing cases with simultaneous different HPyVs as tested by consensus and specific PCR.
Mapping the presence of the different HPyV DNAs according to the sampling scheme (Figure 1), we found that 5/11 RCC samples (45%) from the tumoral core (tc) were positive for one HPyV (3 MCPyV,1 HPyV7, and 1 WUPyV), while in the tumoral transition (tt) 7/11 (63%) tissues contained HPyV (4 MCPyV and 3 HPyV6). Most HPyVs were detected in the non-tumoral RCC tissues (t1–t3) 22/33 (66%), with MCPyV (n = 14) more prevalent than HPyV7 (n = 8) DNA (data summarized in Table 3).

Table 3.
Summary of the sequencing results of the PCR products of the HPyV-consensus and specific PCRs according to their distribution in the kidney resections, as shown in Figure 1; (tc: tumor core; tt: tumor transition; t1: 1 cm; t2: 2 cm; t3: 3 cm distance to tumor core); CM: Centimeter.
2.1.2. HPyV-Specific PCRs
The results of the HPyV-specific PCRs were generally in agreement with the results of the HPyV consensus PCR. By HPyV-specific PCR, 17 (30%) tested tissues were MCPyV-positive, 11 (20%) for HPyV7, and 8 (14%) for HPyV6 (Table 2, Figure 3A). JCPyV sequences were found in 10% (6/55) of the tissues, and BKPyV sequences in 14% (8/55).
2.1.3. Combined PCR Results (HPyV Consensus PCR and HPyV-Specific PCRs)
Both PCR approaches combined reveal that HPyVs are frequently detected in the tissue of RCC kidney resections. Additionally, 78% (43/55) of the tissues tested were positive for at least one HPyV (i.e., MCPyV, HPyV6, HPyV7, BKPyV, JCPyV, and WUyV). Further, 25 tissues (45%) were positive for only one HPyV, 14 (25%) for two HPyVs, 3 (5%) for three HPyVs, and only 1 (1%) tissue specimen was positive for four HPyVs (Figure 3A,B). Of interest, 11 (20%) RCC specimens were completely devoid of HPyV sequences in both PCR approaches (Table 2). Overall, both PCR approaches combined reveal that MCPyV was seen in 24/55 RCC tissues, HPyV7 in 19, and HPyV6 in 8 (Figure 3B). Of interest, 9 RCC specimens harbor both MCPyV and HPyV7, while 2 tissue specimens were positive for HPyV6 and HPyV7.
No significant association was found with the distribution of the detected HPyV sequences and RCC tumor tissue. However, HPyV sequences were more frequently found in the adjacent peritumoral kidney tissues compared to RCC tumor tissues (Table 3).
2.2. Fluorescence In Situ Hybridization (FISH)
Selected cases of MCPyV-DNA PCR-positive (n = 3) and HPyV6-DNA PCR-positive (n = 4) tissues were tested by using FISH. In these tissues (no. 3, 42, 48), specific haphazardly distributed focal punctate nuclear dots were seen for MCPyV (Table 2, Figure 4A), as compared to the positive and negative controls. All results for MCPyV FISH were in agreement with MCPyV DNA PCR and IHC. In addition, 9, 12, 17, and 32 were screened by HPyV6 FISH, and specific haphazardly distributed focal punctate nuclear dots were seen, which also match with HPyV6 DNA PCR and IHC (Table 2, Figure 4B).
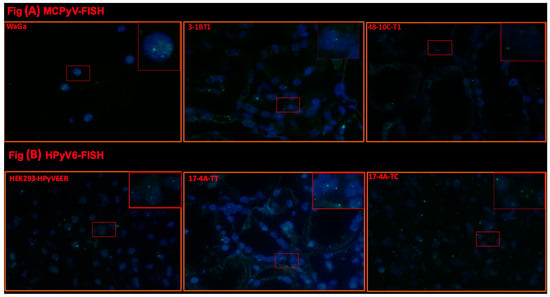
Figure 4.
(A) Detection of MCPyV on DNA in FFPE of RCC and adjacent tissues. Merged green (FITC) and blue (nuclei were counterstained with DAPI) show specific renal cells. Merged green (FITC) and blue (nuclei were counterstained with DAPI) show specific green signals. Example of results of FISH specific nuclear MCPyV in the nuclei of epithelial RCC and the adjacent tissue. WaGa cell lines served as a positive control for the MCPyV probe. The images were taken at 630× magnification, and a red square area was magnified 6× in the top right corner of each figure. (B) Detection of HPyV6 on the DNA in FFPE of RCC and adjacent tissues. Merged green (FITC) and blue (nuclei were counterstained with DAPI) show specific renal cells. Merged green (FITC) and blue (nuclei were counterstained with DAPI) show specific green signals. Example of results of FISH specific nuclear HPyV6 in the nuclei of epithelial RCC and the adjacent tissue. HEK-HPyV6 cell lines served as a positive control for the HPyV6 probe. The images were taken at 630× magnification, and a red square area was magnified 6× in the top right corner of each figure. The representative cases shown above correspond to the case numbers listed in Table 2.
2.3. RNA In Situ Hybridization (RISH)
This technique was also employed for RCC tissues which revealed positivity in consensus, specific DNA PCR, and IHC in both MCPyV (n = 3) and HPyV6 (n = 2) to double confirm and validate the presence of these HPyVs in our RCC cohort from a transcriptional level. Three patients (no. 3, 42, and 48) of MCPyV-RCC-positive specimens (PCR and IHC) showed positive punctate dots (Table 2, Figure 5A). In addition, HPyV6-RCC-positive specimens (PCRs and IHC) also showed some faint and positive punctate dots (no. 12 and 17) (Table 2, Figure 5B).
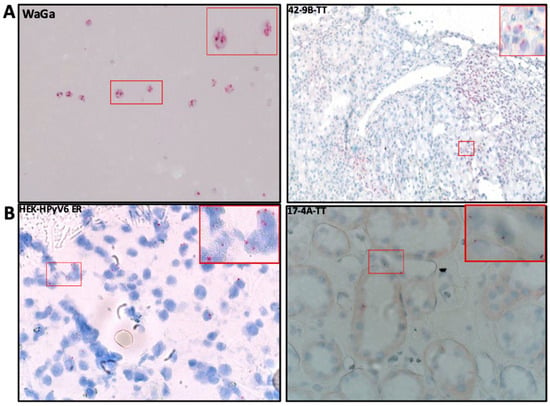
Figure 5.
(A) Detection of MCPyV on the transcriptional level in FFPE RCC tissue by RNA-ISH; the RCC patient tissue section was hybridized with 20 set labeled probes to detect MCPyV LTAg mRNA using an RNAscope RNA in situ hybridization assay. WaGa cell lines served as a positive control for the MCPyV probe. Positive red signals were detected using fast red chromogen. MCPyV LTA transcript seen as red signals were detected using fast red chromogen. MCPyV LTA transcript seen as red signals in RCC and adjacent tissue. The images were taken at 200× magnification, and a red square area was magnified 6× in the top right corner of each figure. (B) Detection of HPyV6 on the transcriptional level in FFPE RCC tissue by RNA-ISH; the RCC patient tissue section was hybridized with 20 set labeled probes to detect HPyV6 LTAg mRNA using an RNAscope RNA in situ hybridization assay. HEK-HPyV6 cell lines served as a positive control for the HPyV6 probe. Positive red signals were detected using fast red chromogen. HPyV6 LTA transcript seen as red signals were detected using fast red chromogen. HPyV6 LTA transcript seen as red signals in RCC and adjacent tissue. The images were taken at 400× magnification, and a red square area was magnified 6× in the top right corner of each figure. The representative cases shown above correspond to the case numbers listed in Table 2.
2.4. HPyV LT-Ag Immunohistochemistry
2.4.1. MCPyV-LTAg
Nuclear CM2B4 LT-Ag immunoreactivity was found in 27/55 (49%) of RCC and non-tumoral RCC tissues (Table 2). Of interest, 23/27 (85%) were overall in agreement with the results of the consensus PCR and/or MCPyV-specific PCRs (M1/M2, LT3, and VP1) (Table 2). Of note, 3/23 (13%) were detected in the tumor core (tc) of RCC as well 5/23 (21%) in tumor transition (tt). In line with the PCR results (Table 2, Figure 6A), 15/23 (65%) non-tumoral RCC tissues (t1–t3) revealed CM2B4 immunoreactivity. Surprisingly, four cases (no. 7, 17, 24, 32) revealed moderate-to-strong nuclear CM2B4 immunoreactivity without any evidence of MCPyV-DNA, as tested by consensus and MCPyV-specific PCR (Table 2, Figure 6A).
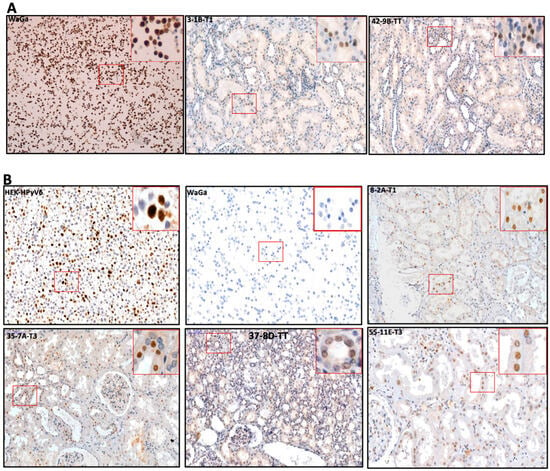
Figure 6.
(A) Detection of MCPyV on the translational level in FFPE of RCC and adjacent tissues. Representative examples of IHC using CM2B4 antibodies show the specific immunoreactivity in the nucleus (brown) of RCC tissues. WaGa cell line served as a positive for MCPyV antibodies. The images were taken at 200× magnification, and a red square area was magnified 6× in the top right corner of each figure. (B) Detection of HPyVs on the translational level in FFPE of RCC and adjacent tissues. Representative examples of IHC using PAb416 antibodies show the specific nuclear immunoreactivity (brown) of RCC tissues. HEK-HPyV6 cell lines were used as positive control, while WaGa cell line served as a negative for PAb416 antibodies. The images were taken at 200× magnification, and a red square area was magnified 6× in the top right corner of each figure. The representative cases shown above correspond to the case numbers listed in Table 2.
2.4.2. PAb416 LTAg Immunoreactivity
Nuclear PAb416 LT-Ag immunoreactivity was found in 22/55 (40%) of RCC and non-tumoral RCC tissues (Table 2). In agreement with the PCR results, 20 (90%) of the RCC tissues containing HPyV DNA (i.e., HPyV6, HPyV7, WUPyV, BKPyV, and JCPyV) (Table 2) revealed CM2B4 immunoreactivity. Of these, 6 (30%) were restricted to tumor transition (tt) and 13 (65%) in the non-tumoral RCC tissues (t1–t3). Interestingly, PAb416 LT-Ag immunoreactivity was found in only one (5%) RCC (tc) (Table 2, Figure 6B). Surprisingly, two cases (no. 10 and 31) revealed weak-to-moderate PAb416 LT-Ag immunoreactivity without any evidence of HPyV-DNA, as tested by HPyV-consensus or HPyV-specific PCR.
3. Discussion
In the present study, we used two different PCR screening tools to determine the presence of HPyV DNA in kidney tissues from RCC resection specimens, including four differentially spaced (transition, 1, 2, and 3 cm; Figure 1) adjacent non-neoplastic tissues.
Using HPyV consensus PCR and HPyV-specific DNA PCR, we frequently detected HPyV in the kidney tissues examined. Although the overall detection rates of HPyV consensus PCR and HPyV-specific PCR were in agreement, slightly more HPvV DNA tended to be found with the latter. Interestingly, in contrast to BKPyV- and JCPyV-specific PCR, HPyV DNA PCR did not detect either virus, although the consensus primers are also designed to amplify BKPyV and JCPyV. When assigning the combined PCR results to tissue localization, we find that HPyV DNA was more frequently found in the adjacent nontumoral tissues and less frequently in the RCC tumor tissues (MCPyV in 14 (25%) compared to HPyV7 in 8 (14%) in the nontumoral tissues). The findings of HPyV DNA in our tissue collection are only partly in line with a recent report by Pyöriä et al. [19] assessing the tissue-resident eukaryotic DNA virome in humans. By integration of quantitative (qPCR) and qualitative (hybrid-capture sequencing) analysis, the authors identified HPyV6 in 84%, JCPyV in 39%, BKPyV and MCPyV in 3%. In this study, we found a surprisingly high percentage of MCPyV DNA-positive tissues. However, the presence of MCPyV DNA has been previously detected in RCC tissues. Loyo et al. reported 19% MCPyV-positive RCCs tested by MCPyV-specific PCR. (19%; ref. [36]). The most likely explanations for these differences compared with the results of Loyo [36] and Pyöriä et al. [19] are the different technical approach and the patient group included. The study by Pyöriä et al. [19] did not include RCC patients.
Using MCPyV-FISH and RNAscope analyses, we detected viral DNA and RNA in RCC tissues (Figure 4A and Figure 5A, Table 2) at the single-cell level in a histomorphologic context, showing good agreement with the PCR results. These results confirm our findings not only at the DNA level but also at the RNA level. Moreover, in the same cases, IHC showed immunoreactivity, confirming the presence of MCPyV and possibly indicating MCPyV replication (Figure 6A). We used the anti-MCPyV-LTAg antibody CM2B4, which detects the expression of MCPyV-LTAg [37,38]. Interestingly, four tissues showed “specific” immunoreactivity for CM2B4, but were completely devoid of MCPyV nucleic acids in all other assays. This suggests that interpretation of CM2B4 IHC in tissues other than Merkel cell carcinoma should be taken with caution and should always be supported by at least one additional technical procedure, e.g., MCPyV-specific PCR, FISH, or RISH. A possible cross-reaction of CM2B4 with cellular antigens could explain this immunoreactivity in these tissues. Based on the results of HPyV consensus PCR, there is no evidence to suggest that a previously unknown PyV related to MCPyV could be the reason for this immunoreactivity.
HPyVs, particularly BKPyV and JCPyV, have long been suspected to be associated with tumorigenesis in human renal cell carcinoma [17,36,37,38,39,40,41,42]. However, a possible contribution of BKPyV to RCC tumorigenesis has only been demonstrated in a very limited number of cases by demonstrating viral genome integration into the tumor genome [43,44]. In the context of the high prevalence of BKPyV in the genitourinary system, especially in the transplant setting, this has been interpreted as a rare biological accident of viral DNA integration [43]. The overall finding of HPyV DNA, especially in the peritumoral non-neoplastic tissues in our study, together with the findings of Pyöriä et al. [19], possibly suggests reactivation of HPyV DNA secondary to RCC. The frequent detection of HPyV DNA in renal tissue from RCC, including results from FISH, RISH, and IHC, strongly suggests that renal tissues serve as a potential reservoir for HPyV latency in humans. It has been shown that BKPyV can infect the renal tubule epithelium and remain in latency there. Our results also strongly suggest that other HPyVs, e.g., MCPyV and HPyV6/7, also infect renal tubular epithelium and may remain in latency until possible reactivation, e.g., in the setting of immunosuppression.
Other interesting results of this study are that HPyV6 was restricted to the tumor transition (tt) region, and that only one RCC tested positive for WUPyV DNA in the tumor core of the RCC tissue (Table 3, Figure 3A). Of note, 9/55 (16%) RCC tissues were completely free of HPyV6 by all techniques.
On the protein level, the PAb416 IHC antibody detected HPyV-LTAg accumulation in non-tumoral tissues of RCC in twelve RCC cases. Six RCC cases also showed LTAg positivity in tumor transition. Only one case of RCC was positive in the tumor core (Table 2).
Our results suggest that MCPyV, HPyV7, HPyV6, BKPyV, JCPyV, and WUPyV can potentially infect both RCC and surrounding tumor tissues. In this study, all six HPyVs were shown to be present in kidney tissues. We observed that MCPyV and HPyV7 were more abundant in neoplastic and non-neoplastic cells than HPyV6, BKPyV, JCPyV, and WUPyV in our subset of RCC samples.
In summary, this study is the first to not only map HPyVs at various distances of RCC tissues but also report the presence of MCPyV and HPyV6 on a single-cell level. We utilized various molecular techniques to examine the presence of these viruses from DNA to protein levels. A role for these HPyVs in renal carcinogenesis based on the distribution of HPyVs in the RCC tissues investigated seems to be unlikely. Whether our results may suggest an indirect link between these HPyVs and carcinogenesis through, e.g., inflammation [45,46,47,48], needs further investigation. However, based on the HPyV distribution and heterogeneity, causal indications are currently insufficient. Importantly, MCPyV, HPyV7, HPyV6, and WUPyV are present in the human kidney tissues of RCC patients, pointing to the kidney as a latency reservoir of these HPyVs. The frequent finding of HPyVs, particularly MCPyV and HPyV7, in kidney tissues may also indicate a possible involvement of these in other kidney diseases.
4. Materials and Methods
4.1. Patients and Tissues
Formalin-fixed paraffin-embedded (FFPE) tissues from kidney resections of 11 RCC patients (3 female and 8 males; mean age 71.6 years; range 43–85 years) were included in this study. Tissues were collected at the Department of Pathology, MUMC+, Maastricht, The Netherlands, and included 8 clear cell renal carcinomas (CCRCCs) and 3 papillary RCCs (PRCCs). From each of the 11 RCCs, 5 FFPE blocks were taken from different locations (i.e., tc: tumor core, tt: tumor transition, t1: 1 cm distance to tumor core, t2: 2 cm distance to tumor core, and t3: 3 cm distance to tumor core; see also Figure 1), totaling 55 paraffin blocks. A total of 5/11 (45%) patients were diagnosed with stage I, and 6/11 (54%) with stage III. The clinico-pathological data of this RCC cohort are summarized in Figure 7 and Table 2.
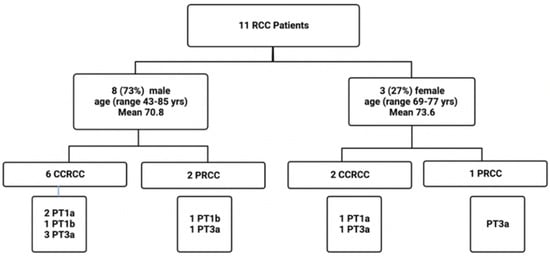
Figure 7.
Flow chart depicting the details of the clinicopathologic parameters of this RCC cohort. yrs, years; CCRCC, clear cell renal cell carcinoma; PRCC, papillary renal cell carcinoma.
This study was approved by the Medical Ethics Review Committee of the Maastricht UMC+, The Netherlands (Ref no. 2021-2789). Histopathology including immunohistochemistry (IHC) was independently reviewed by three experienced pathologists (IVS, VW, and AzH).
4.2. DNA Extraction
DNA extraction was performed as previously described [49]. Five consecutive 10 µm thick sections were cut from each FFPE tissue. After deparaffinization with xylene, the tissues were lysed with proteinase-K and incubated overnight at 56 °C until the tissue completely dissolved. The DNA was then extracted using the protocol of Genomic DNA from a tissue kit by Macherey-Nagel (Dueren, Germany). Purified DNA was measured in a spectrophotometer (Nanodrop, 2000; Thermo Scientific, Wilmington, DE, USA). Spectrophotometry (Nanodrop 2000; Thermo Scientific, Wilmington, DE, USA) and specimen control size (SCS) ladder DNA PCR were used to assess the quality and integrity of purified DNA. According to the assessment of all specimens by SCS ladder PCR [50], all specimens revealed sufficient DNA quality for further HPyVs testing.
4.3. HPyV PCR
PCRs were performed with 125 ng of genomic DNA using AmpliTaq Gold (Applied Biosystems SimpliAmp Thermal Cycler; Thermo Fisher Scientific, Landsmeer, the Netherlands) DNA polymerase in a final volume of 25 µL. Negative controls with nuclease-free water or DNA isolated from tissue-free FFPE blocks were included.
4.3.1. Consensus PCR
HPyV consensus PCR was carried out as previously described [51]. Here, we tailed the HPyV consensus primers with M13 forward and reverse primers to ease DNA sequencing (Table 1).
4.3.2. HPyV-Specific PCRs
HPyV-specific PCRs were performed using primer sets targeting various regions of the HPyV6, HPyV7, MCPyV, JCPyV, and BKPyV genomes, as previously described [23,28]. All HPyV primer sequences used in this study are summarized in Table 1.
4.4. Sequence Analyses
The HPyV PCR products were sequenced by automated nucleotide sequencing in an ABI 3130XL genetic analyzer (ABI). Subsequently, the sequences were analyzed with the reference sequences of Polyomaviridae (taxid:151341) retrieved from the NCBI Entrez Nucleotide database, using the Blast program. Multiple sequence alignments, with Clustal Omega algorithm (by The European Bioinformatics Institute, Hinxton, Cambridgeshire, UK, https://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 18 June 2023), were performed against the positive control (MCPyV, GenBank: EU375804.1, HPyV6, GenBank: HM011560, HPyV7, GenBank: HM011566, BKPyV, GenBank: NC_001538.1, JCPyV, GenBank: NC_001699.1, WUPyV, GenBank: NC_009539.1) sequence in order to compare each HPyV-positive result.
4.5. Construction of HPyV6 ER Lentiviral Vector and Generation of HPyV6 ER-Expressing HEK Cell Line
The following plasmids were used in this study: pLVX-TRE3G-ZsGreen1, pLVX-Tet3G, Lenti-X™ Packaging Single Shots (VSV-G). All plasmids were purchased from Takara bio company, San Jose, CA, USA. The transfer plasmids containing genes coding for HIV-1 LTRs, lentiviral packaging signal (Ψ), WPRE, and GFP were constructed by inserting the amplified PCR products HPyV6 early region (ER) (Addgene: pHPyV6-607a Plasmid#24727) into EcoRl and Mlul restriction sites of the pLVX-TRE3G-ZsGreenl-HPyV6ER-WPRE. Lentiviral particles were produced by Lenti-X™ Packaging Single Shots (VSV-G) with the transfer plasmid co-transfection of HEK239T cells. The packaged recombinant lentiviruses were harvested from the supernatant at 48 h and 72 h post-transfection. RT-qPCR measured the lentivirus titer. The next day, the cells in each well were transduced with packaged recombinant lentivirus LVX-TRE3G-ZsGreenl-HPyV6ER-WPRE or without recombinant lentivirus LVX-TRE3G-ZsGreenl-WPRE with lentivirus LVX-Tet3G at a MOI (multiplicity of infection) of 10 in DMEM medium containing 10% FBS with TransDux™ and MAX Enhancer (System Biosciences, Cat: LV860A-1, Palo Alto, CA, USA). The wells were incubated at 5% CO2 at 37 °C. After 48 h, the transduction medium was removed and replaced with a fresh medium containing 1 μg/mL of doxycycline. These cells were selected using puromycin (2 μg/mL). Upon reaching a specific cell count, the cells underwent appropriate fixation processes and were embedded into paraffin.
4.6. Fluorescence In Situ Hybridization (FISH)
To confirm the presence of the viral DNA at the single-cell level, selected cases of HPyVs DNA PCR-positive RCC tissues were tested by MCPyV- and HPyV6-FISH, as previously described [37,52]. In brief, FISH was performed on 3 μm thick FFPE sections, according to Hopman et al. with slight modifications in pre-treatment and procedure to adapt to kidney tissues [38]. We also validated the sensitivity and specificity of the probes by adding hybridization buffer instead of probe to the same positive sections to confirm real signals. WaGa and MCC26 were used as controls for MCPyV test. HEK293 cells expressing HPyV6 LT-Ag and HEK293 cells were used as controls for HPyV6 FISH. Slides were scanned manually, and FISH signals were detected using a DM 5000 B fluorescence microscope (Leica, Wetzlar, Germany) equipped with DAPI, TR (Texas red), and FITC filters. All photos were recorded with a Leica DC 300 Fx camera (Leica). FISH fluorescence intensity, signal numbers, and sizes for strong and weak nuclear FISH signals were evaluated independently by three investigators (G.M., E.J.S., A.z.H.) according to the criteria in Hafkamp et al. [53]. Cross-hybridization was excluded by performing a HPyV6 FISH probe on the MCPyV-positive WaGa cell line and a MCPyV FISH probe with HEK293-HPyV6, which did not show any specific hybridization signals.
4.7. RNA In Situ Hybridization (RISH)
To confirm the findings of HPyVs at the transcriptional level (mRNA), the RNAscope technique, which uses complementary RNA probes to target specific expressions of HPyV mRNA, was employed in selected MCPyV, HPyV6, and HPyV7-DNA PCR- and IHC-positive cases.
A total of 20 pairs of 50 bp pooled probes were designed by Advanced Cell Diagnostics (ACD) for HPyV6 and HPyV7 (LT&sT-Ag) [51], and 14 pairs for MCPyV (LT&sT-Ag) [34]. The sections were pre-treated using the RNAscope® HD Red 2·5 Kit (Advanced Cell Diagnostics, Cat No. 322350, Newark, CA, USA), according to the manufacturer’s instructions.
HEK293 cells expressing HPyV6 and HPyV7 early proteins were used as positive controls for HPyV6 and HPyV7, and HEK293 empty block cells were used as a negative control. The WaGa cell line and MCC26 were utilized as positive and negative controls, respectively, for MCPyV to validate our probes and the pre-treatment quality and efficiency. 3DHISTECH’s PANNORAMIC 1000 DX scanner (Budapest, Öv u. 3., Hungary) was used to scan and record images of all the RISH slides, which were then reviewed and graded in accordance with the ACD protocols [54].
4.8. Immunohistochemistry (IHC)
All immunohistochemical procedures were performed on a Dako Autostainer Link 48, using the EnVision FLEX Visualization Kit (K8008, DAKO, Carpinteria, CA, USA) according to standard diagnostic routine protocols and the manufacturers’ instructions. In short, consecutive 3–5 μm thick FFPE sections slides were deparaffinized in xylene and rehydrated in a series of ethanol solutions. Endogenous peroxidase activity was blocked by incubation of the slides within hydrogen peroxide for 5 min. Epitope retrieval was performed using high buffer, pH 9, at 95 °C for 10 min and controlled cooling in 95 °C to 85 °C in an antigen retrieval pre-treatment chamber (Dako PT Link PT20027 Pre-Treatment Module).
MCPyV immunohistochemistry (IHC) was performed with a monoclonal antibody (clone: CM2B4, dilution 1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), primarily detecting the LT-antigen expression in exon 2 of MCPyV with a cysteine C-terminus; this antibody is more specific and sensitive because of the epitope region (SRSRKPSSNASRGA) that differs from other HPyVs, as demonstrated by Moshiri et al. [55,56] (Figure S1A,B, Table S1A).
To validate HPyVs positivity as shown by consensus and specific PCR (i.e., BKPyV, JCPyV, HPyV6, HPyV7, WUyV), IHC was performed using a monoclonal antibody (mouse anti-SV40 large T antigen; Calbiochem Inc., San Diego, CA, USA, cat # DP02, clone: PAb416, dilution 1:100). The PAb416 antibody detects and cross-reacts with the LT antigen of HPyVs exon 2, except for with the MCPyV large T antigen because of the dissimilar epitope-binding sites [56] (Figure S2, Table S1B).
The CM2B4 antibody was incubated after addition to FFPE section slides for 20 min at RT, but PAb416 was incubated overnight at 4C.
Human embryonic kidney 293 cells (HEK293) were transduced with HPyV-6 and HPyV-7 early region and were used as positive controls for PAb416; meanwhile, the WaGa cell line was used as positive control for CM2B4. Moreover, the WaGa cell line and HEK293 empty cells were used as negative controls, respectively, for the PAb416 and CM2B4 antibodies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25158213/s1.
Author Contributions
Conceptualization, G.M., F.K., A.z.H., K.M.S., V.W., E.-J.M.S. and I.S.; methodology, G.M., S.S., F.K. and I.S.; investigation, G.M., S.S., F.K. and I.S.; data curation, F.K., G.M. and I.S.; writing—original draft preparation, G.M., F.K. and A.z.H.; writing—review and editing, G.M., S.S., F.K., K.M.S., K.S., K.L., D.R., D.L., A.z.H. and I.S.; visualization, G.M., S.S., F.K. and I.S.; resources, K.M.S., K.L., D.R. and I.S.; principal investigator, supervision, and project administration, V.W., I.S. and A.z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Review Committee of the Maastricht UMC+, The Netherlands (Ref no. 2021–2789).
Informed Consent Statement
Not applicable. Tissues of patients who object to the usage of their tissues for research purposes are automatically excluded from research databases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Kathuria-Prakash, N.; Drolen, C.; Hannigan, C.A.; Drakaki, A. Immunotherapy and Metastatic Renal Cell Carcinoma: A Review of New Treatment Approaches. Life 2021, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Hes, O.; Williamson, S.R.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1392–1424. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. Jama 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk factors for renal cell carcinoma in the VITAL study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.M.; Hofmann, J.N.; Cho, E.; Pollak, M.N.; Chow, W.H.; Purdue, M.P. Circulating levels of obesity-related markers and risk of renal cell carcinoma in the PLCO cancer screening trial. Cancer Causes Control 2017, 28, 801–807. [Google Scholar] [CrossRef]
- Webster, B.R.; Gopal, N.; Ball, M.W. Tumorigenesis Mechanisms Found in Hereditary Renal Cell Carcinoma: A Review. Genes 2022, 13, 2122. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Infections Causing Human Cancer; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- zur Hausen, H. Viruses in human cancers. Science 1991, 254, 1167–1173. [Google Scholar] [CrossRef]
- Kim, K.H.; Han, E.M.; Lee, E.S.; Park, H.S.; Kim, I.; Kim, Y.S. Epstein-Barr virus infection in sarcomatoid renal cell carcinoma tissues. BJU Int. 2005, 96, 547–552. [Google Scholar] [CrossRef]
- Farhadi, A.; Behzad-Behbahani, A.; Geramizadeh, B.; Sekawi, Z.; Rahsaz, M.; Sharifzadeh, S. High-risk human papillomavirus infection in different histological subtypes of renal cell carcinoma. J. Med. Virol. 2014, 86, 1134–1144. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Z.; Jian, Z.; Wei, X. The association between hepatitis C virus infection and renal cell cancer, prostate cancer, and bladder cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10833. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Y.; Ozdemir, E.; Ozercan, H.I.; Etem, E.O.; Aker, F.; Toraman, Z.A.; Seyrek, A.; Firdolas, F. Potential relationship between BK virus and renal cell carcinoma. J. Med. Virol. 2013, 85, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Szymanski, J.; Slavcheva, E.; Rao, A.; Kelly, A.; Jones, K.; Jaffers, G. BK virus associated renal cell carcinoma: Case presentation with optimized PCR and other diagnostic tests. Am. J. Transplant. 2007, 7, 1666–1671. [Google Scholar] [CrossRef]
- Pyöriä, L.; Pratas, D.; Toppinen, M.; Hedman, K.; Sajantila, A.; Perdomo, M.F. Unmasking the tissue-resident eukaryotic DNA virome in humans. Nucleic Acids Res. 2023, 51, 3223–3239. [Google Scholar] [CrossRef]
- Namdari, S.; Chong, P.P.; Behzad-Behbahani, A.; Geramizadeh, B.; Nazhvani, A.D.; Sekawi, Z.; Farhadi, A. Human herpesvirus 6A and 6B and polyomavirus JC and BK infections in renal cell carcinoma and their relationship with p53, p16INK4a, Ki-67, and nuclear factor-kappa B expression. Microbiol. Immunol. 2022, 66, 510–518. [Google Scholar] [CrossRef]
- Nickeleit, V.; Klimkait, T.; Binet, I.F.; Dalquen, P.; Del Zenero, V.; Thiel, G.; Mihatsch, M.J.; Hirsch, H.H. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N. Engl. J. Med. 2000, 342, 1309–1315. [Google Scholar] [CrossRef]
- DeCaprio, J.A.; Garcea, R.L. A cornucopia of human polyomaviruses. Nat. Rev. Microbiol. 2013, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.D.; Field, A.M.; Coleman, D.V.; Hulme, B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971, 1, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Padgett, B.L.; Walker, D.L.; ZuRhein, G.M.; Eckroade, R.J.; Dessel, B.H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971, 1, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Straif, K. Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 2012, 13, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Dalianis, T.; Hirsch, H.H. Human polyomaviruses in disease and cancer. Virology 2013, 437, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nickeleit, V.; Hirsch, H.H.; Binet, I.F.; Gudat, F.; Prince, O.; Dalquen, P.; Thiel, G.; Mihatsch, M.J. Polyomavirus infection of renal allograft recipients: From latent infection to manifest disease. J. Am. Soc. Nephrol. 1999, 10, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Nickeleit, V.; Singh, H.K. Polyomaviruses and disease: Is there more to know than viremia and viruria? Curr. Opin. Organ Transplant. 2015, 20, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Dobson, S. BK and JC virus: A review. J. Infect. 2014, 68 (Suppl. 1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Orellana, J.; Kwun, H.J.; Artusi, S.; Chang, Y.; Moore, P.S. Sirolimus and Other Mechanistic Target of Rapamycin Inhibitors Directly Activate Latent Pathogenic Human Polyomavirus Replication. J. Infect. Dis. 2021, 224, 1160–1169. [Google Scholar] [CrossRef]
- Gheit, T.; Dutta, S.; Oliver, J.; Robitaille, A.; Hampras, S.; Combes, J.D.; McKay-Chopin, S.; Le Calvez-Kelm, F.; Fenske, N.; Cherpelis, B.; et al. Isolation and characterization of a novel putative human polyomavirus. Virology 2017, 506, 45–54. [Google Scholar] [CrossRef]
- Schowalter, R.M.; Pastrana, D.V.; Pumphrey, K.A.; Moyer, A.L.; Buck, C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010, 7, 509–515. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Wang, L.; Harms, P.W.; Palanisamy, N.; Carskadon, S.; Cao, X.; Siddiqui, J.; Patel, R.M.; Zelenka-Wang, S.; Durham, A.B.; Fullen, D.R.; et al. Age and Gender Associations of Virus Positivity in Merkel Cell Carcinoma Characterized Using a Novel RNA In Situ Hybridization Assay. Clin. Cancer Res. 2017, 23, 5622–5630. [Google Scholar] [CrossRef]
- Kassem, A.; Schöpflin, A.; Diaz, C.; Weyers, W.; Stickeler, E.; Werner, M.; Zur Hausen, A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008, 68, 5009–5013. [Google Scholar] [CrossRef] [PubMed]
- Rennspiess, D.; Pujari, S.; Keijzers, M.; Abdul-Hamid, M.A.; Hochstenbag, M.; Dingemans, A.M.; Kurz, A.K.; Speel, E.J.; Haugg, A.; Pastrana, D.V.; et al. Detection of human polyomavirus 7 in human thymic epithelial tumors. J. Thorac. Oncol. 2015, 10, 360–366. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Klufah, F.; Mobaraki, G.; Chteinberg, E.; Alharbi, R.A.; Winnepenninckx, V.; Speel, E.J.M.; Rennspiess, D.; Olde Damink, S.W.; Neumann, U.P.; Kurz, A.K.; et al. High Prevalence of Human Polyomavirus 7 in Cholangiocarcinomas and Adjacent Peritumoral Hepatocytes: Preliminary Findings. Microorganisms 2020, 8, 1125. [Google Scholar] [CrossRef]
- Haugg, A.M.; Rennspiess, D.; zur Hausen, A.; Speel, E.J.; Cathomas, G.; Becker, J.C.; Schrama, D. Fluorescence in situ hybridization and qPCR to detect Merkel cell polyomavirus physical status and load in Merkel cell carcinomas. Int. J. Cancer 2014, 135, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Haugg, A.M.; Speel, E.J.; Pantulu, N.D.; Pallasch, C.; Kurz, A.K.; Kvasnicka, H.M.; Cathomas, G.; Wendtner, C.M.; zur Hausen, A. Fluorescence in situ hybridization confirms the presence of Merkel cell polyomavirus in chronic lymphocytic leukemia cells. Blood 2011, 117, 5776–5777. [Google Scholar] [CrossRef]
- Hopman, A.H.; Kamps, M.A.; Smedts, F.; Speel, E.J.; Herrington, C.S.; Ramaekers, F.C. HPV in situ hybridization: Impact of different protocols on the detection of integrated HPV. Int. J. Cancer 2005, 115, 419–428. [Google Scholar] [CrossRef]
- Hafkamp, H.C.; Manni, J.J.; Haesevoets, A.; Voogd, A.C.; Schepers, M.; Bot, F.J.; Hopman, A.H.; Ramaekers, F.C.; Speel, E.J. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer 2008, 122, 2656–2664. [Google Scholar] [CrossRef]
- Anderson, C.M.; Zhang, B.; Miller, M.; Butko, E.; Wu, X.; Laver, T.; Kernag, C.; Kim, J.; Luo, Y.; Lamparski, H.; et al. Fully Automated RNAscope In Situ Hybridization Assays for Formalin-Fixed Paraffin-Embedded Cells and Tissues. J. Cell Biochem. 2016, 117, 2201–2208. [Google Scholar] [CrossRef]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef]
- Toptan, T.; Yousem, S.A.; Ho, J.; Matsushima, Y.; Stabile, L.P.; Fernández-Figueras, M.T.; Bhargava, R.; Ryo, A.; Moore, P.S.; Chang, Y. Survey for human polyomaviruses in cancer. JCI Insight 2016, 1, e85562. [Google Scholar] [CrossRef]
- Loyo, M.; Guerrero-Preston, R.; Brait, M.; Hoque, M.O.; Chuang, A.; Kim, M.S.; Sharma, R.; Liégeois, N.J.; Koch, W.M.; Califano, J.A.; et al. Quantitative detection of Merkel cell virus in human tissues and possible mode of transmission. Int. J. Cancer 2010, 126, 2991–2996. [Google Scholar] [CrossRef]
- Jiang, M.; Abend, J.R.; Tsai, B.; Imperiale, M.J. Early events during BK virus entry and disassembly. J. Virol. 2009, 83, 1350–1358. [Google Scholar] [CrossRef]
- Bersanelli, M.; Casartelli, C.; Buti, S.; Porta, C. Renal cell carcinoma and viral infections: A dangerous relationship? World J. Nephrol. 2022, 11, 1–12. [Google Scholar] [CrossRef]
- Garayeva, N.; Demir, E.; Dirim, A.B.; Safak, S.; Artan, A.S.; Ozluk, Y.; Kílícaslan, I.; Turkmen, A. Expression of JC virus in a kidney transplant recipient with renal cell carcinoma. Nefrologia 2024, 44, 288–289. [Google Scholar] [CrossRef]
- Knöll, A.; Stoehr, R.; Jilg, W.; Hartmann, A. Low frequency of human polyomavirus BKV and JCV DNA in urothelial carcinomas of the renal pelvis and renal cell carcinomas. Oncol. Rep. 2003, 10, 487–491. [Google Scholar] [CrossRef]
- Kenan, D.J.; Mieczkowski, P.A.; Burger-Calderon, R.; Singh, H.K.; Nickeleit, V. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J. Pathol. 2015, 237, 379–389. [Google Scholar] [CrossRef]
- Kenan, D.J.; Mieczkowski, P.A.; Latulippe, E.; Côté, I.; Singh, H.K.; Nickeleit, V. BK Polyomavirus Genomic Integration and Large T Antigen Expression: Evolving Paradigms in Human Oncogenesis. Am. J. Transplant. 2017, 17, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Oncogenic DNA viruses. Oncogene 2001, 20, 7820–7823. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. The search for infectious causes of human cancers: Where and why. Virology 2009, 392, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Howley, P.M. Gordon Wilson Lecture: Infectious Disease Causes of Cancer: Opportunities for Prevention and Treatment. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 117–132. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).