Early and Late Effects of Low-Dose X-ray Exposure in Human Fibroblasts: DNA Repair Foci, Proliferation, Autophagy, and Senescence
Abstract
1. Introduction
2. Results
2.1. Early Effects
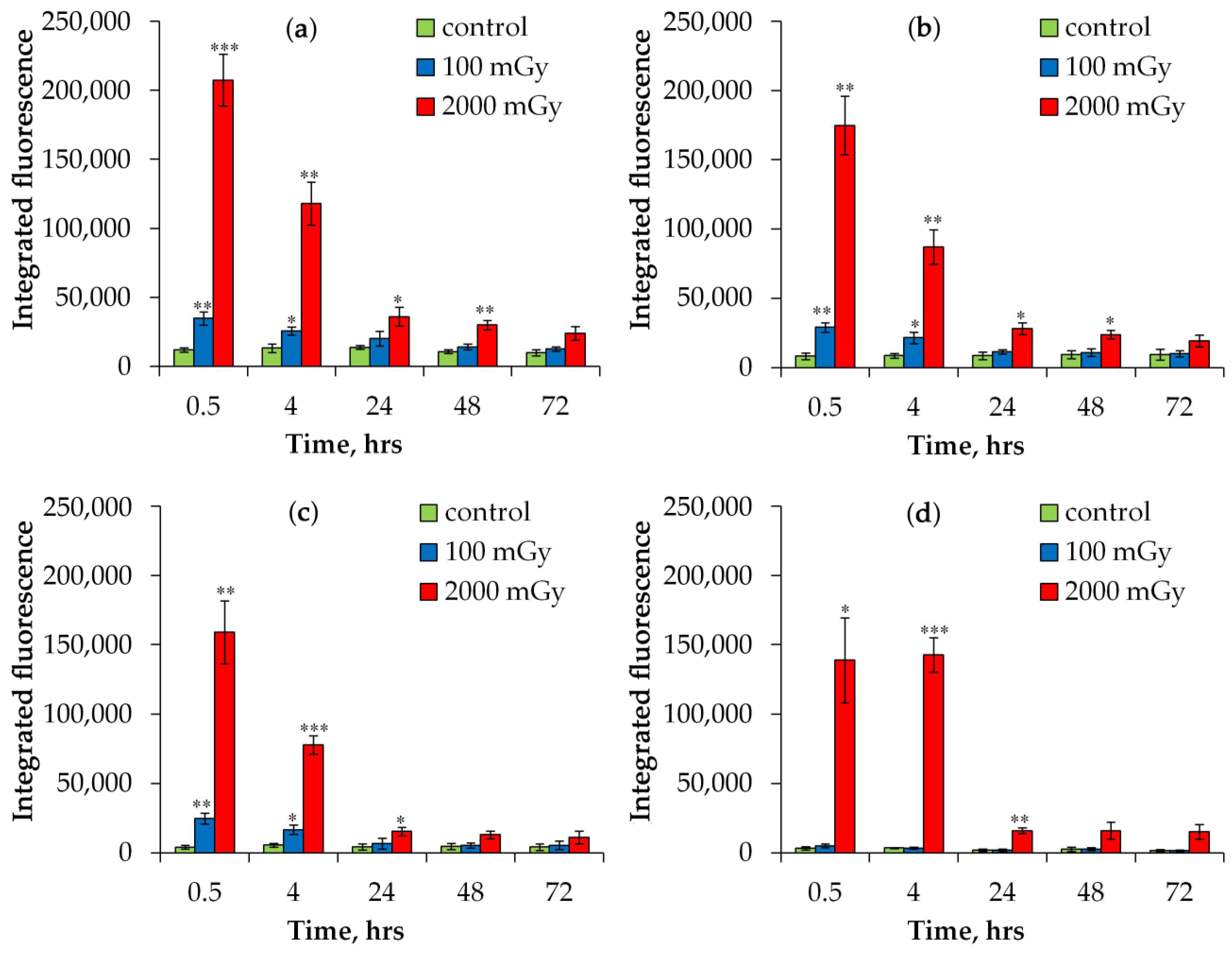
2.1.1. DNA Repair Foci
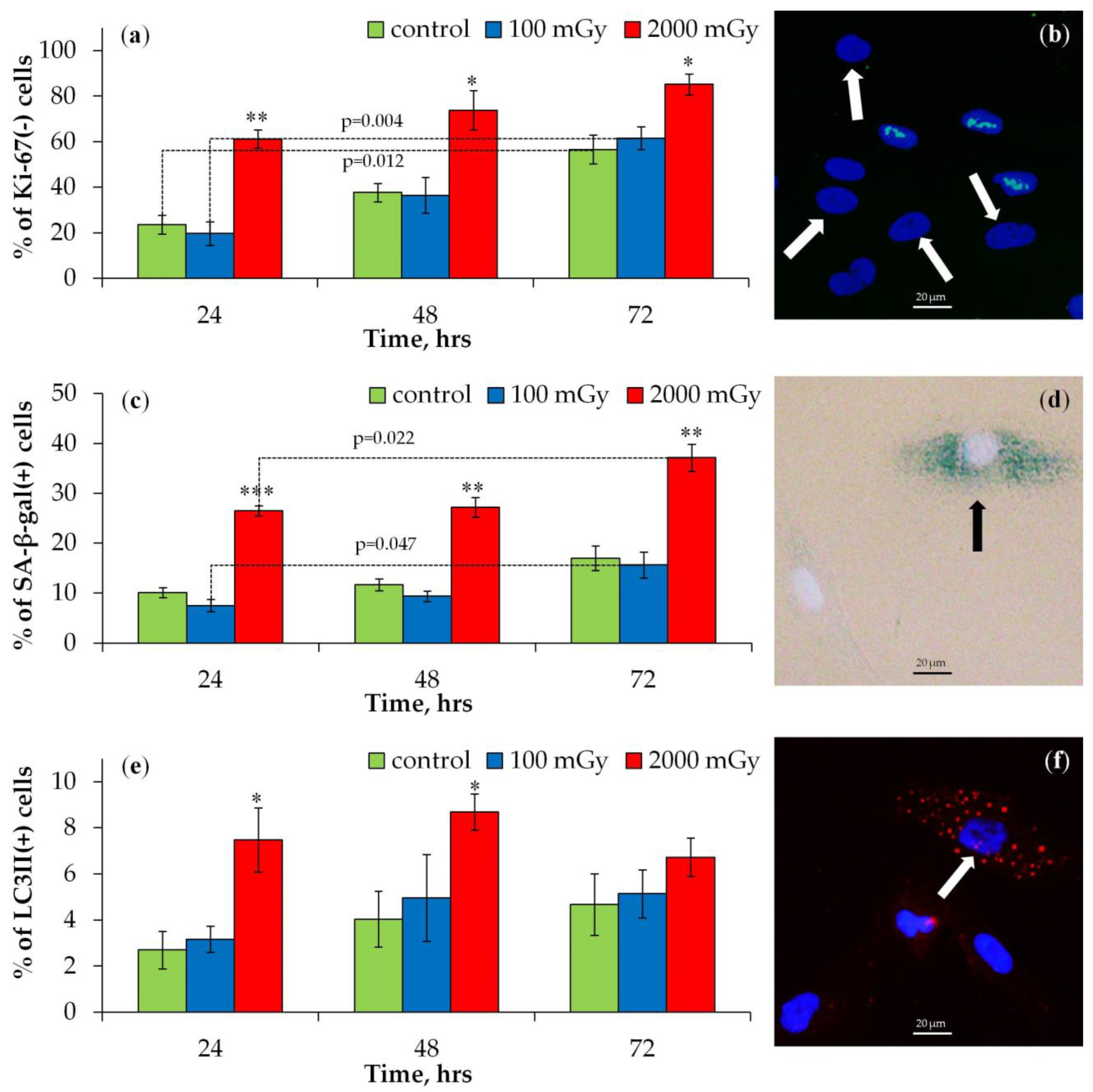
2.1.2. Proliferation, Senescence, and Autophagy
2.2. Late Effects
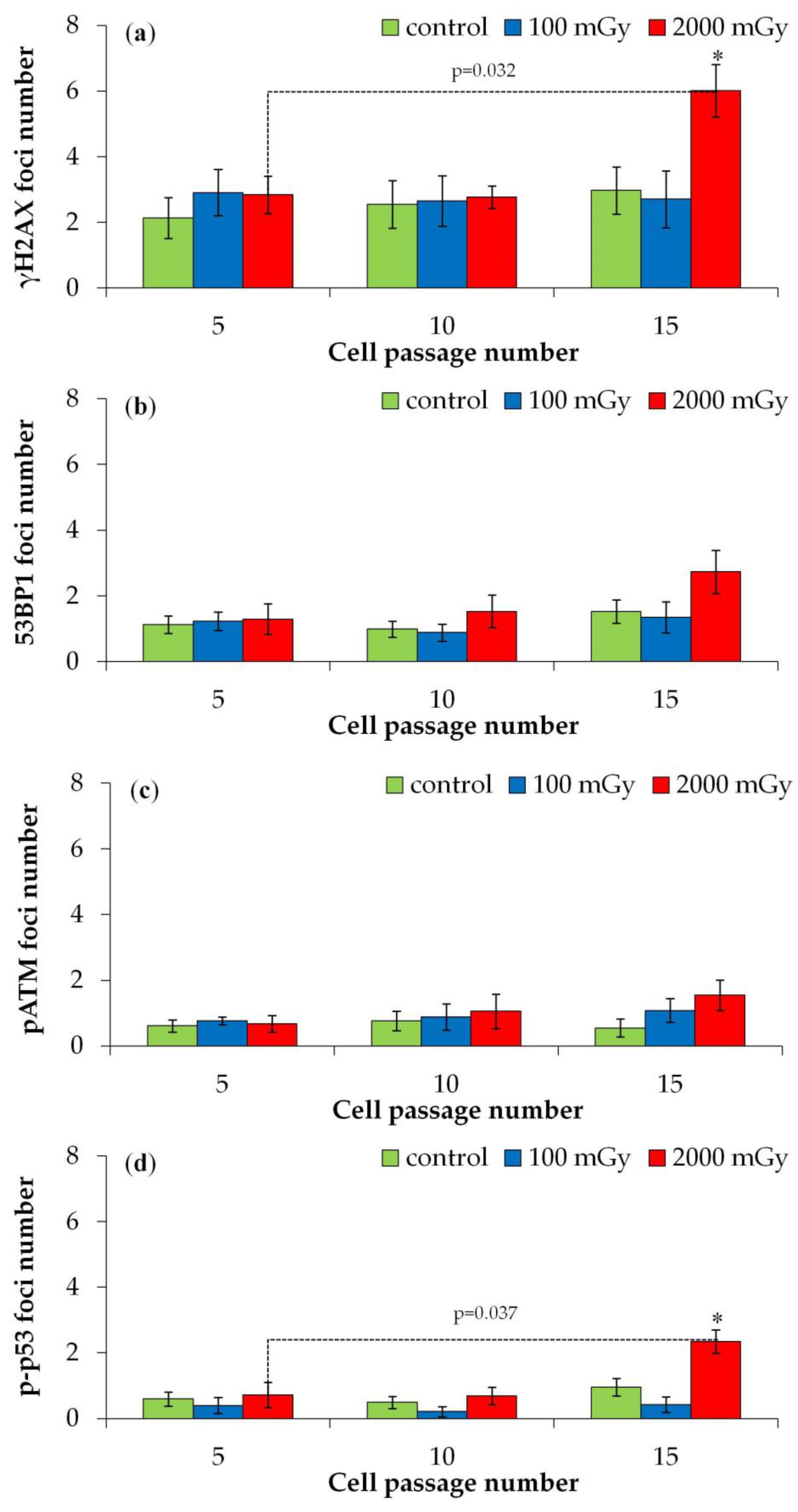
2.2.1. DNA Repair Foci in Later Passages
2.2.2. Proliferation, Senescence, and Autophagy in Later Passages
3. Discussion
- (1)
- The fusion of individual foci to form larger “repair centers”. The existence of repair centers was demonstrated in the work of T. Neumaier et al. and was used by the authors to explain a similar reduction in the quantitative output of DNA repair foci with increasing radiation dose [46].
- (2)
- It has been shown that with increasing radiation dose, the contribution of the fast mechanism of non-homologous end joining increases [47].
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation
4.3. Immunocytochemistry
4.4. Analysis of Senescence-Associated β-Galactosidase-Positive Cells
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Abalo, K.D.; Rage, E.; Leuraud, K.; Richardson, D.B.; Le Pointe, H.D.; Laurier, D.; Bernier, M.O. Early life ionizing radiation exposure and cancer risks: Systematic review and meta-analysis. Pediatr. Radiol. 2021, 51, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nawa, T. Low-dose CT screening for lung cancer reduced lung cancer mortality in Hitachi City. Int. J. Radiat. Biol. 2019, 95, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Rampinelli, C.; De Marco, P.; Origgi, D.; Maisonneuve, P.; Casiraghi, M.; Veronesi, G.; Spaggiari, L.; Bellomi, M. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: Secondary analysis of trial data and risk-benefit analysis. BMJ 2017, 356, j347. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research, T.; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Bezak, E.; Le, H.; Kempson, I. DNA Double Strand Break and Response Fluorescent Assays: Choices and Interpretation. Int. J. Mol. Sci. 2024, 25, 2227. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Ramachandran, C.; Melnick, S.J. Multidrug resistance in human tumors--molecular diagnosis and clinical significance. Mol. Diagn. 1999, 4, 81–94. [Google Scholar] [CrossRef]
- Podralska, M.; Sajek, M.P.; Bielicka, A.; Zurawek, M.; Ziolkowska-Suchanek, I.; Izykowska, K.; Kolenda, T.; Kazimierska, M.; Kasprzyk, M.E.; Sura, W.; et al. Identification of ATM-dependent long non-coding RNAs induced in response to DNA damage. DNA Repair 2024, 135, 103648. [Google Scholar] [CrossRef] [PubMed]
- Muraki, K.; Nyhan, K.; Han, L.; Murnane, J.P. Mechanisms of telomere loss and their consequences for chromosome instability. Front. Oncol. 2012, 2, 135. [Google Scholar] [CrossRef]
- Gaziev, A.; Shaikhaev, G. Limited Repair of Critical DNA Damage in Cells Exposed to Low Dose Radiation. In Current Topics in Ionizing Radiation Research; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Grudzenski, S.; Raths, A.; Conrad, S.; Rube, C.E.; Lobrich, M. Inducible response required for repair of low-dose radiation damage in human fibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 14205–14210. [Google Scholar] [CrossRef]
- Bushmanov, A.; Vorobyeva, N.; Molodtsova, D.; Osipov, A.N. Utilization of DNA double-strand breaks for biodosimetry of ionizing radiation exposure. Environ. Adv. 2022, 8, 100207. [Google Scholar] [CrossRef]
- Barbieri, S.; Babini, G.; Morini, J.; Friedland, W.; Buonanno, M.; Grilj, V.; Brenner, D.J.; Ottolenghi, A.; Baiocco, G. Predicting DNA damage foci and their experimental readout with 2D microscopy: A unified approach applied to photon and neutron exposures. Sci. Rep. 2019, 9, 14019. [Google Scholar] [CrossRef]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA damage foci: Meaning and significance. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E.; Costes, S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y. Radiation-induced DNA repair foci: Spatio-temporal aspects of formation, application for assessment of radiosensitivity and biological dosimetry. Mutat. Res. 2010, 704, 132–141. [Google Scholar] [CrossRef]
- Wanotayan, R.; Chousangsuntorn, K.; Petisiwaveth, P.; Anuttra, T.; Lertchanyaphan, W.; Jaikuna, T.; Jangpatarapongsa, K.; Uttayarat, P.; Tongloy, T.; Chousangsuntorn, C.; et al. A deep learning model (FociRad) for automated detection of gamma-H2AX foci and radiation dose estimation. Sci. Rep. 2022, 12, 5527. [Google Scholar] [CrossRef]
- Raavi, V.; Perumal, V.; Paul, S.F.D. Potential application of gamma-H2AX as a biodosimetry tool for radiation triage. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108350. [Google Scholar] [CrossRef] [PubMed]
- Lobrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. gammaH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Jakl, L.; Markova, E.; Kolarikova, L.; Belyaev, I. Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes. Genes 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Falaschi, A.; Chiaramonte, A.; Testi, S.; Scarpato, R. Dual Immunofluorescence of gammaH2AX and 53BP1 in Human Peripheral Lymphocytes. J. Vis. Exp. 2023, 197, e65472. [Google Scholar] [CrossRef]
- Kocher, S.; Volquardsen, J.; Perugachi Heinsohn, A.; Petersen, C.; Roggenbuck, D.; Rothkamm, K.; Mansour, W.Y. Fully automated counting of DNA damage foci in tumor cell culture: A matter of cell separation. DNA Repair 2021, 102, 103100. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Kowalczyk, A.; Janecka-Widla, A.; Kabat, D.; Szatkowski, W.; Biesaga, B. Low-Dose Hypersensitive Response for Residual pATM and gammaH2AX Foci in Normal Fibroblasts of Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Ulyanenko, S.; Pustovalova, M.; Koryakin, S.; Beketov, E.; Lychagin, A.; Ulyanenko, L.; Kaprin, A.; Grekhova, A.; Ozerova, A.M.; Ozerov, I.V.; et al. Formation of γH2AX and pATM Foci in Human Mesenchymal Stem Cells Exposed to Low Dose-Rate Gamma-Radiation. Int. J. Mol. Sci. 2019, 20, 2645. [Google Scholar] [CrossRef]
- Luo, M.; Chen, L.; Zheng, J.; Wang, Q.; Huang, Y.; Liao, F.; Jiang, Z.; Zhang, C.; Shen, G.; Wu, J.; et al. Mitigation of radiation-induced pulmonary fibrosis by small-molecule dye IR-780. Free Radic. Biol. Med. 2021, 164, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Rodel, F.; Fournier, C.; Wiedemann, J.; Merz, F.; Gaipl, U.S.; Frey, B.; Keilholz, L.; Seegenschmiedt, M.H.; Rodel, C.; Hehlgans, S. Basics of Radiation Biology When Treating Hyperproliferative Benign Diseases. Front. Immunol. 2017, 8, 519. [Google Scholar] [CrossRef]
- Kosmacek, E.A.; Oberley-Deegan, R.E. Adipocytes protect fibroblasts from radiation-induced damage by adiponectin secretion. Sci. Rep. 2020, 10, 12616. [Google Scholar] [CrossRef]
- Wieder, R. Fibroblasts as Turned Agents in Cancer Progression. Cancers 2023, 15, 2014. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-associated fibroblasts and resistance to anticancer therapies: Status, mechanisms, and countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Fu, Y.; Kuang, J.; Zhao, B.; Zhang, L.; Lin, J.; Lin, S.; Wu, D.; Xie, G. Cancer-associated fibroblasts induce growth and radioresistance of breast cancer cells through paracrine IL-6. Cell Death Discov. 2023, 9, 6. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017, 77, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Lleres, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e1105. [Google Scholar] [CrossRef]
- Maier, A.B.; Westendorp, R.G.; Van Heemst, D. Beta-galactosidase activity as a biomarker of replicative senescence during the course of human fibroblast cultures. Ann. N. Y. Acad. Sci. 2007, 1100, 323–332. [Google Scholar] [CrossRef]
- Osipov, A.; Chigasova, A.; Yashkina, E.; Ignatov, M.; Fedotov, Y.; Molodtsova, D.; Vorobyeva, N.; Osipov, A.N. Residual Foci of DNA Damage Response Proteins in Relation to Cellular Senescence and Autophagy in X-Ray Irradiated Fibroblasts. Cells 2023, 12, 1209. [Google Scholar] [CrossRef]
- Leontieva, O.V.; Demidenko, Z.N.; Blagosklonny, M.V. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc. Natl. Acad. Sci. USA 2014, 111, 8832–8837. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Baeyens, A.; Abrantes, A.M.; Ahire, V.; Ainsbury, E.A.; Baatout, S.; Baselet, B.; Botelho, M.F.; Boterberg, T.; Chevalier, F.; Da Pieve, F.; et al. Basic Concepts of Radiation Biology. In Radiobiology Textbook; Springer: Cham, Switzerland, 2023; pp. 25–81. [Google Scholar] [CrossRef]
- Osipov, A.N.; Grekhova, A.; Pustovalova, M.; Ozerov, I.V.; Eremin, P.; Vorobyeva, N.; Lazareva, N.; Pulin, A.; Zhavoronkov, A.; Roumiantsev, S.; et al. Activation of homologous recombination DNA repair in human skin fibroblasts continuously exposed to X-ray radiation. Oncotarget 2015, 6, 26876–26885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yachi, Y.; Yoshii, Y.; Matsuya, Y.; Mori, R.; Oikawa, J.; Date, H. Track Structure Study for Energy Dependency of Electrons and X-rays on DNA Double-Strand Break Induction. Sci. Rep. 2019, 9, 17649. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, M.; Puskin, J.; Hertel, N.; Eckerman, K. An empirical method for deriving RBE values associated with electrons, photons and radionuclides. Radiat. Prot. Dosim. 2015, 167, 664–670. [Google Scholar] [CrossRef]
- Neumaier, T.; Swenson, J.; Pham, C.; Polyzos, A.; Lo, A.T.; Yang, P.; Dyball, J.; Asaithamby, A.; Chen, D.J.; Bissell, M.J.; et al. Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells. Proc. Natl. Acad. Sci. USA 2012, 109, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, M.; Rothkamm, K.; Lobrich, M. Physical and biological parameters affecting DNA double strand break misrejoining in mammalian cells. Radiat. Prot. Dosim. 2002, 99, 129–132. [Google Scholar] [CrossRef]
- Ruprecht, N.; Hungerbühler, M.N.; Böhm, I.B.; Heverhagen, J.T. Improved identification of DNA double strand breaks: γ-H2AX-epitope visualization by confocal microscopy and 3D reconstructed images. Radiat. Environ. Biophys. 2019, 58, 295–302. [Google Scholar] [CrossRef]
- Ingram, S.P.; Warmenhoven, J.W.; Henthorn, N.T.; Chadiwck, A.L.; Santina, E.E.; McMahon, S.J.; Schuemann, J.; Kirkby, N.F.; Mackay, R.I.; Kirkby, K.J.; et al. A computational approach to quantifying miscounting of radiation-induced double-strand break immunofluorescent foci. Commun. Biol. 2022, 5, 700. [Google Scholar] [CrossRef]
- Belov, O.; Chigasova, A.; Pustovalova, M.; Osipov, A.; Eremin, P.; Vorobyeva, N.; Osipov, A.N. Dose-Dependent Shift in Relative Contribution of Homologous Recombination to DNA Repair after Low-LET Ionizing Radiation Exposure: Empirical Evidence and Numerical Simulation. Curr. Issues Mol. Biol. 2023, 45, 7352–7373. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Scott, A.; Murray, D. New insights into p53 signaling and cancer cell response to DNA damage: Implications for cancer therapy. J. Biomed. Biotechnol. 2012, 2012, 170325. [Google Scholar] [CrossRef]
- Al Rashid, S.T.; Dellaire, G.; Cuddihy, A.; Jalali, F.; Vaid, M.; Coackley, C.; Folkard, M.; Xu, Y.; Chen, B.P.; Chen, D.J.; et al. Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res. 2005, 65, 10810–10821. [Google Scholar] [CrossRef]
- Zyuzikov, N.A.; Coates, P.J.; Parry, J.M.; Lorimore, S.A.; Wright, E.G. Lack of Nontargeted Effects in Murine Bone Marrow after Low-DoseIn VivoX Irradiation. Radiat. Res. 2011, 175, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gu, J.; Yu, D.; Wang, G.; Zhou, L.; Zhang, X.; Zhao, Y.; Chen, X.; Zheng, S.; Liu, Q.; et al. Low-Dose Radiation Induces Cell Proliferation in Human Embryonic Lung Fibroblasts but not in Lung Cancer Cells: Importance of ERK1/2 and AKT Signaling Pathways. Dose Response 2016, 14, 1559325815622174. [Google Scholar] [CrossRef] [PubMed]
- Velegzhaninov, I.O.; Ermakova, A.V.; Klokov, D.Y. Low dose ionizing irradiation suppresses cellular senescence in normal human fibroblasts. Int. J. Radiat. Biol. 2018, 94, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Berardinelli, F.; Antoccia, A.; Buonsante, R.; Gerardi, S.; Cherubini, R.; De Nadal, V.; Tanzarella, C.; Sgura, A. The role of telomere length modulation in delayed chromosome instability induced by ionizing radiation in human primary fibroblasts. Environ. Mol. Mutagen. 2013, 54, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hovest, M.G.; Bruggenolte, N.; Hosseini, K.S.; Krieg, T.; Herrmann, G. Senescence of human fibroblasts after psoralen photoactivation is mediated by ATR kinase and persistent DNA damage foci at telomeres. Mol. Biol. Cell 2006, 17, 1758–1767. [Google Scholar] [CrossRef]
- Guo, L.; Xie, B.; Mao, Z. Autophagy in premature senescent cells is activated via AMPK pathway. Int. J. Mol. Sci. 2012, 13, 3563–3582. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, J.W.; Jeoung, J.A.; Kim, M.S.; Kang, C. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol. Cells 2017, 40, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. Autophagy and senescence: A partnership in search of definition. Autophagy 2013, 9, 808–812. [Google Scholar] [CrossRef]
- Pustovalova, M.; Astrelina capital Te, C.; Grekhova, A.; Vorobyeva, N.; Tsvetkova, A.; Blokhina, T.; Nikitina, V.; Suchkova, Y.; Usupzhanova, D.; Brunchukov, V.; et al. Residual gammaH2AX foci induced by low dose x-ray radiation in bone marrow mesenchymal stem cells do not cause accelerated senescence in the progeny of irradiated cells. Aging 2017, 9, 2397–2410. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, A.; Chigasova, A.; Yashkina, E.; Ignatov, M.; Vorobyeva, N.; Zyuzikov, N.; Osipov, A.N. Early and Late Effects of Low-Dose X-ray Exposure in Human Fibroblasts: DNA Repair Foci, Proliferation, Autophagy, and Senescence. Int. J. Mol. Sci. 2024, 25, 8253. https://doi.org/10.3390/ijms25158253
Osipov A, Chigasova A, Yashkina E, Ignatov M, Vorobyeva N, Zyuzikov N, Osipov AN. Early and Late Effects of Low-Dose X-ray Exposure in Human Fibroblasts: DNA Repair Foci, Proliferation, Autophagy, and Senescence. International Journal of Molecular Sciences. 2024; 25(15):8253. https://doi.org/10.3390/ijms25158253
Chicago/Turabian StyleOsipov, Andrey, Anna Chigasova, Elizaveta Yashkina, Maxim Ignatov, Natalia Vorobyeva, Nikolay Zyuzikov, and Andreyan N. Osipov. 2024. "Early and Late Effects of Low-Dose X-ray Exposure in Human Fibroblasts: DNA Repair Foci, Proliferation, Autophagy, and Senescence" International Journal of Molecular Sciences 25, no. 15: 8253. https://doi.org/10.3390/ijms25158253
APA StyleOsipov, A., Chigasova, A., Yashkina, E., Ignatov, M., Vorobyeva, N., Zyuzikov, N., & Osipov, A. N. (2024). Early and Late Effects of Low-Dose X-ray Exposure in Human Fibroblasts: DNA Repair Foci, Proliferation, Autophagy, and Senescence. International Journal of Molecular Sciences, 25(15), 8253. https://doi.org/10.3390/ijms25158253





