LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response
Abstract
:1. Introduction
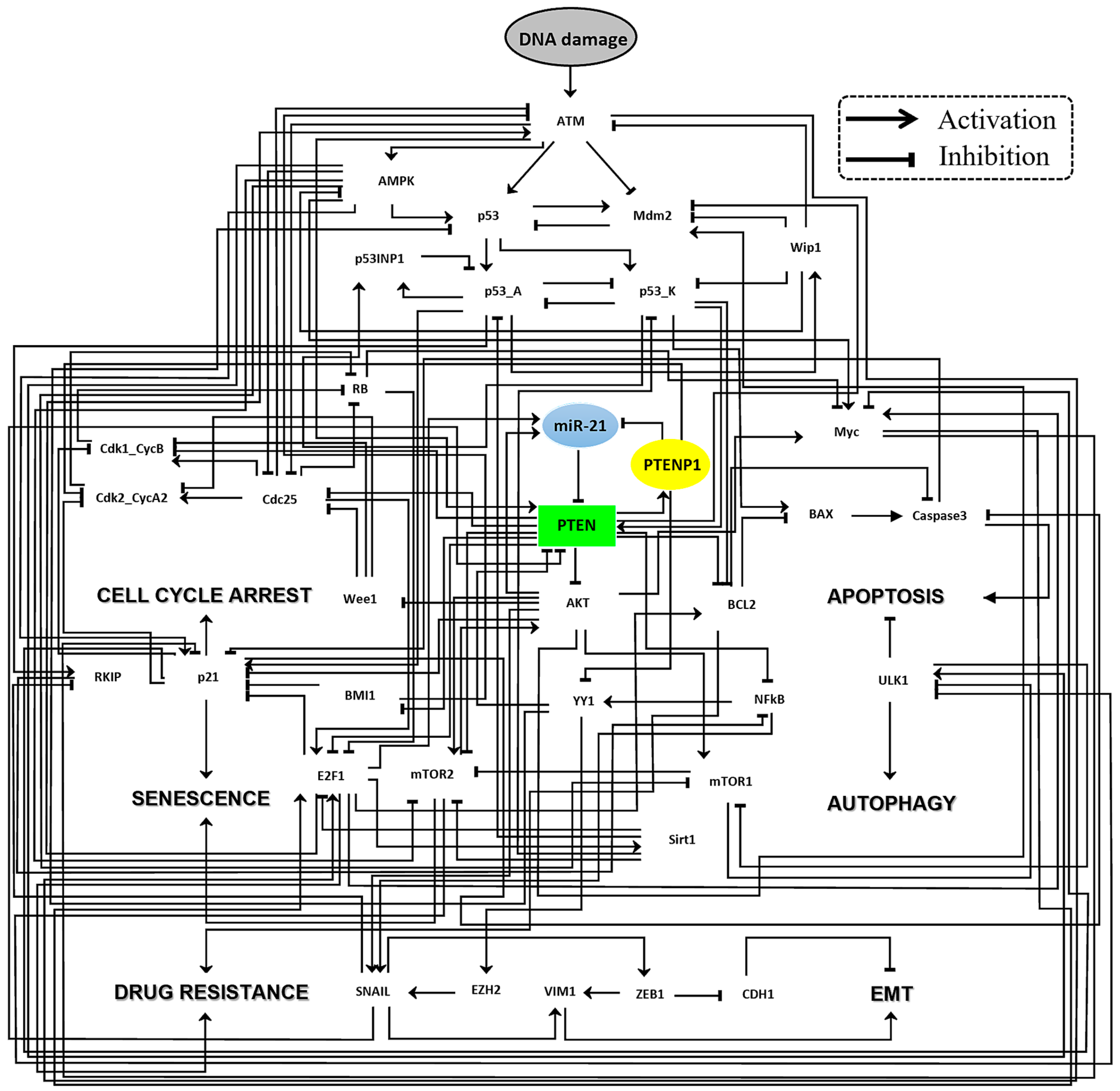
2. Results
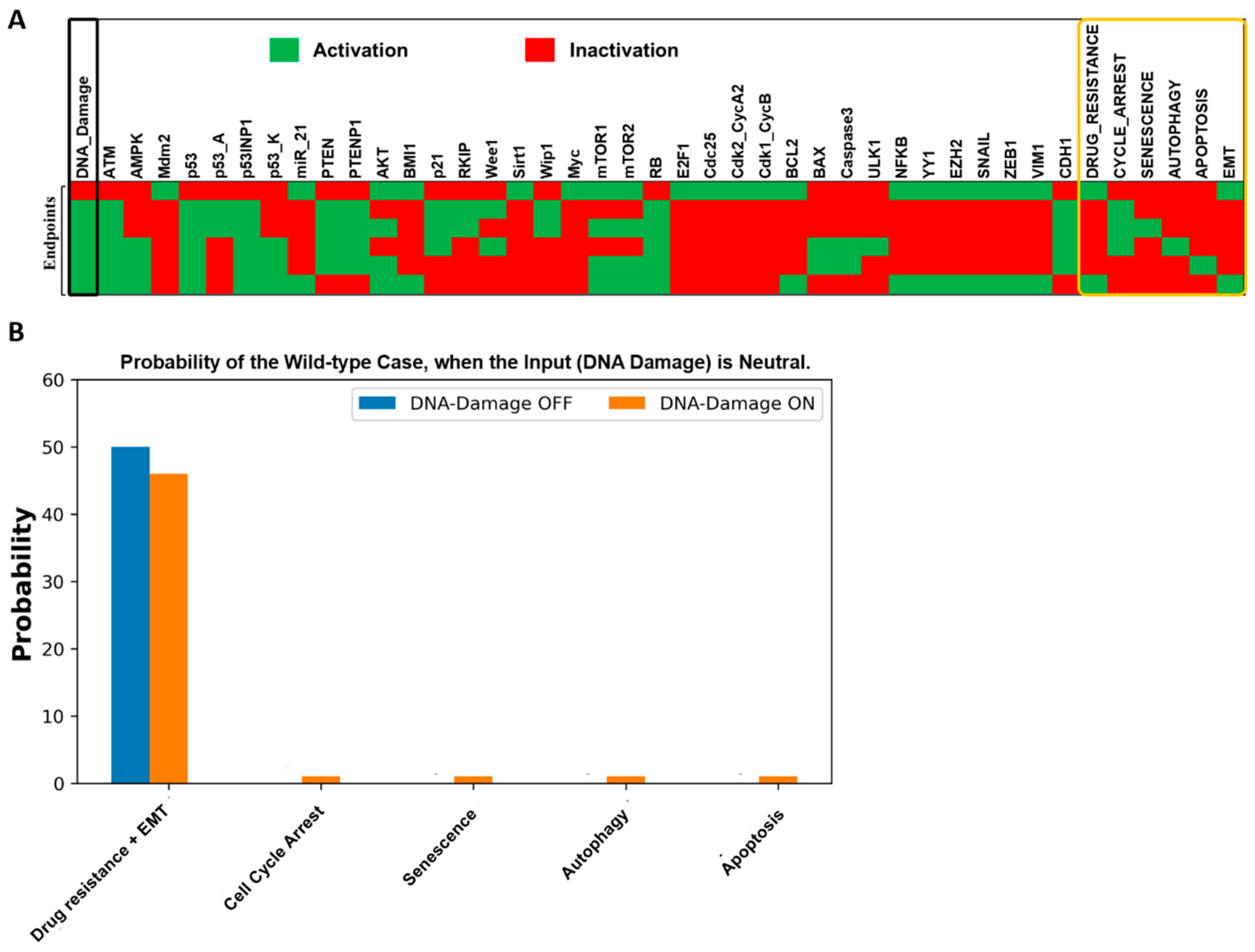
2.1. Fixed Point Analysis of the Wild-Type Scenario in the Boolean Network
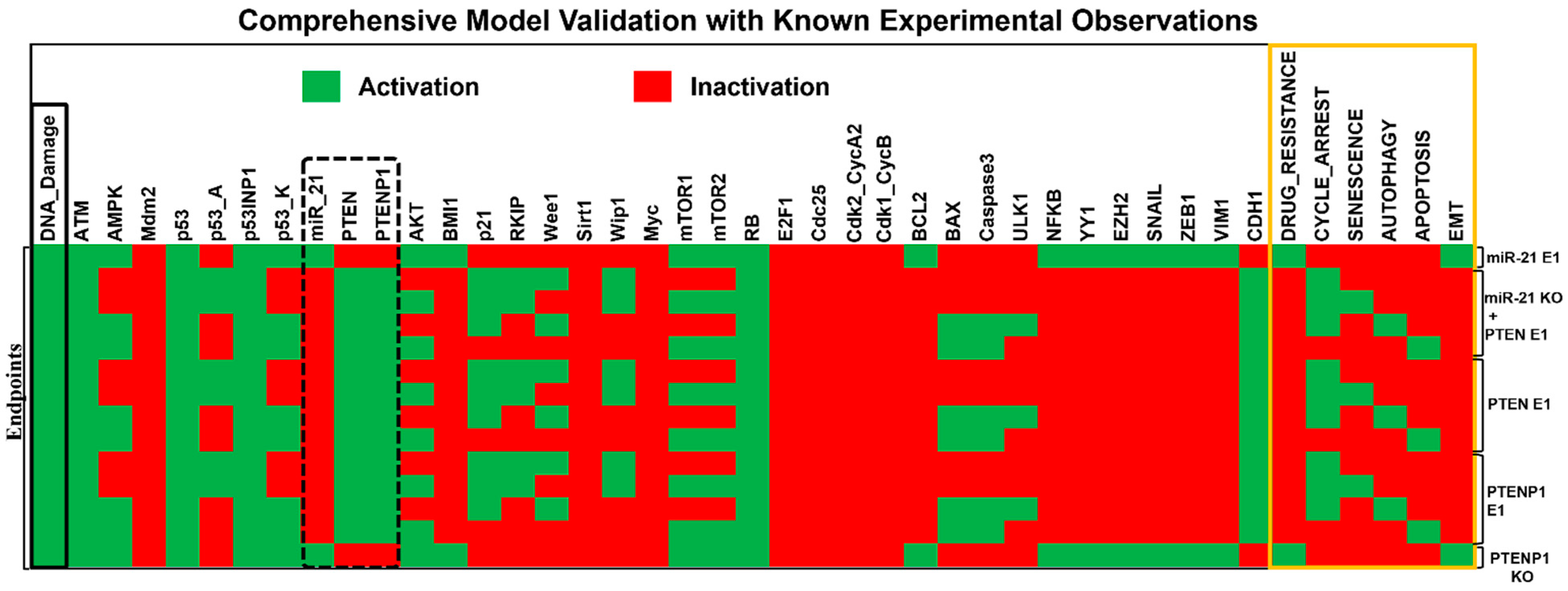
2.2. Dynamic Model Validation: In Silico Perturbation against Known Experimental Findings
| Cancer Type and Their Corresponding Cell Lines | Experimental Observation | References |
|---|---|---|
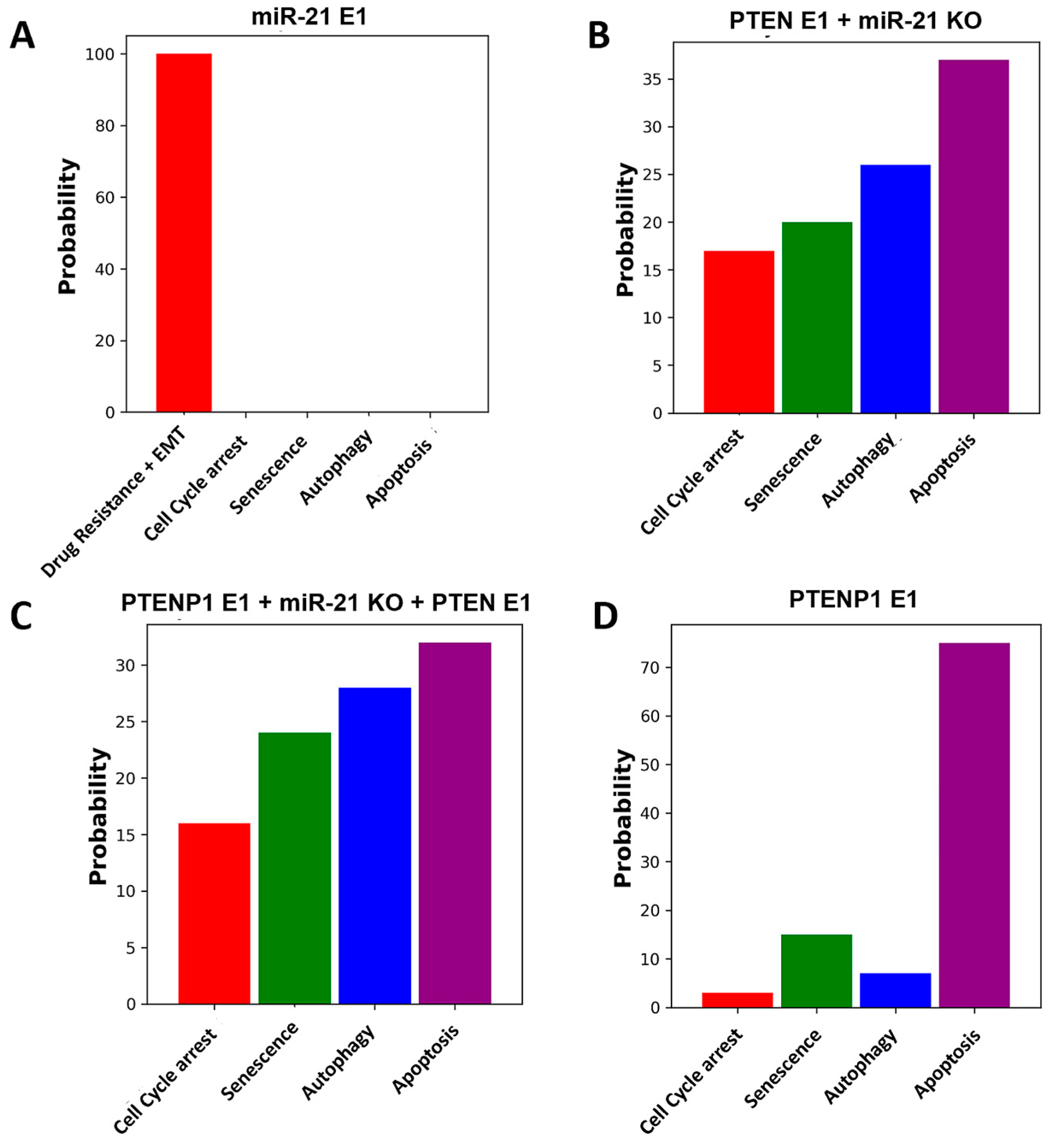
| Breast Cancer (cell lines MCF-7, MDA-MB-231, and ZR75-1). HCC (cell lines HepG2, SK-HEP1, SNU-182, SNU-449, PLC/PRF-5, KIM-1, KYN-1, KYN-2, KYN-3, HAK-1A, and HAK-1B). OSCC (cell lines SCC15, SCC25, Cal-27, HEK 293, and Tca-8113). | Overexpression (E1) of miR-21 inhibits PTEN and activates the AKT pathway, leading to proliferation, drug resistance, and EMT. Knockdown (KO) of miR-21 and upregulation (E1) of PTEN inhibits proliferation, drug resistance, and EMT. Upregulation (E1) of PTEN induces G2/M cell cycle arrest, apoptosis, and autophagy. Knockdown (KO) of PTENP1 induces drug resistance and EMT, while overexpression (E1) inhibits EMT and drug resistance. Overexpression (E1) of PTENP1 induces cell cycle arrest from the S to G2 phase and apoptosis. Overexpression (E1) of PTENP1 is involved in activating autophagy in hepatocellular carcinoma. | [9,19,21,22,23,24,25,26,27,30] |
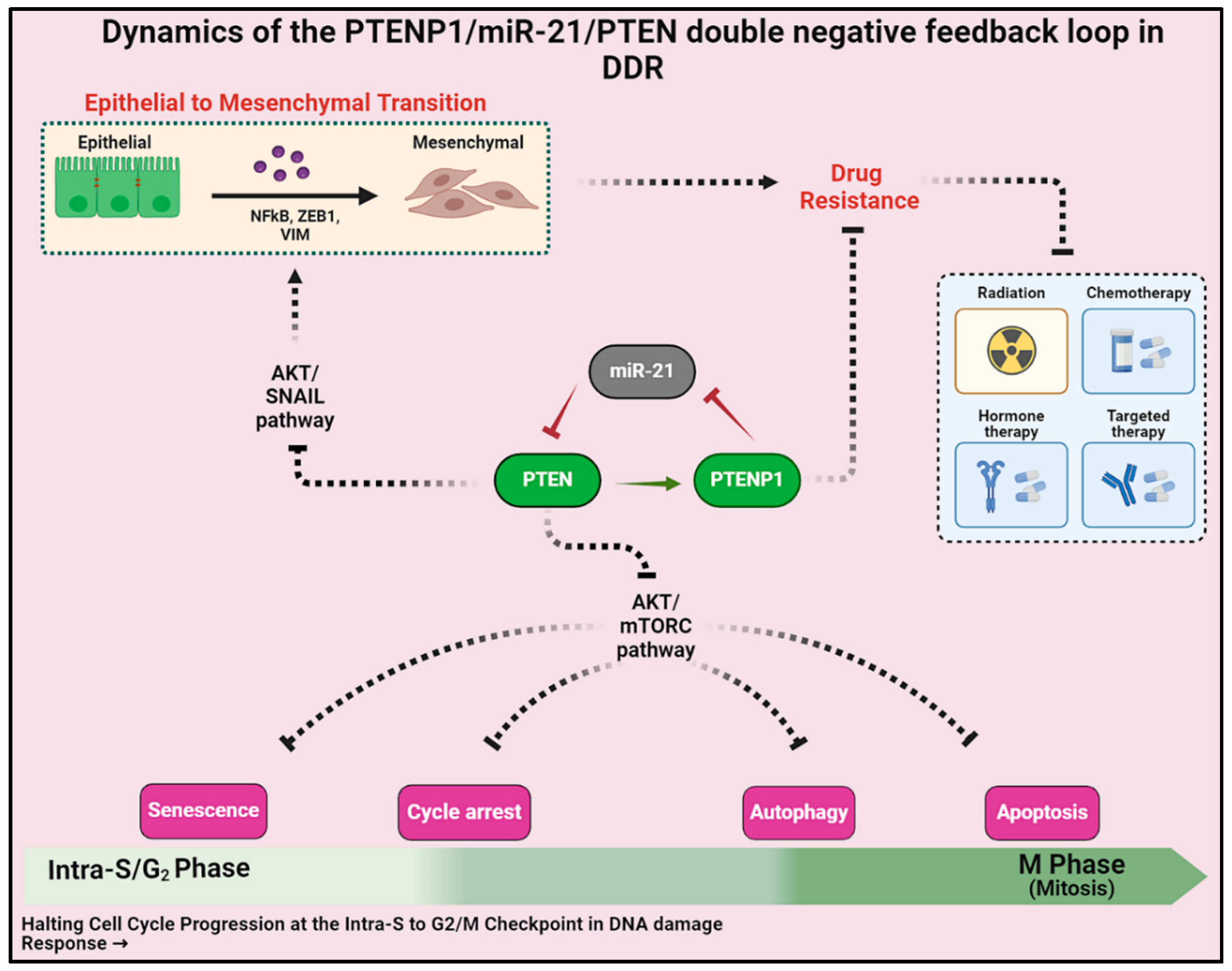
2.3. Exploring the Influence of the miR-21/PTEN and PTENP1 Axis on Cell Fate, Drug Resistance, and EMT
2.4. Mapping Molecular Circuits: Patterns and Dynamics
2.5. Network-Based Therapeutic Strategies for Combating Drug Resistance and EMT
3. Discussion
4. Materials and Methods
4.1. Mapping the Gene Regulatory Network Landscape in Cancer Cells: A Fusion of Public Databases and Tools
4.2. Developing Dynamic Boolean Network Models, Rules, and Simulations from PubMed-Based Insights
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Entezari, M.; Taheriazam, A.; Orouei, S.; Fallah, S.; Sanaei, A.; Hejazi, E.S.; Kakavand, A.; Rezaei, S.; Heidari, H.; Behroozaghdam, M.; et al. LncRNA-miRNA Axis in Tumor Progression and Therapy Response: An Emphasis on Molecular Interactions and Therapeutic Interventions. Biomed. Pharmacother. 2022, 154, 113609. [Google Scholar] [CrossRef] [PubMed]
- Chawra, H.S.; Agarwal, M.; Mishra, A.; Chandel, S.S.; Singh, R.P.; Dubey, G.; Kukreti, N.; Singh, M. MicroRNA-21’s Role in PTEN Suppression and PI3K/AKT Activation: Implications for Cancer Biology. Pathol.-Res. Pract. 2024, 254, 155091. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yang, J.; Chen, M.; Cui, L.; Wang, T.; Gao, W.; Tian, J.; Wei, R. MicroRNA-21 as a Diagnostic Marker for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Pak. J. Med. Sci. 2019, 35, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, Y.; Xu, M.; Zhang, X.; Zhou, Y.; Xu, M. miR-21 Promotes Cell Migration and Invasion of Hepatocellular Carcinoma by Targeting KLF5. Oncol. Lett. 2019, 17, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yao, W.; Gumireddy, K.; Li, A.; Wang, J.; Xiao, W.; Chen, K.; Xiao, H.; Li, H.; Tang, K.; et al. Pseudogene PTENP1 Functions as a Competing Endogenous RNA to Suppress Clear-Cell Renal Cell Carcinoma Progression. Mol. Cancer Ther. 2014, 13, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A Coding-Independent Function of Gene and Pseudogene mRNAs Regulates Tumour Biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-Q.; Yang, X.-W.; Chen, Y.-B.; Zhang, D.-W.; Jiang, X.-F.; Xue, P. Exosomal miR-21 Regulates the TETs/PTENp1/PTEN Pathway to Promote Hepatocellular Carcinoma Growth. Mol. Cancer 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ren, W.; Zhang, L.; Li, S.; Kong, X.; Zhang, H.; Dong, J.; Cai, G.; Jin, C.; Zheng, D.; et al. PTENp1, a Natural Sponge of miR-21, Mediates PTEN Expression to Inhibit the Proliferation of Oral Squamous Cell Carcinoma. Mol. Carcinog. 2017, 56, 1322–1334. [Google Scholar] [CrossRef]
- Fragni, M.; Bonini, S.A.; Bettinsoli, P.; Bodei, S.; Generali, D.; Bottini, A.; Spano, P.F.; Memo, M.; Sigala, S. The miR-21/PTEN/Akt Signaling Pathway Is Involved in the Anti-Tumoral Effects of Zoledronic Acid in Human Breast Cancer Cell Lines. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 529–538. [Google Scholar] [CrossRef]
- Li, L.-Q.; Li, X.-L.; Wang, L.; Du, W.-J.; Guo, R.; Liang, H.-H.; Liu, X.; Liang, D.-S.; Lu, Y.-J.; Shan, H.-L.; et al. Matrine Inhibits Breast Cancer Growth Via miR-21/PTEN/Akt Pathway in MCF-7 Cells. Cell. Physiol. Biochem. 2012, 30, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Cell Cycle Arrest Is Not Senescence. Aging 2011, 3, 94–101. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Geroconversion: Irreversible Step to Cellular Senescence. Cell Cycle 2014, 13, 3628–3635. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of Cell Cycle Transcription during G1 and S Phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Jung, H.-Y.; Park, S.H.; Kang, S.Y.; Yi, M.-R.; Um, H.D.; Hong, S.H. Combination of PTEN and γ-Ionizing Radiation Enhances Cell Death and G2/M Arrest Through Regulation of AKT Activity and P21 Induction in Non–Small-Cell Lung Cancer Cells. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, L.; Zhang, L.; Xu, A.; Li, Z.; Xu, Y.; He, P.; Wu, M.; Wei, F.; Wang, C. PTEN Enhances G2/M Arrest in Etoposide-Treated MCF-7 Cells through Activation of the ATM Pathway. Oncol. Rep. 2016, 35, 2707–2714. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhang, J.-H.; Xia, Q.-J.; Sun, Q.; Li, Z.-K.; Zhang, J.-G.; Tang, M.-S.; Dong, M.-S. Long Non-Coding RNA PTENP1 Inhibits Proliferation and Migration of Breast Cancer Cells via AKT and MAPK Signaling Pathways. Oncol. Lett. 2017, 14, 4659. [Google Scholar] [CrossRef]
- Chen, K.; Shou, L.-M.; Lin, F.; Duan, W.-M.; Wu, M.-Y.; Xie, X.; Xie, Y.-F.; Li, W.; Tao, M. Artesunate Induces G2/M Cell Cycle Arrest through Autophagy Induction in Breast Cancer Cells. Anticancer Drugs 2014, 25, 652–662. [Google Scholar] [CrossRef]
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer Effect of Curcumin Inhibits Cell Growth through miR-21/PTEN/Akt Pathway in Breast Cancer Cell. Oncol. Lett. 2017, 13, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, J.; Jiang, F.; Li, Y.; Chang, G.; Ma, H. Inhibition of miR-21 Promotes Cell Apoptosis in Oral Squamous Cell Carcinoma by Upregulating PTEN. Oncol. Rep. 2018, 40, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ranjan, N.; Jiang, L.; Ansari, A.H.; Degyatoreva, N.; Ahluwalia, S.; Arya, D.P.; Maiti, S. Fine-Tuning miR-21 Expression and Inhibition of EMT in Breast Cancer Cells Using Aromatic-Neomycin Derivatives. Mol. Ther. Nucleic Acids 2021, 27, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Alqosaibi, A.I.; Abdel-Ghany, S. Vorinostat Induces G2/M Cell Cycle Arrest in Breast Cancer Cells via Upregulation of PTEN. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qin, T.; Mao, J.; Zhang, J.; Fan, S.; Lu, Y.; Sun, Z.; Zhang, Q.; Song, B.; Li, L. PTENP1/miR-20a/PTEN Axis Contributes to Breast Cancer Progression by Regulating PTEN via PI3K/AKT Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 256. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; et al. Bergapten Drives Autophagy through the Up-Regulation of PTEN Expression in Breast Cancer Cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, L.; Cao, D.; Zhang, H. Suppression of miR-21 Expression Inhibits Cell Proliferation and Migration of Liver Cancer Cells by Targeting Phosphatase and Tensin Homolog (PTEN). Med. Sci. Monit. 2018, 24, 3571–3577. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Dong, X.; Zhai, B.; Jiang, X.; Dong, D.; Li, B.; Jiang, H.; Xu, S.; Sun, X. MiR-21 Mediates Sorafenib Resistance of Hepatocellular Carcinoma Cells by Inhibiting Autophagy via the PTEN/Akt Pathway. Oncotarget 2015, 6, 28867–28881. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, H.; Zhang, L.; Gong, L.; Wu, G.; Ni, J.; Tang, X. Suppression of miR-21-3p Enhances TRAIL-Mediated Apoptosis in Liver Cancer Stem Cells by Suppressing PI3K/Akt/Bad Cascade via Regulating PTEN. Cancer Manag. Res. 2019, 11, 955–968. [Google Scholar] [CrossRef]
- Reyes, J.; Chen, J.-Y.; Stewart-Ornstein, J.; Karhohs, K.W.; Mock, C.S.; Lahav, G. Fluctuations in P53 Signaling Allow Escape from Cell-Cycle Arrest. Mol. Cell 2018, 71, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Sarin, N.; Engel, F.; Kalayda, G.V.; Mannewitz, M.; Cinatl, J., Jr.; Rothweiler, F.; Michaelis, M.; Saafan, H.; Ritter, C.A.; Jaehde, U.; et al. Cisplatin Resistance in Non-Small Cell Lung Cancer Cells Is Associated with an Abrogation of Cisplatin-Induced G2/M Cell Cycle Arrest. PLoS ONE 2017, 12, e0181081. [Google Scholar] [CrossRef]
- Thieffry, D. Dynamical Roles of Biological Regulatory Circuits. Brief. Bioinform. 2007, 8, 220–225. [Google Scholar] [CrossRef]
- Dillen, A.; Bui, I.; Jung, M.; Agioti, S.; Zaravinos, A.; Bonavida, B. Regulation of PD-L1 Expression by YY1 in Cancer: Therapeutic Efficacy of Targeting YY1. Cancers 2024, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, X.; Qian, X.; Wang, H.; Yang, C.; Brinkman, K.L.; Serrano-Gonzalez, M.; Jope, R.S.; Zhou, B.; Engler, D.A.; et al. Activation of the ATM-Snail Pathway Promotes Breast Cancer Metastasis. J. Mol. Cell Biol. 2012, 4, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, A.; Faheem, M.M.; Katoch, A.; Kumar, A.; Jamwal, V.L.; Nayak, D.; Golani, A.; Rasool, R.U.; Ahmad, S.M.; et al. Vimentin Activation in Early Apoptotic Cancer Cells Errands Survival Pathways during DNA Damage Inducer CPT Treatment in Colon Carcinoma Model. Cell Death Dis. 2019, 10, 467. [Google Scholar] [CrossRef]
- Chatr-Aryamontri, A.; Oughtred, R.; Boucher, L.; Rust, J.; Chang, C.; Kolas, N.K.; O’Donnell, L.; Oster, S.; Theesfeld, C.; Sellam, A.; et al. The BioGRID Interaction Database: 2017 Update. Nucleic Acids Res. 2017, 45, D369–D379. [Google Scholar] [CrossRef] [PubMed]
- Naldi, A.; Hernandez, C.; Abou-Jaoudé, W.; Monteiro, P.T.; Chaouiya, C.; Thieffry, D. Logical Modeling and Analysis of Cellular Regulatory Networks With GINsim 3.0. Front. Physiol. 2018, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Abou-Jaoudé, W.; Traynard, P.; Monteiro, P.T.; Saez-Rodriguez, J.; Helikar, T.; Thieffry, D.; Chaouiya, C. Logical Modeling and Dynamical Analysis of Cellular Networks. Front. Genet. 2016, 7, 94. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Barbé-Tuana, F.M.; Mombach, J.C.M. Integrative Data Modeling from Lung and Lymphatic Cancer Predicts Functional Roles for miR-34a and miR-16 in Cell Fate Regulation. Sci. Rep. 2020, 10, 2511. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Towards DNA-Damage Induced Autophagy: A Boolean Model of P53-Induced Cell Fate Mechanisms. DNA Repair. 2020, 96, 102971. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. ATM/miR-34a-5p Axis Regulates a P21-Dependent Senescence-Apoptosis Switch in Non-Small Cell Lung Cancer: A Boolean Model of G1/S Checkpoint Regulation. FEBS Lett. 2020, 594, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hashimoto, R.F. Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer. Biomolecules 2022, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Panda, P.K.; Hashimoto, R.F.; Samal, S.K.; Mishra, S.; Verma, S.K.; Mishra, Y.K.; Ahuja, R. Dynamical Modeling of miR-34a, miR-449a, and miR-16 Reveals Numerous DDR Signaling Pathways Regulating Senescence, Autophagy, and Apoptosis in HeLa Cells. Sci. Rep. 2022, 12, 4911. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Panda, P.K.; Luo, W.; Hashimoto, R.F.; Ahuja, R. Network Analysis Reveals That the Tumor Suppressor lncRNA GAS5 Acts as a Double-Edged Sword in Response to DNA Damage in Gastric Cancer. Sci. Rep. 2022, 12, 18312. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Panda, P.K.; Silveira, D.A.; Ahuja, R.; Hashimoto, R.F. Quadra-Stable Dynamics of P53 and PTEN in the DNA Damage Response. Cells 2023, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M.; Hashimoto, R.F. The lncRNA DLX6-AS1/miR-16-5p Axis Regulates Autophagy and Apoptosis in Non-Small Cell Lung Cancer: A Boolean Model of Cell Death. Non-Coding RNA Res. 2023, 8, 605–614. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Hashimoto, R.F. A Boolean Model of the Oncogene Role of FAM111B in Lung Adenocarcinoma. Comput. Biol. Chem. 2023, 106, 107926. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.A.; Gupta, S.; da Cunha Jaeger, M.; Brunetto de Farias, C.; Mombach, J.C.M.; Sinigaglia, M. A Logical Model of Ewing Sarcoma Cell Epithelial-to-Mesenchymal Transition Supports the Existence of Hybrid Cellular Phenotypes. FEBS Lett. 2023, 597, 2446–2460. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Piedade, G.P.S.; Ostrowski, M.P.; Mombach, J.C.M.; Hashimoto, R.F. A Dynamic Boolean Network Reveals That the BMI1 and MALAT1 Axis Is Associated with Drug Resistance by Limiting miR-145-5p in Non-Small Cell Lung Cancer. Non-Coding RNA Res. 2024, 9, 185–193. [Google Scholar] [CrossRef]
- Barnum, K.J.; O’Connell, M.J. Cell Cycle Regulation by Checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lin, F.T.; Nevins, J.R. Selective Induction of E2F1 in Response to DNA Damage, Mediated by ATM-Dependent Phosphorylation. Genes. Dev. 2001, 15, 1833–1844. [Google Scholar] [PubMed]
- Zhang, X.-P.; Liu, F.; Wang, W. Two-Phase Dynamics of P53 in the DNA Damage Response. Proc. Natl. Acad. Sci. USA 2011, 108, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Lev Bar-Or, R.; Maya, R.; Segel, L.A.; Alon, U.; Levine, A.J.; Oren, M. Generation of Oscillations by the P53-Mdm2 Feedback Loop: A Theoretical and Experimental Study. Proc. Natl. Acad. Sci. USA 2000, 97, 11250–11255. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; White, E. P53-Dependent Apoptosis Pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Q.; Zhang, L.; Shi, D.; Wang, H.; Wang, S.; Bian, B. Folate-Targeted PTEN/AKT/P53 Signaling Pathway Promotes Apoptosis in Breast Cancer Cells. Pteridines 2020, 31, 158–164. [Google Scholar] [CrossRef]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN Transcription by P53. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Abdellatif, M. AKT-Ing via microRNA. Cell Cycle 2010, 9, 3233–3237. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, J.; Yi, Y.; Jiang, S.; Jin, P.; Xia, X.; Ma, F. Transcription Factors and Methylation Drive Prognostic miRNA Dysregulation in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 691115. [Google Scholar] [CrossRef]
- Coller, H.A.; Forman, J.J.; Legesse-Miller, A. “Myc’ed Messages”: Myc Induces Transcription of E2F1 While Inhibiting Its Translation via a microRNA Polycistron. PLoS Genet. 2007, 3, e146. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Hou, X.; Li, Z.; Kabra, N.; Ma, Y.; Nemoto, S.; Finkel, T.; Gu, W.; Cress, W.D.; et al. Interactions between E2F1 and SirT1 Regulate Apoptotic Response to DNA Damage. Nat. Cell Biol. 2006, 8, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Bačević, K.; Lossaint, G.; Achour, T.N.; Georget, V.; Fisher, D.; Dulić, V. Cdk2 Strengthens the Intra-S Checkpoint and Counteracts Cell Cycle Exit Induced by DNA Damage. Sci. Rep. 2017, 7, 13429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fujita, N.; Tsuruo, T. Caspase-Mediated Cleavage of p21Waf1/Cip1 Converts Cancer Cells from Growth Arrest to Undergoing Apoptosis. Oncogene 1999, 18, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Baritaki, S.; Militello, L.; Malaponte, G.; Bevelacqua, Y.; Bonavida, B. The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes. Cancer 2010, 1, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sohn, D.; Essmann, F.; Schulze-Osthoff, K. The Multiple Battles Fought by Anti-Apoptotic P21. Cell Cycle 2007, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Murashige, D.S.; Humphrey, S.J.; James, D.E. A Positive Feedback Loop between Akt and mTORC2 via SIN1 Phosphorylation. Cell Rep. 2015, 12, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic Acid Causes Inactivating Phosphorylation of cdc25A/cdc25C-Cdc2 via ATM-Chk2 Activation, Leading to Cell Cycle Arrest, and Induces Apoptosis in Human Prostate Carcinoma DU145 Cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Holczer, M.; Hajdú, B.; Lőrincz, T.; Szarka, A.; Bánhegyi, G.; Kapuy, O. A Double Negative Feedback Loop between mTORC1 and AMPK Kinases Guarantees Precise Autophagy Induction upon Cellular Stress. Int. J. Mol. Sci. 2019, 20, 5543. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Zhang, P.; Chen, W.-D.; Li, D.-D.; Wu, X.-Q.; Deng, R.; Jiao, L.; Li, X.; Ji, J.; Feng, G.-K.; et al. ATM-Mediated PTEN Phosphorylation Promotes PTEN Nuclear Translocation and Autophagy in Response to DNA-Damaging Agents in Cancer Cells. Autophagy 2015, 11, 239–252. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S.F.W. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS ONE 2017, 12, e0169622. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Tang, Z.; Zhou, Y.; Qiao, L. Possible Regulatory Role of Snail in NF-κB-Mediated Changes in E-Cadherin in Gastric Cancer. Oncol. Rep. 2013, 29, 993–1000. [Google Scholar] [CrossRef]
- Prasad, P.; Vasas, A.; Hohmann, J.; Bishayee, A.; Sinha, D. Cirsiliol Suppressed Epithelial to Mesenchymal Transition in B16F10 Malignant Melanoma Cells 5 through Alteration of the PI3K/Akt/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 608. [Google Scholar] [CrossRef]
- He, E.; Pan, F.; Li, G.; Li, J. Fractionated Ionizing Radiation Promotes Epithelial-Mesenchymal Transition in Human Esophageal Cancer Cells through PTEN Deficiency-Mediated Akt Activation. PLoS ONE 2015, 10, e0126149. [Google Scholar] [CrossRef]
- Malaney, P.; Palumbo, E.; Semidey-Hurtado, J.; Hardee, J.; Stanford, K.; Kathiriya, J.J.; Patel, D.; Tian, Z.; Allen-Gipson, D.; Davé, V. PTEN Physically Interacts with and Regulates E2F1-mediated Transcription in Lung Cancer. Cell Cycle 2018, 17, 947–962. [Google Scholar] [CrossRef]
- Fan, C.; He, L.; Kapoor, A.; Rybak, A.P.; De Melo, J.; Cutz, J.-C.; Tang, D. PTEN inhibits BMI1 function independently of its phosphatase activity. Mol. Cancer 2009, 8, 98. [Google Scholar] [CrossRef]
- Ginjala, V.; Nacerddine, K.; Kulkarni, A.; Oza, J.; Hill, S.J.; Yao, M.; Citterio, E.; van Lohuizen, M.; Ganesan, S. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol. Cell. Biol. 2011, 31, 1972–1982. [Google Scholar] [CrossRef]
- Verduzco, D.; Dovey, J.S.; Shukla, A.A.; Kodym, E.; Skaug, B.A.; Amatruda, J.F. Multiple isoforms of CDC25 oppose ATM activity to maintain cell proliferation during vertebrate development. Mol. Cancer Res. 2012, 10, 1451–1461. [Google Scholar] [CrossRef]
- Blanco-Aparicio, C.; Renner, O.; Leal, J.F.M.; Carnero, A. PTEN, more than the AKT pathway. Carcinogenesis 2007, 28, 1379–1386. [Google Scholar] [CrossRef]
- Dong, J.; Zhai, B.; Sun, W.; Hu, F.; Cheng, H.; Xu, J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS ONE 2017, 12, e0185088. [Google Scholar] [CrossRef]
- Escrivà, M.; Peiró, S.; Herranz, N.; Villagrasa, P.; Dave, N.; Montserrat-Sentís, B.; Murray, S.A.; Francí, C.; Gridley, T.; Virtanen, I.; et al. Repression of PTEN Phosphatase by Snail1 Transcriptional Factor during Gamma Radiation-Induced Apoptosis. Mol. Cell Biol. 2008, 28, 1528–1540. [Google Scholar] [CrossRef]
- Yi, C.; Li, G.; Wang, W.; Sun, Y.; Zhang, Y.; Zhong, C.; Stovall, D.B.; Li, D.; Shi, J.; Sui, G. Disruption of YY1-EZH2 Interaction Using Synthetic Peptides Inhibits Breast Cancer Development. Cancers 2021, 13, 2402. [Google Scholar] [CrossRef]
- Bonavida, B. (Ed.) Chapter 13—The role of YY1 in drug resistant cancer: Involvement of the YY1/PTEN/PP2A/H2Ax/Rad51 axis. In YY1 in the Control of the Pathogenesis and Drug Resistance of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 225–242. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, N.; Findley, H.W.; Zhou, M. Loss of PTEN Expression Induces NF-kB Via PI3K/Akt Pathway Involving Resistance to Chemotherapy in Acute Lymphoblastic Leukemia Cell Lines. Blood 2004, 104, 4438. [Google Scholar] [CrossRef]
- Wang, H.; Hertlein, E.; Bakkar, N.; Sun, H.; Acharyya, S.; Wang, J.; Carathers, M.; Davuluri, R.; Guttridge, D.C. NF-κB Regulation of YY1 Inhibits Skeletal Myogenesis through Transcriptional Silencing of Myofibrillar Genes. Mol. Cell Biol. 2007, 27, 4374–4387. [Google Scholar] [CrossRef]
- Bonavida, B. Linking Autophagy and the Dysregulated NFκB/SNAIL/YY1/RKIP/PTEN Loop in Cancer: Therapeutic Implications. Crit. Rev. Oncog. 2018, 23, 307–320. [Google Scholar] [CrossRef]

| Predictive Feedback Loops |
|---|
| Positive feedback loops |
| PTEN/PTENP1/miR-21 |
| PTEN/E2F1/miR-21 |
| PTEN/BMI1/ATM |
| PTEN/Cdc25/ATM |
| PTEN/AKT/SNAIL |
| PTEN/PTENP1/YY1 |
| PTEN/NFkB/YY1 |
| PTEN/NFkB/SNAIL |
| Negative feedback loop |
| PTEN/E2F1/ATM |
| Positive Circuits | Perturbations | Phenotypes |
|---|---|---|
| PTEN/PTENP1/miR-21 | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| PTEN/E2F1/miR-21 | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| PTEN/BMI1/ATM | Various | EMT, Senescence, Drug Resistance, Cell Cycle Arrest, Autophagy, Apoptosis. |
| PTEN/Cdc25/ATM | Various | EMT, Senescence, Drug Resistance, Cell Cycle Arrest, Autophagy, Apoptosis. |
| PTEN/AKT/SNAIL | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| PTEN/PTENP1/YY1 | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| PTEN/NFkB/YY1 | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| PTEN/NFkB/SNAIL | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
| Negative Circuit | Perturbations | Phenotypes |
| PTEN/E2F1/ATM | Various | Drug Resistance, EMT, Cell Cycle Arrest, Senescence, Autophagy, Apoptosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Silveira, D.A.; Lorenzoni, P.R.; Mombach, J.C.M.; Hashimoto, R.F. LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response. Int. J. Mol. Sci. 2024, 25, 8264. https://doi.org/10.3390/ijms25158264
Gupta S, Silveira DA, Lorenzoni PR, Mombach JCM, Hashimoto RF. LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response. International Journal of Molecular Sciences. 2024; 25(15):8264. https://doi.org/10.3390/ijms25158264
Chicago/Turabian StyleGupta, Shantanu, Daner A. Silveira, Pedro R. Lorenzoni, Jose Carlos M. Mombach, and Ronaldo F. Hashimoto. 2024. "LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response" International Journal of Molecular Sciences 25, no. 15: 8264. https://doi.org/10.3390/ijms25158264






