Genome-Wide Analysis and Expression Profiling of Lectin Receptor-like Kinase Genes in Watermelon (Citrullus lanatus)
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification of LecRLKs in Watermelon
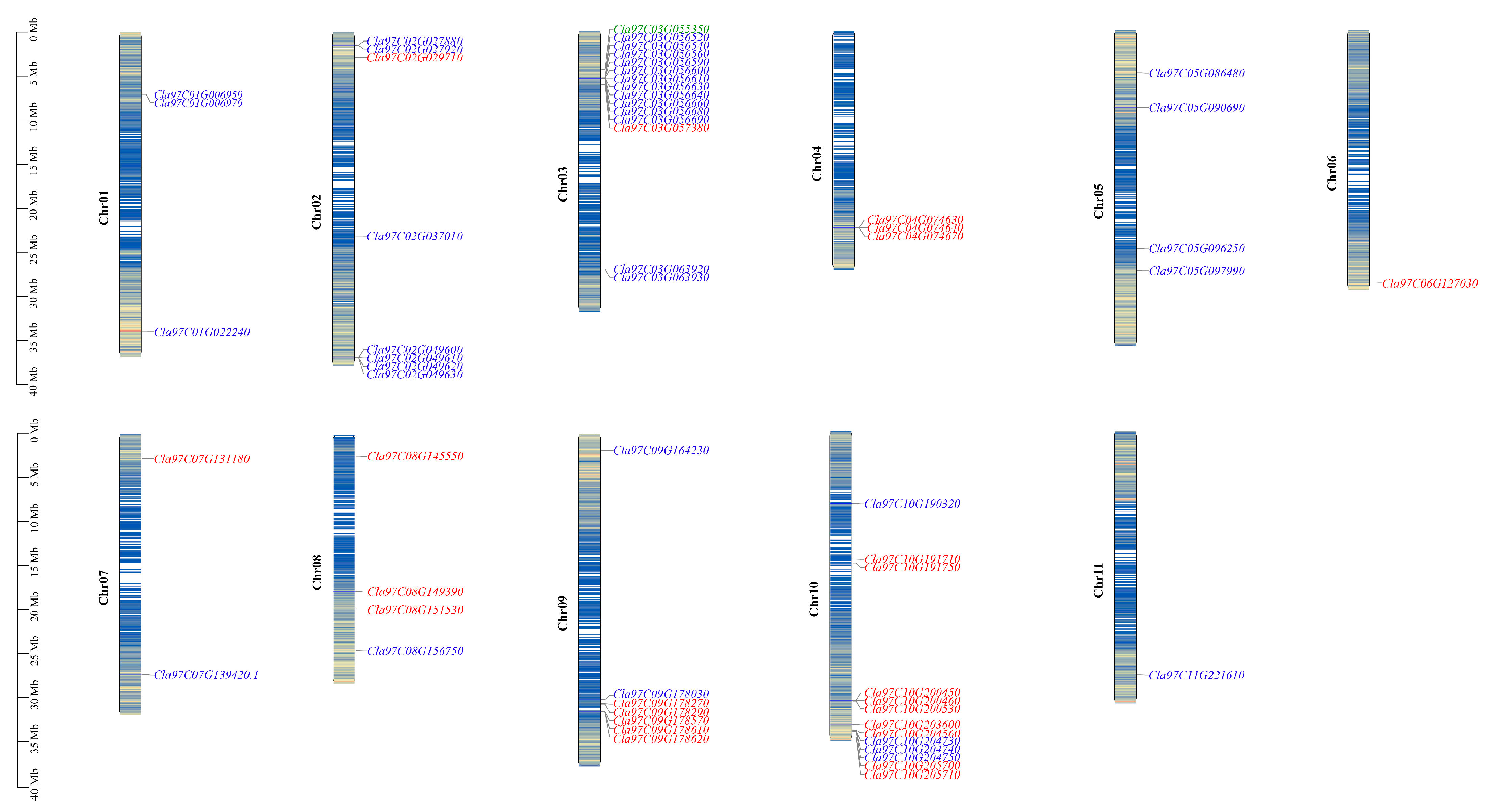
2.2. Chromosomal Location and Gene Duplication of ClaLecRLK Genes
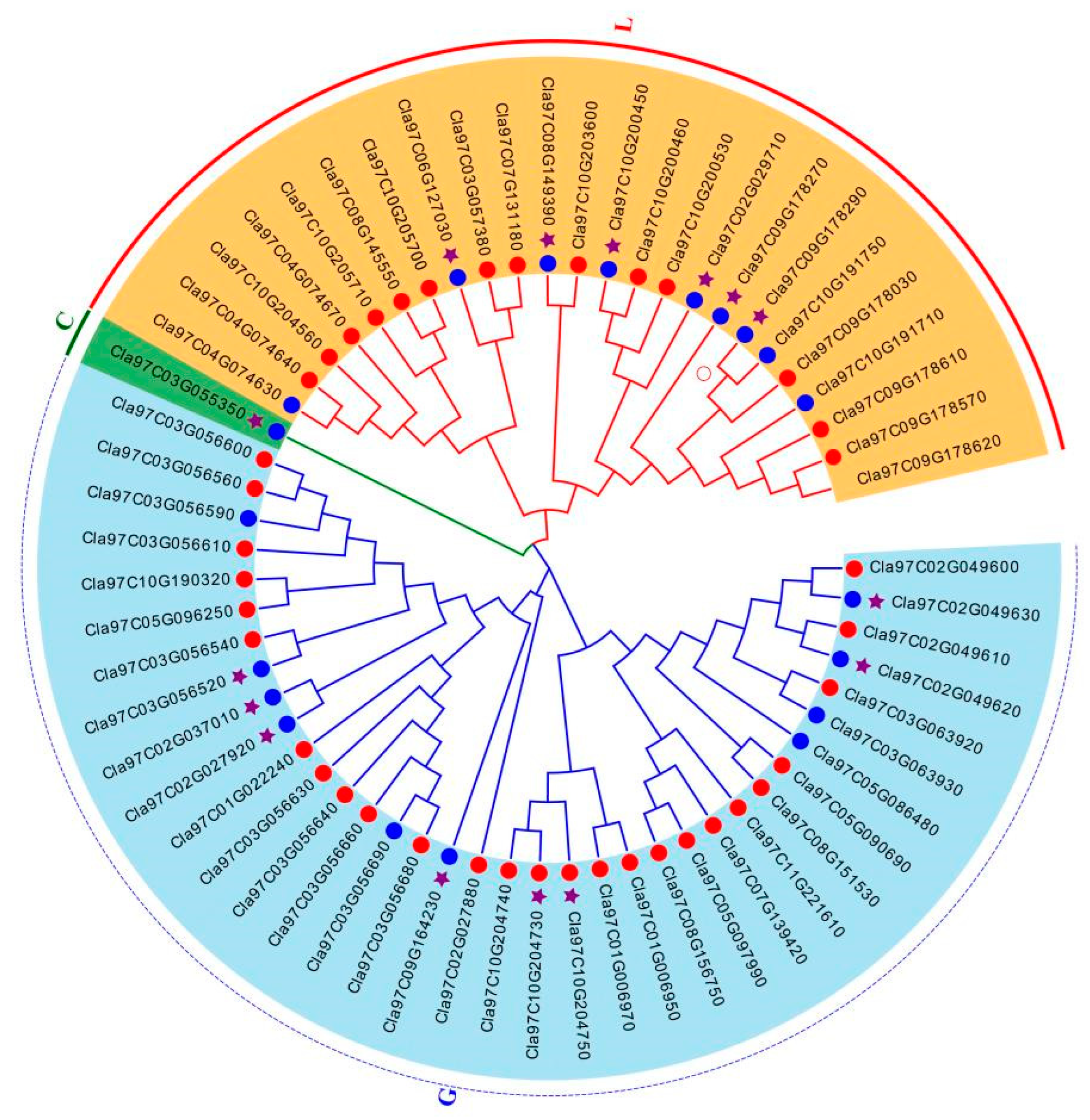
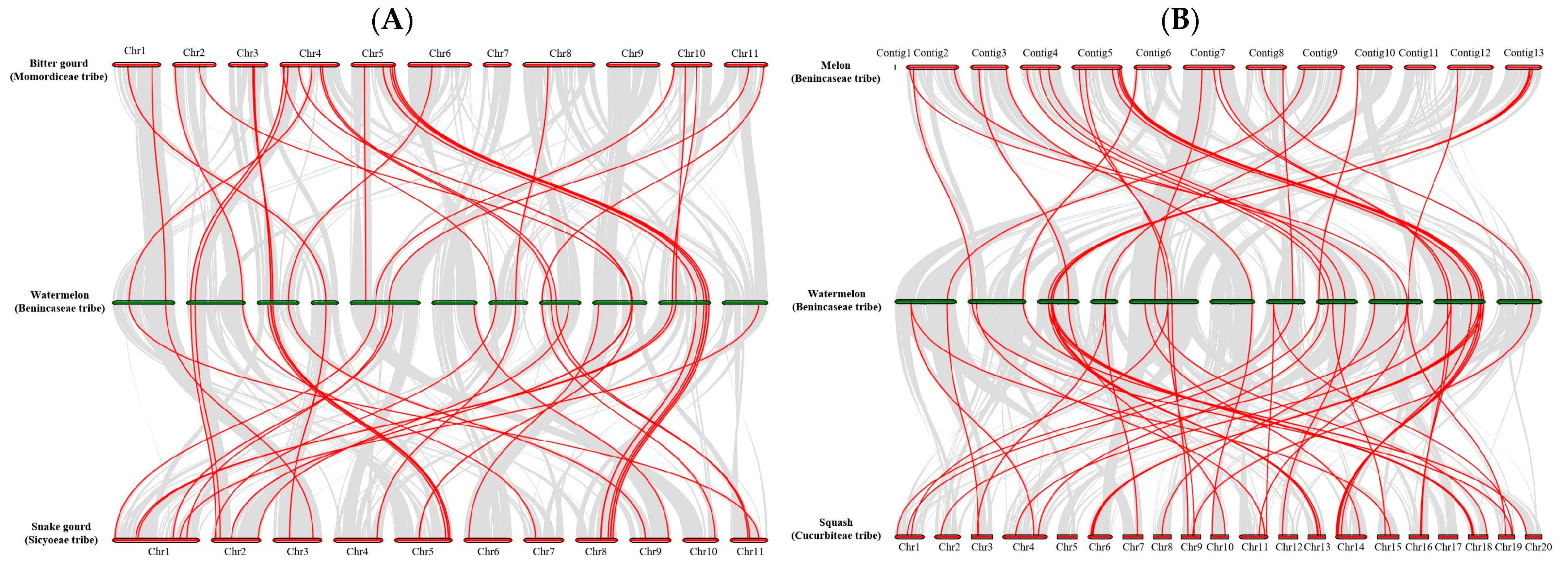
2.3. Analysis of Phylogenetic and Synteny in ClaLecRLK Genes
2.4. Gene Structure and Protein Motif Analysis of ClaLecRLK Genes
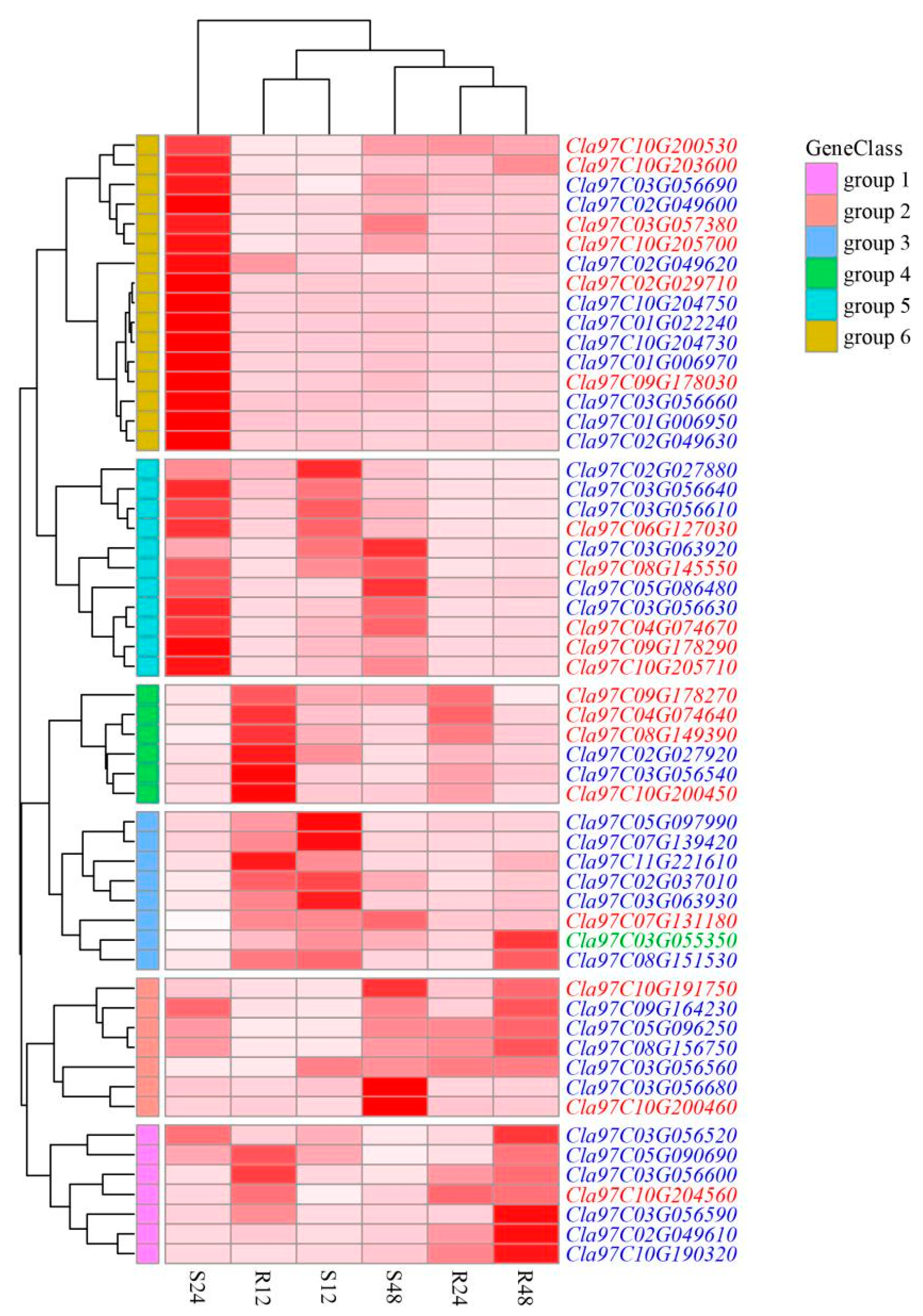
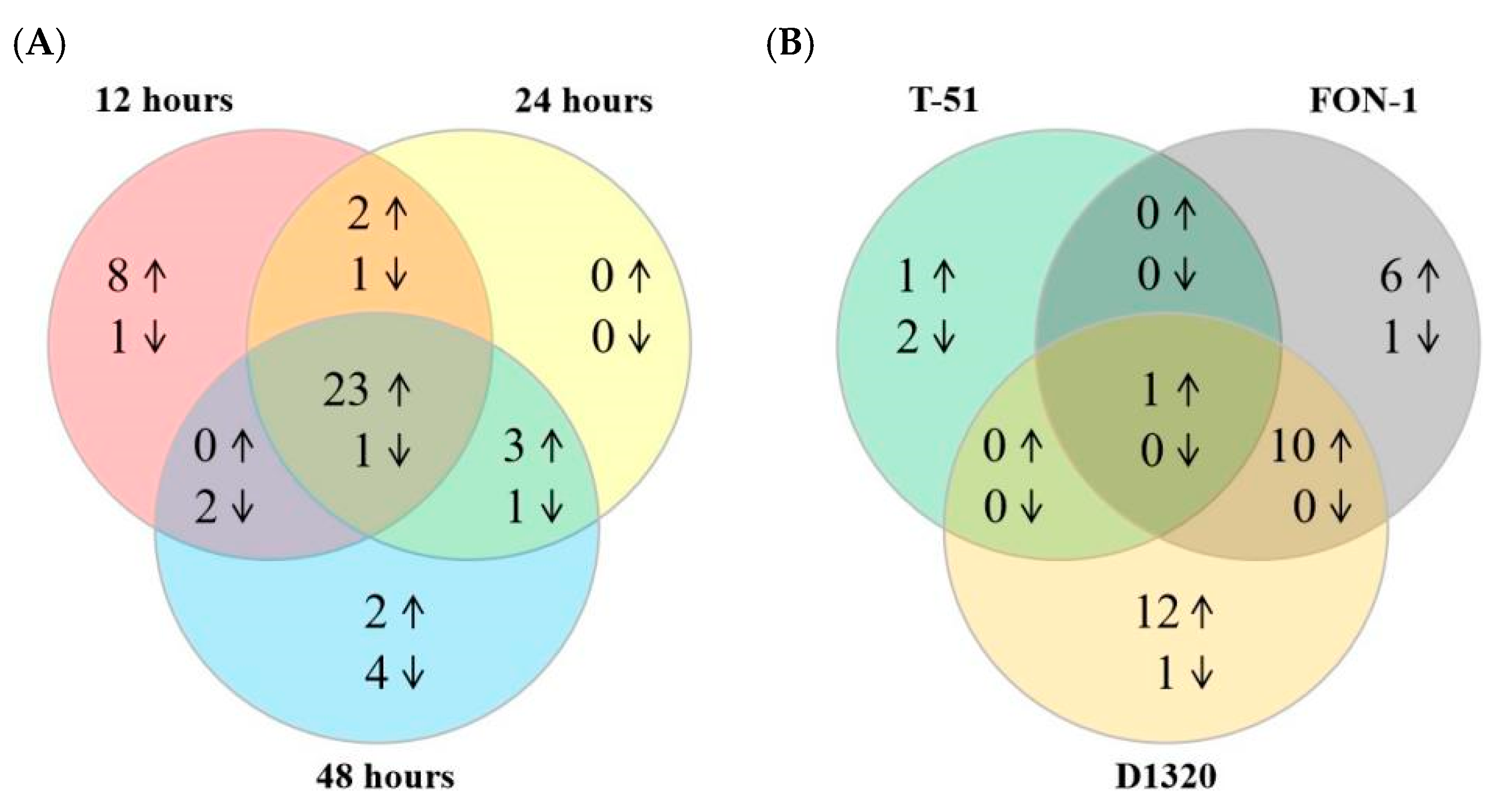
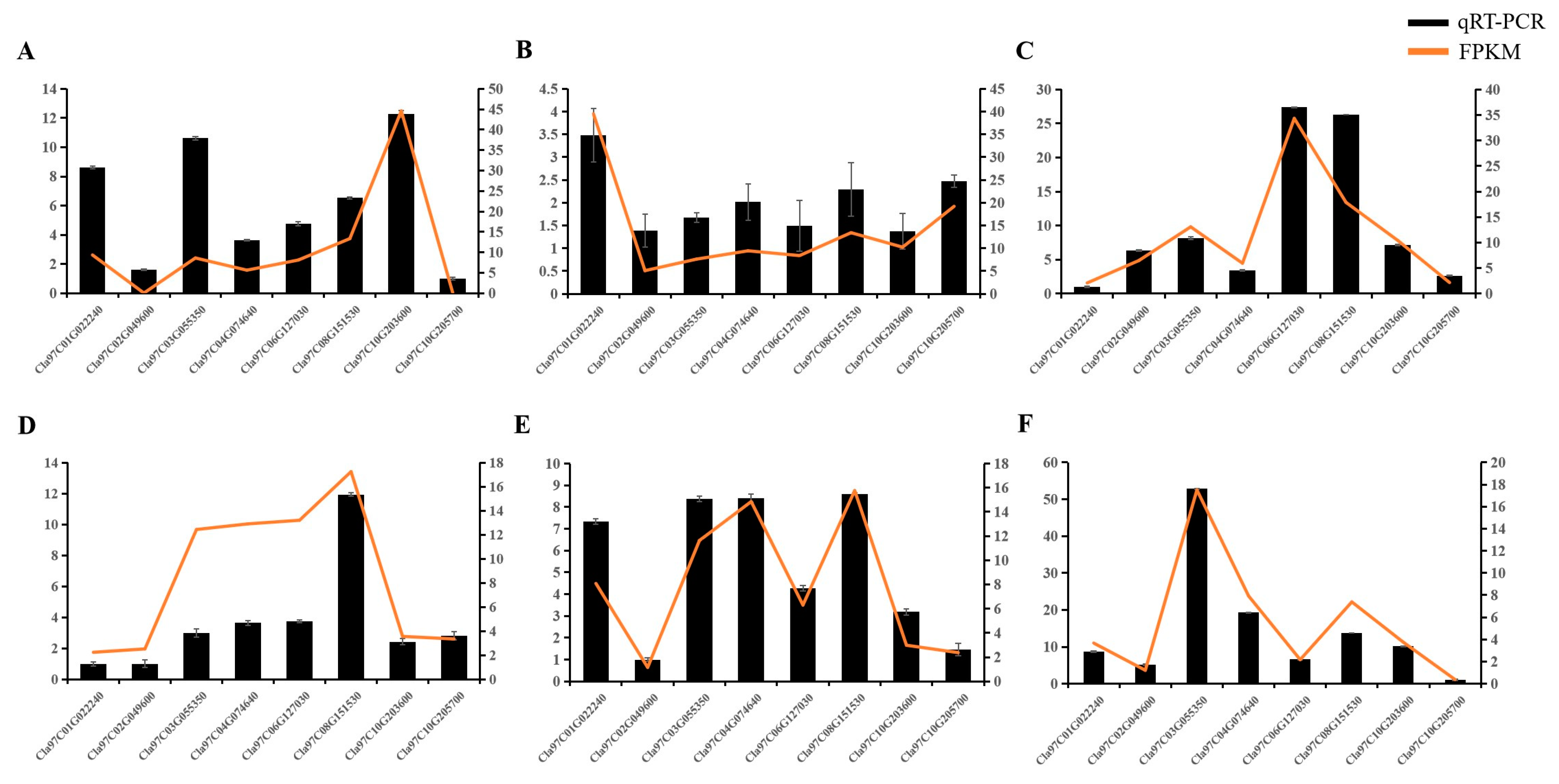
2.5. Response of LecRLK Genes to Fungal Infection in Watermelon
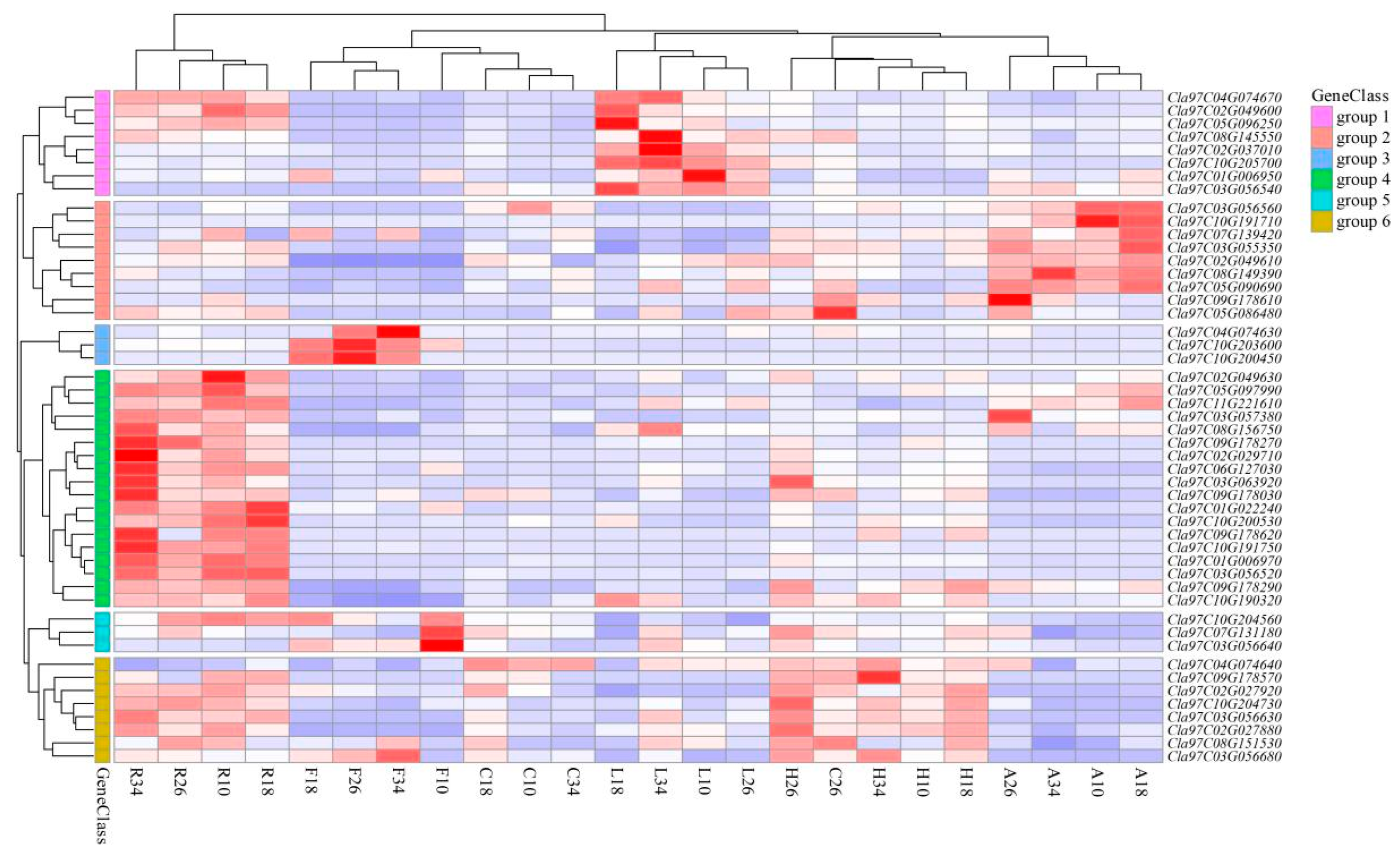
2.6. Tissue-Specific Expression of ClaLecRLK Genes
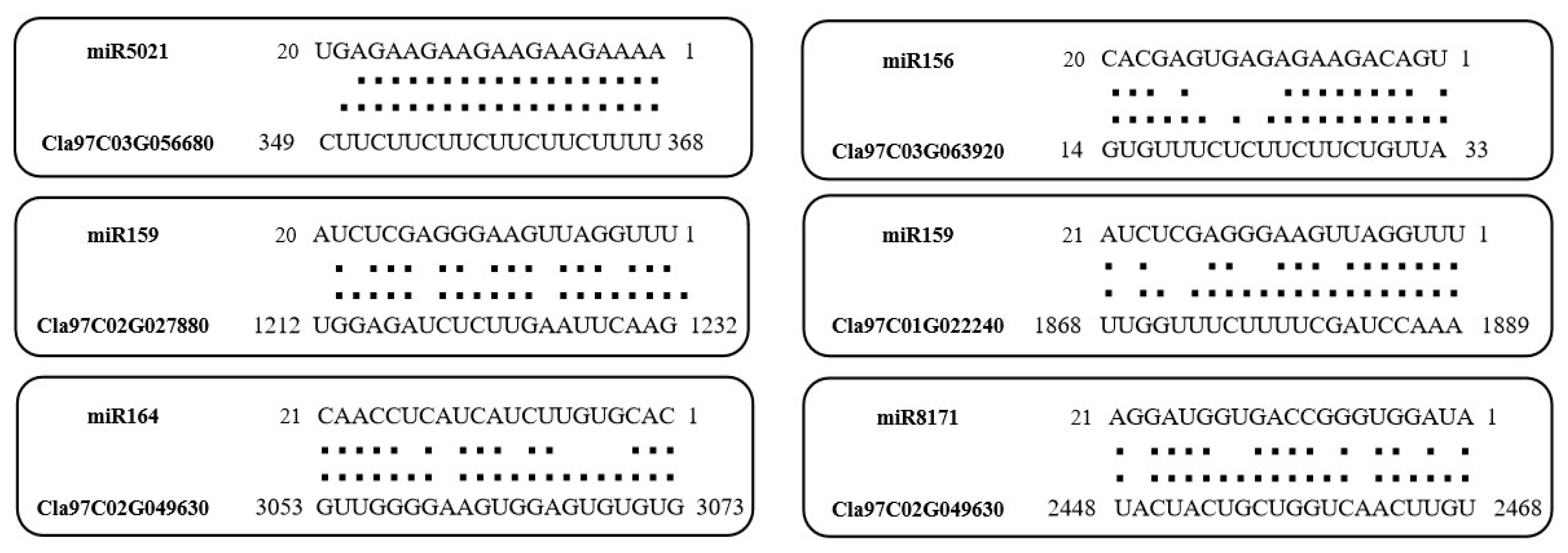
2.7. Prediction Analysis of ClaLecRLK Genes Targeted by miRNA
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Fungal Culture
4.2. Identification of LecRLK Genes in Different Plants
4.3. Analysis of Phylogenetic and Gene Structure
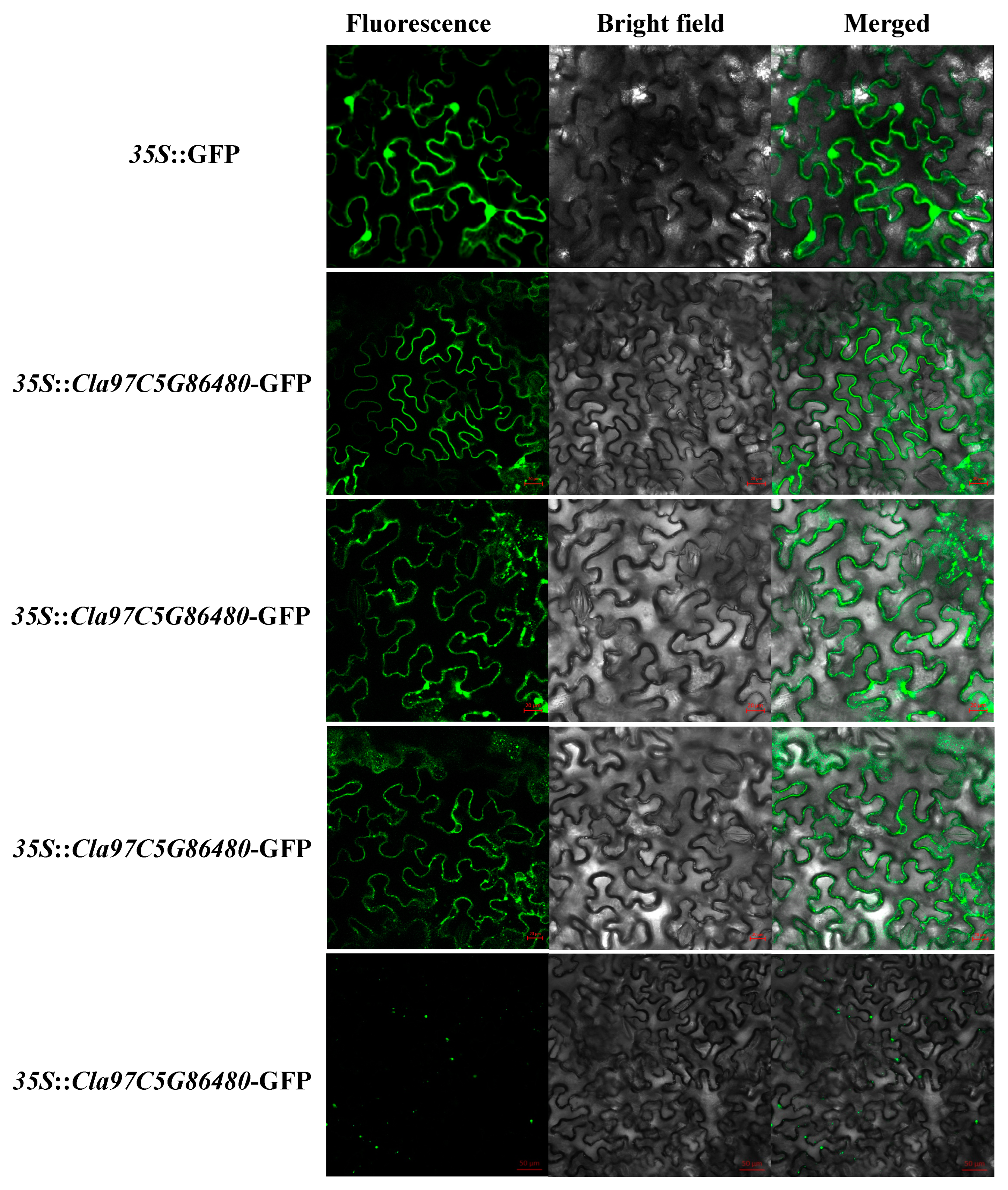
4.4. Tissue-Specific Expression Pattern Analysis and Subcellular Localization
4.5. Chromosome Localization and Duplication Analyses
4.6. Analysis of Conserved Motif and Synteny
4.7. RNA-Seq of Fungal Inoculation
4.8. Prediction of Binding Sites of the ClaLecRLK Genes Targeted by miRNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Vaid, N.; Macovei, A.; Tuteja, N. Knights in action: Lectin receptor-like kinases in plant development and stress responses. Mol. Plant 2013, 6, 1405–1418. [Google Scholar] [CrossRef]
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, G.; Xiong, L.R.; Sun, J.X.; Chen, Y.; Guo, C.L.; Yu, Y.; He, H.L.; Cai, R.; Pan, J.S. Genome-Wide Identification and Characterization of Lectin Receptor-like Kinase Gene Family in Cucumber and Expression Profiling Analysis under Different Treatments. Genes 2020, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Tanksley, S.D.; Loaiza-Figueroa, F. Gametophytic self-incompatibility is controlled by a single major locus on chromosome 1 in Lycopersicon peruvianum. Proc. Natl. Acad. Sci. USA 1985, 82, 5093–5096. [Google Scholar] [CrossRef]
- Cambi, A.; Koopman, M.; Figdor, C.G. How C-type lectins detect pathogens. Cell Microbiol. 2005, 7, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, J.; Chen, D.; Lei, C.; Zou, Y.; Zhai, W.; Liu, G.; Xu, J.; Ling, Z.; Cao, G.; et al. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006, 46, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.; Serres, J.; Dabos, P.; Canut, H.; Barre, A.; Rougé, P.; Lescure, B. Characterization of the Arabidopsis lecRK-a genes: Members of a superfamily encoding putative receptors with an extracellular domain homologous to legume lectins. Plant Mol. Biol. 1999, 39, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, X.; Wang, Y.; Nie, J.; Yang, Y.; Sun, J.; Du, H.; Zhu, W.; Pan, J.; Chen, Y.; et al. Identification and mapping of ts (tender spines), a gene involved in soft spine development in Cucumis sativus. Theor. Appl. Genet. 2018, 131, 1–12. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, G.; Zhao, J.L.; Zhang, L.Q.; Ai, L.F.; Han, Y.F.; Sun, D.Y.; Zhang, S.W.; Sun, Y. The Receptor-like Kinase SIT1 Mediates Salt Sensitivity by Activating MAPK3/6 and Regulating Ethylene Homeostasis in Rice. Plant Cell 2014, 26, 2538–2553. [Google Scholar] [CrossRef]
- Kanzaki, H.; Saitoh, H.; Takahashi, Y.; Berberich, T.; Ito, A.; Kamoun, S.; Terauchi, R. NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta 2008, 228, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G. The Nicotiana attenuata LECTIN RECEPTOR KINASE 1 is involved in the perception of insect feeding. Plant Signal Behav. 2011, 6, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Zheng, Y.; Guo, J.; Yuan, S.; Fu, A.; Bai, C.; Zhao, X.; Zheng, S.; Wen, C.; et al. Cucurbitaceae genome evolution, gene function and molecular breeding. Hortic. Res. 2022, 9, uhab057. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.; Heibl, C.; Renner, S.S. Gourds afloat: A dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc. Biol. Sci. 2009, 276, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Denna, D.W. Expression of determinate habit in cucumbers (Cucumis sativus L.). Amer Soc. Hort. Sci. J. 1971, 96, 277–279. [Google Scholar] [CrossRef]
- Nie, J.T.; Wang, Y.L.; He, H.L.; Guo, C.L.; Zhu, W.Y.; Pan, J.; Li, D.D.; Lian, H.L.; Pan, J.S.; Cai, R. Loss-of-Function Mutations in Confer Durable Powdery Mildew Resistance in Cucumber (L.). Front. Plant Sci. 2015, 6, 1155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Labbé, J.; Muchero, W.; Yang, X.; Jawdy, S.S.; Kennedy, M.; Johnson, J.; Sreedasyam, A.; Schmutz, J.; Tuskan, G.A.; et al. Genome-wide analysis of lectin receptor-like kinases in Populus. BMC Genom. 2016, 17, 699. [Google Scholar] [CrossRef] [PubMed]
- Mehan, M.R.; Freimer, N.B.; Ophoff, R.A. A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. Hum. Genom. 2004, 1, 335–344. [Google Scholar] [CrossRef]
- Kent, W.J.; Baertsch, R.; Hinrichs, A.; Miller, W.; Haussler, D. Evolution’s cauldron: Duplication, deletion, and rearrangement in the mouse and human genomes. Proc. Natl. Acad. Sci. USA 2003, 100, 11484–11489. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lambel, S.; Lanini, B.; Vivoda, E.; Fauve, J.; Patrick Wechter, W.; Harris-Shultz, K.R.; Massey, L.; Levi, A. A major QTL associated with Fusarium oxysporum race 1 resistance identified in genetic populations derived from closely related watermelon lines using selective genotyping and genotyping-by-sequencing for SNP discovery. Theor. Appl. Genet. 2014, 127, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Kwon, O.K.; Jeong, A.R.; Lee, H.; Moon, H.; Lee, O.N.; Hong, J.K.; Park, C.J. Population Structure of Stagonosporopsis Species Associated with Cucurbit Gummy Stem Blight in Korea. Plant Pathol. J. 2022, 38, 522–532. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological Control and Plant Growth Promotion by Volatile Organic Compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Vaid, N.; Pandey, P.K.; Tuteja, N. Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice. Plant Mol. Biol. 2012, 80, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Everts, K.L. Suppression of Fusarium Wilt of Watermelon by Soil Amendment with Hairy Vetch. Plant Dis. 2004, 88, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P. From Native Plants in Central Europe to Cultivated Crops Worldwide: The Emergence of Didymella bryoniae as a Cucurbit Pathogen. HortScience 2011, 46, 532–535. [Google Scholar] [CrossRef]
- You, J.; Zhang, J.; Wu, M.; Yang, L.; Li, G. Multiple Criteria-based Screening of Trichoderma isolates for Biological Control of Botrytis cinerea on Tomato. Biol. Control 2016, 101, 31–38. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Wyler, M.; Schmid, M.W.; Kadota, Y.; Shirasu, K. Evolutionary trajectory of pattern recognition receptors in plants. Nat. Commun. 2024, 15, 308. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Heal, R.; Wyler, M.; Schmid, M.W.; Jones, J.D.G. Concerted expansion and contraction of immune receptor gene repertoires in plant genomes. Nat. Plants 2022, 8, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, G.; Zhang, Q.; Yu, Y.; Qin, P.C.; Pang, J.A.; Sun, J.X.; Zhang, K.Y.; He, H.L.; Cai, R. Comparative Transcriptome Analysis of Hard and Tender Fruit Spines of Cucumber to Identify Genes Involved in the Morphological Development of Fruit Spines. Front. Plant Sci. 2022, 13, 797433. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xue, S.; Shan, L.; Fan, S.; Sun, L.; Dong, Y.; Li, S.; Gao, Y.; Qi, Y.; Yang, L.; et al. The CsTM alters multicellular trichome morphology and enhances resistance against aphid by interacting with CsTIP1;1 in cucumber. J. Adv. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, S.; Yu, B. microRNA biogenesis, degradation and activity in plants. Cell Mol. Life Sci. 2015, 72, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ye, M.; Sang, M.; Wu, R. A Regulatory Network for miR156-SPL Module in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6166. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, D.; Palatnik, J.F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: A conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 2020, 53, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.M. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.S.; Eddy, S.R.; Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 2010, 11, 431. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–d496. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Gu, Z.; Cavalcanti, A.; Chen, F.C.; Bouman, P.; Li, W.H. Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol. Biol. Evol. 2002, 19, 256–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.; Yu, J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]

| Gene ID | Gene | CDS | Protein | MW | pI | Localization | Exon | Intron | |
|---|---|---|---|---|---|---|---|---|---|
| (bp) | (bp) | (aa) | (KDa) | ||||||
| C-type | Cla97C03G055350 | 2533 | 1689 | 562 | 63.4 | 9.26 | PM | 5 | 4 |
| L-type | Cla97C02G029710 | 2349 | 2076 | 691 | 77.7 | 6.25 | PM | 2 | 1 |
| Cla97C03G057380 | 2052 | 2052 | 683 | 75.7 | 6.13 | PM | 1 | 0 | |
| Cla97C04G074630 | 707 | 588 | 195 | 22.1 | 10.04 | PM | 3 | 2 | |
| Cla97C04G074640 | 1983 | 1983 | 660 | 73.4 | 6.66 | PM | 1 | 0 | |
| Cla97C04G074670 | 5405 | 2046 | 681 | 76.2 | 6.49 | PM | 2 | 1 | |
| Cla97C06G127030 | 1971 | 1971 | 656 | 73.2 | 5.16 | PM | 1 | 0 | |
| Cla97C07G131180 | 2055 | 2055 | 684 | 76.0 | 5.58 | PM | 1 | 0 | |
| Cla97C08G145550 | 1992 | 1992 | 663 | 73.8 | 6.12 | PM | 1 | 0 | |
| Cla97C08G149390 | 3450 | 2247 | 748 | 83.0 | 6.74 | PM | 2 | 1 | |
| Cla97C09G178030 | 2031 | 2031 | 676 | 74.9 | 5.44 | PM | 1 | 0 | |
| Cla97C09G178270 | 2121 | 2121 | 706 | 79.7 | 6.18 | PM | 1 | 0 | |
| Cla97C09G178290 | 2130 | 2130 | 709 | 79.2 | 6.22 | PM | 1 | 0 | |
| Cla97C09G178570 | 1971 | 1971 | 656 | 73.2 | 5.93 | PM | 1 | 0 | |
| Cla97C09G178610 | 1944 | 1944 | 647 | 73.1 | 6.99 | PM | 1 | 0 | |
| Cla97C09G178620 | 1974 | 1974 | 657 | 73.6 | 5.89 | PM | 1 | 0 | |
| Cla97C10G191710 | 2602 | 1677 | 558 | 62.5 | 6.27 | PM | 2 | 1 | |
| Cla97C10G191750 | 2162 | 2079 | 692 | 77.3 | 5.97 | PM | 2 | 1 | |
| Cla97C10G200450 | 1989 | 1989 | 662 | 74.2 | 7.92 | PM | 1 | 0 | |
| Cla97C10G200460 | 2001 | 2001 | 666 | 74.4 | 5.88 | PM | 1 | 0 | |
| Cla97C10G200530 | 2028 | 2028 | 675 | 75.2 | 5.32 | PM | 1 | 0 | |
| Cla97C10G203600 | 2178 | 2178 | 725 | 78.2 | 5.79 | PM | 1 | 0 | |
| Cla97C10G204560 | 2148 | 2052 | 683 | 74.9 | 6.45 | PM | 2 | 1 | |
| Cla97C10G205700 | 2004 | 2004 | 667 | 74.2 | 6.65 | PM | 1 | 0 | |
| Cla97C10G205710 | 2046 | 2046 | 681 | 75.2 | 5.78 | PM | 1 | 0 | |
| Range | 707–5405 | 588–2247 | 195–748 | 22.1–83 | 5.16–10.04 | 1–3 | 1–2 | ||
| Average | 2221 | 1968 | 655 | 73.0 | 6.33 | 1.3 | 0.3 | ||
| G-type | Cla97C01G006950 | 2466 | 2466 | 821 | 91.6 | 8.37 | Ec | 1 | 0 |
| Cla97C01G006970 | 2394 | 2394 | 797 | 88.9 | 8.72 | Ec | 1 | 0 | |
| Cla97C01G022240 | 3318 | 2580 | 859 | 96.2 | 5.30 | PM | 7 | 6 | |
| Cla97C02G027880 | 2493 | 2493 | 830 | 92.3 | 6.77 | PM | 1 | 0 | |
| Cla97C02G027920 | 3740 | 3096 | 1031 | 116.2 | 5.65 | PM | 8 | 7 | |
| Cla97C02G037010 | 2772 | 2433 | 810 | 92.0 | 8.35 | PM | 5 | 4 | |
| Cla97C02G049600 | 2424 | 2424 | 807 | 90.1 | 5.70 | PM | 1 | 0 | |
| Cla97C02G049610 | 2409 | 2409 | 802 | 90.1 | 5.90 | PM | 1 | 0 | |
| Cla97C02G049620 | 2481 | 2481 | 826 | 92.7 | 6.00 | PM | 1 | 0 | |
| Cla97C02G049630 | 5749 | 4557 | 1518 | 171.1 | 6.41 | Ec | 2 | 1 | |
| Cla97C03G056520 | 4511 | 2553 | 850 | 96.3 | 7.37 | PM | 7 | 6 | |
| Cla97C03G056540 | 4213 | 2505 | 834 | 94.0 | 6.87 | PM | 7 | 6 | |
| Cla97C03G056560 | 3185 | 2427 | 808 | 90.2 | 5.78 | PM | 7 | 6 | |
| Cla97C03G056590 | 3255 | 2421 | 806 | 91.5 | 7.49 | PM | 7 | 6 | |
| Cla97C03G056600 | 3111 | 2418 | 805 | 90.7 | 5.95 | PM | 7 | 6 | |
| Cla97C03G056610 | 3314 | 2445 | 814 | 92.1 | 6.17 | PM | 7 | 6 | |
| Cla97C03G056630 | 3007 | 2466 | 821 | 92.8 | 6.19 | PM | 7 | 6 | |
| Cla97C03G056640 | 3119 | 2532 | 843 | 95.7 | 7.99 | PM | 7 | 6 | |
| Cla97C03G056660 | 10,301 | 2049 | 682 | 77.0 | 5.86 | PM | 6 | 5 | |
| Cla97C03G056680 | 3185 | 2553 | 850 | 95.9 | 6.00 | PM | 7 | 6 | |
| Cla97C03G056690 | 2598 | 2145 | 714 | 80.8 | 7.77 | PM | 4 | 3 | |
| Cla97C03G063920 | 2448 | 2448 | 815 | 91.2 | 5.20 | PM | 1 | 0 | |
| Cla97C03G063930 | 2361 | 2361 | 786 | 89.8 | 5.88 | PM | 1 | 0 | |
| Cla97C05G086480 | 1979 | 1926 | 641 | 72.5 | 6.12 | PM | 2 | 1 | |
| Cla97C05G090690 | 5800 | 2604 | 867 | 95.4 | 5.83 | PM | 5 | 4 | |
| Cla97C05G096250 | 4180 | 2433 | 810 | 90.7 | 6.78 | PM | 7 | 6 | |
| Cla97C05G097990 | 2466 | 2466 | 821 | 90.3 | 5.74 | PM | 1 | 0 | |
| Cla97C07G139420 | 2869 | 2460 | 819 | 92.3 | 7.99 | PM | 2 | 1 | |
| Cla97C08G151530 | 2556 | 2556 | 851 | 92.7 | 5.94 | PM | 1 | 0 | |
| Cla97C08G156750 | 2466 | 2466 | 821 | 90.5 | 5.70 | PM | 1 | 0 | |
| Cla97C09G164230 | 4117 | 2703 | 900 | 102.6 | 6.08 | PM | 7 | 6 | |
| Cla97C10G190320 | 15,727 | 2457 | 818 | 92.6 | 8.21 | PM | 7 | 6 | |
| Cla97C10G204730 | 2436 | 2436 | 811 | 91.1 | 5.74 | PM | 1 | 0 | |
| Cla97C10G204740 | 2439 | 2439 | 812 | 91.3 | 5.14 | PM | 1 | 0 | |
| Cla97C10G204750 | 2391 | 2391 | 796 | 89.5 | 6.19 | PM | 1 | 0 | |
| Cla97C11G221610 | 4269 | 2604 | 867 | 95.6 | 6.08 | PM | 2 | 1 | |
| Range | 1979–15,727 | 1926–4557 | 641–1518 | 72.5–171.1 | 5.14–8.72 | 1–8 | 0–7 | ||
| Average | 3682 | 2517 | 838 | 94.1 | 6.48 | 3.90 | 2.90 | ||
| Range | 707–15,727 | 588–4557 | 195–1518 | 63.4–171.1 | 5.14–10.04 | ||||
| Average | 3088 | 2287 | 761 | 85.3 | 6.46 |
| Species | Dicot | Monocot | |||||
|---|---|---|---|---|---|---|---|
| Bitter Gourd | Snake Gourd | Monk Gourd | Melon | Watermelon | Squash | Rice | |
| Tribe | Momordiceae | Sicyoeae | Siraitieae | Benincaseae | Benincaseae | Cucurbiteae | |
| Gene ID | MC02G0084 | Tan0002591 | Sgr014680 | MELO3C014624 | Cla97C02G049630 | CmoCh20G000680 | |
| MC03Gnew0349 | Tan0014355 | Sgr029435 | MELO3C002524 | Cla97C03G056520 | CmoCh13G007160 | ||
| MC03G0790 | Tan0016767 | Sgr019525 | MELO3C002606 | Cla97C03G057380 | CmoCh13G006610 | ||
| CmoCh18G003480 | |||||||
| MC01G0434 | Tan0008913 | Sgr026531 | MELO3C003146 | Cla97C04G074670 | CmoCh07G010510 | ||
| MC11G0930 | Tan0015648 | Sgr028234 | MELO3C018168 | Cla97C05G096250 | CmoCh01G012240 | Os01G0784700 | |
| CmoCh09G009010 | Os04G0633200 | ||||||
| MC08G0987 | Tan0015848 | Sgr015781 | MELO3C017881 | Cla97C07G139420 | CmoCh01G001500 | ||
| MC04G1328 | Tan0003615 | Sgr014359 | MELO3C008394 | Cla97C08G149390 | CmoCh04G025490 | ||
| MC10G0972 | Tan0002296 | Sgr020073 | MELO3C016574 | Cla97C10G190320 | CmoCh16G008700 | Os05G0501400 | |
| MC10G0515 | Tan0018666 | Sgr020674 | MELO3C014953 | Cla97C10G191750 | CmoCh04G009160 | ||
| CmoCh16G007980 | |||||||
| MC05Gnew0379 | Tan0005166 | Sgr022715 | MELO3C009237 | Cla97C10G204560 | CmoCh06G002850 | ||
| CmoCh14G001210 | |||||||
| Subfamily | Gene ID | Fungi | Regulated Direction | |||
|---|---|---|---|---|---|---|
| T51 | FON-1 | D1320 | Up | Down | ||
| L-type | Cla97C10G205710 | ✔ | ✔ | ✔ | ||
| Cla97C09G178290 | ✔ | ✔ | ✔ | |||
| Cla97C02G029710 | ✔ | ✔ | ||||
| Cla97C04G074670 | ✔ | ✔ | ||||
| Cla97C08G145550 | ✔ | ✔ | ||||
| Cla97C10G200450 | ✔ | ✔ | ||||
| Cla97C09G178030 | ✔ | ✔ | ✔ | |||
| Cla97C10G191750 | ✔ | ✔ | ||||
| Cla97C09G178270 | ✔ | ✔ | ||||
| Cla97C10G205700 | ✔ | ✔ | ||||
| Cla97C10G203600 | ✔ | |||||
| Cla97C08G149390 | ✔ | ✔ | ||||
| Cla97C03G057380 | ✔ | ✔ | ||||
| G-type | Cla97C05G096250 | ✔ | ✔ | ✔ | ||
| Cla97C01G022240 | ✔ | ✔ | ✔ | |||
| Cla97C02G037010 | ✔ | ✔ | ✔ | |||
| Cla97C02G027880 | ✔ | ✔ | ||||
| Cla97C03G056660 | ✔ | ✔ | ||||
| Cla97C03G056640 | ✔ | ✔ | ✔ | |||
| Cla97C08G151530 | ✔ | ✔ | ✔ | ✔ | ||
| Cla97C10G204750 | ✔ | ✔ | ||||
| Cla97C01G006970 | ✔ | ✔ | ||||
| Cla97C10G204730 | ✔ | ✔ | ✔ | |||
| Cla97C03G063920 | ✔ | ✔ | ||||
| Cla97C09G164230 | ✔ | ✔ | ||||
| Cla97C02G049610 | ✔ | ✔ | ||||
| Cla97C03G063930 | ✔ | ✔ | ||||
| Cla97C03G056630 | ✔ | ✔ | ||||
| Cla97C05G086480 | ✔ | ✔ | ✔ | |||
| Cla97C03G056680 | ✔ | ✔ | ✔ | |||
| Cla97C03G056690 | ✔ | ✔ | ||||
| Cla97C10G190320 | ✔ | ✔ | ||||
| Cla97C08G156750 | ✔ | ✔ | ||||
| Cla97C02G049610 | ✔ | ✔ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, D.; Wang, G.; You, J.; Zhu, L.; Yang, H.; Cao, B.; Gu, W.; Li, C. Genome-Wide Analysis and Expression Profiling of Lectin Receptor-like Kinase Genes in Watermelon (Citrullus lanatus). Int. J. Mol. Sci. 2024, 25, 8257. https://doi.org/10.3390/ijms25158257
Lv D, Wang G, You J, Zhu L, Yang H, Cao B, Gu W, Li C. Genome-Wide Analysis and Expression Profiling of Lectin Receptor-like Kinase Genes in Watermelon (Citrullus lanatus). International Journal of Molecular Sciences. 2024; 25(15):8257. https://doi.org/10.3390/ijms25158257
Chicago/Turabian StyleLv, Duo, Gang Wang, Jiaqi You, Lihua Zhu, Hongjuan Yang, Biting Cao, Weihong Gu, and Chaohan Li. 2024. "Genome-Wide Analysis and Expression Profiling of Lectin Receptor-like Kinase Genes in Watermelon (Citrullus lanatus)" International Journal of Molecular Sciences 25, no. 15: 8257. https://doi.org/10.3390/ijms25158257





