Peripheral Blood Mononuclear Cells and Serum Cytokines in Patients with Lupus Nephritis after COVID-19
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Patients
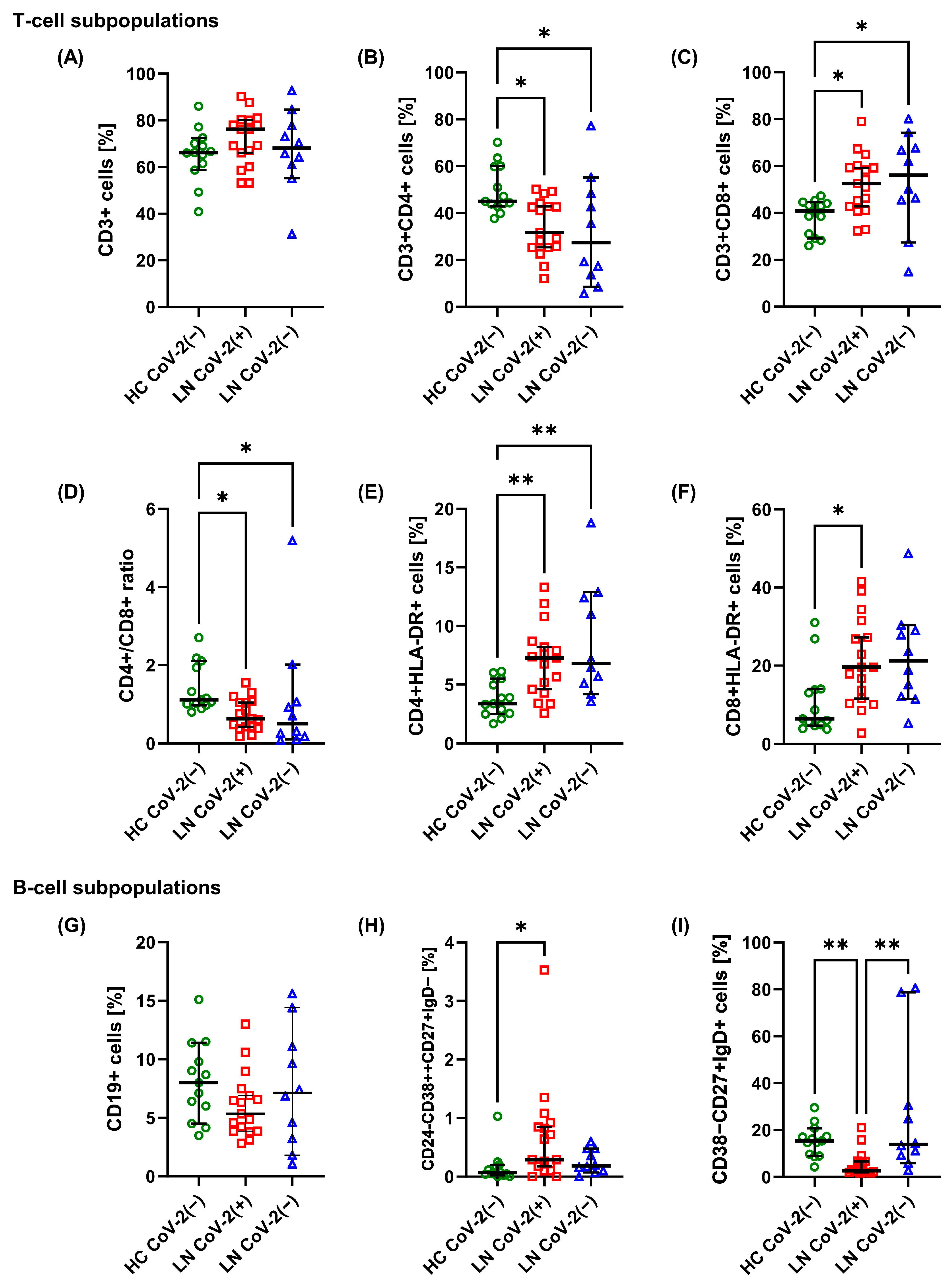
2.2. Changes in T-Cell and B-Cell Subpopulations
2.3. Changes in Monocyte and DC Subpopulations
2.4. Changes in Serum Cytokines, SARS-CoV-2 Spike Ig, and Nucleocapsid
3. Discussion
4. Materials and Methods
4.1. Study Groups
4.2. Determination of T-Cell, B-Cell, and Monocyte Subpopulations Ex Vivo
4.3. Determination of DC Subpopulations in PBMCs
4.4. Cytokine Measurement in Serum Samples
4.5. SARS-CoV-2 Spike Ig and Nucleocapsid Measurement in Serum Samples
4.6. Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danza, A.; Ruiz-Irastorza, G. Infection risk in systemic lupus erythematosus patients: Susceptibility factors and preventive strategies. Lupus 2013, 22, 1286–1294. [Google Scholar] [CrossRef]
- Quaglia, M.; Merlotti, G.; De Andrea, M.; Borgogna, C.; Cantaluppi, V. Viral Infections and Systemic Lupus Erythematosus: New Players in an Old Story. Viruses 2021, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; Corona-Castro, C.C.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Infectious processes and systemic lupus erythematosus. Immunology 2019, 158, 153–160. [Google Scholar] [CrossRef]
- Janahi, E.M.A.; Das, S.; Bhattacharya, S.N.; Haque, S.; Akhter, N.; Jawed, A.; Wahid, M.; Mandal, R.K.; Lohani, M.; Areeshi, M.Y.; et al. Cytomegalovirus aggravates the autoimmune phenomenon in systemic autoimmune diseases. Microb. Pathog. 2018, 120, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.L.; Wan, S.A.; Ling, G.R. Severe infections in systemic lupus erythematosus: Disease pattern and predictors of infection-related mortality. Clin. Rheumatol. 2018, 37, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Qian, Y.; Jin, X.H.; Yu, H.R.; Du, L.; Wu, H.; Chen, H.L.; Shi, Y.Q. COVID-19 in patients with systemic lupus erythematosus: A systematic review. Lupus 2022, 31, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Yurkovich, M.; Vostretsova, K.; Chen, W.; Aviña-Zubieta, J.A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: A meta-analysis of observational studies. Arthritis Care Res. 2014, 66, 608–616. [Google Scholar] [CrossRef]
- Doria, A.; Canova, M.; Tonon, M.; Zen, M.; Rampudda, E.; Bassi, N.; Atzeni, F.; Zampieri, S.; Ghirardello, A. Infections as triggers and complications of systemic lupus erythematosus. Autoimmun. Rev. 2008, 8, 24–28. [Google Scholar] [CrossRef]
- Garcia-Cirera, S.; Calvet, J.; Berenguer-Llergo, A.; Pradenas, E.; Marfil, S.; Massanella, M.; Mateu, L.; Trinité, B.; Llop, M.; Arévalo, M.; et al. Glucocorticoids’ treatment impairs the medium-term immunogenic response to SARS-CoV-2 mRNA vaccines in Systemic Lupus Erythematosus patients. Sci. Rep. 2022, 12, 14772. [Google Scholar] [CrossRef]
- Carvalho, J.S.; dos Reis Neto, E.T.; Kakehasi, A.M.; Ribeiro, S.L.; Studart, S.A.; Martins, F.P.; Cavalheiro do Espírito Santo, R.; Ranzolin, A.; Fernandino, D.C.; Dinis, V.G.; et al. ReumaCoV Brasil Registry. Factors associated with poor outcomes in SLE patients with COVID-19: Data from ReumaCoV-Brazil register. Lupus 2023, 32, 42–53. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. ISARIC4C investigators. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.; Washer, L.; Marder, W.; Kahlenberg, J.M. Patients with lupus with COVID-19: University of Michigan experience. Ann. Rheum. Dis. 2021, 80, e35. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef]
- Yang, X.; Dai, T.; Zhou, X.; Qian, H.; Guo, R.; Lei, L.; Zhang, X.; Zhang, D.; Shi, L.; Cheng, Y.; et al. Naturally activated adaptive immunity in COVID-19 patients. J. Cell Mol. Med. 2020, 24, 12457–12463. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Radrizzani, D.; Viganò, P.; Mazzone, A.; Brando, B. Decrease of Non-Classical and Intermediate Monocyte Subsets in Severe Acute SARS-CoV-2 Infection. Cytom. A 2020, 97, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef] [PubMed]
- Taeschler, P.; Adamo, S.; Deng, Y.; Cervia, C.; Zurbuchen, Y.; Chevrier, S.; Raeber, M.E.; Hasler, S.; Bächli, E.; Rudiger, A.; et al. T-cell recovery and evidence of persistent immune activation 12 months after severe COVID-19. Allergy 2022, 77, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.; Hopkins, F.R.; Göransson, R.; Svanberg, C.; Shankar, E.M.; Hjorth, M.; Nilsdotter-Augustinsson, Å.; Sjöwall, J.; Nyström, S.; Larsson, M. T cell perturbations persist for at least 6 months following hospitalization for COVID-19. Front. Immunol. 2022, 13, 931039. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Domingo, S.; Vidal, X.; Cortés-Hernández, J. Humoral and cellular response in convalescent COVID-19 lupus patients. Sci. Rep. 2022, 12, 13787. [Google Scholar] [CrossRef]
- Wang, H.; Lan, L.; Chen, J.; Xiao, L.; Han, F. Peripheral blood T-cell subset and its clinical significance in lupus nephritis patients. Lupus Sci. Med. 2022, 9, e000717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zeng, Y.; Li, J.; Wang, C.; Li, W.; He, Z.; Ye, J.; Li, F.; Chen, Y.; Lin, X.; et al. Phenotypical changes and clinical significance of CD4+/CD8+ T cells in SLE. Lupus Sci. Med. 2022, 9, e000660. [Google Scholar] [CrossRef] [PubMed]
- Byazrova, M.; Yusubalieva, G.; Spiridonova, A.; Efimov, G.; Mazurov, D.; Baranov, K.; Baklaushev, V.; Filatov, A. Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19. Clin. Transl. Immunol. 2021, 10, e1245. [Google Scholar] [CrossRef]
- Ahern, D.J.; Ai, Z.; Ainsworth, M.; Allan, C.; Allcock, A.; Angus, B.; Ansari, A.M.; Arancibia-Cárcamo, C.V.; Aschenbrenner, D.; Attar, M.; et al. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell 2022, 185, 916–938.e58. [Google Scholar] [CrossRef]
- Boulanger, M.; Molina, E.; Wang, K.; Kickler, T.; Xu, Y.; Garibaldi, B.T. Peripheral Plasma Cells Associated with Mortality Benefit in Severe COVID-19: A Marker of Disease Resolution. Am. J. Med. 2021, 134, 1029–1033. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Vick, S.C.; Frutoso, M.; Mair, F.; Konecny, A.J.; Greene, E.; Wolf, C.R.; Logue, J.K.; Franko, N.M.; Boonyaratanakornkit, J.; Gottardo, R.; et al. A regulatory T cell signature distinguishes the immune landscape of COVID-19 patients from those with other respiratory infections. Sci. Adv. 2021, 7, eabj0274. [Google Scholar] [CrossRef]
- Caldrer, S.; Mazzi, C.; Bernardi, M.; Prato, M.; Ronzoni, N.; Rodari, P.; Angheben, A.; Piubelli, C.; Tiberti, N. Regulatory T Cells as Predictors of Clinical Course in Hospitalised COVID-19 Patients. Front. Immunol. 2021, 12, 789735. [Google Scholar] [CrossRef]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals with Long-COVID: Identification of Diagnostic Biomarkers. Front. Immunol. 2022, 13, 848886. [Google Scholar] [CrossRef]
- Utrero-Rico, A.; Ruiz-Ruigómez, M.; Laguna-Goya, R.; Arrieta-Ortubay, E.; Chivite-Lacaba, M.; González-Cuadrado, C.; Lalueza, A.; Almendro-Vazquez, P.; Serrano, A.; Aguado, J.M.; et al. A Short Corticosteroid Course Reduces Symptoms and Immunological Alterations Underlying Long-COVID. Biomedicines 2021, 9, 1540. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Vitallé, J.; Gasca-Capote, C.; Gutierrez-Valencia, A.; Trujillo-Rodriguez, M.; Serna-Gallego, A.; Muñoz-Muela, E.; Jiménez-Leon, M.L.R.; Rafii-El-Idrissi Benhnia, M.; Rivas-Jeremias, I.; et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell Mol. Immunol. 2021, 18, 2128–2139. [Google Scholar] [CrossRef]
- Liu, K. Dendritic Cells. Encycl. Cell Biol. 2016, 3, 741–749. [Google Scholar]
- Manz, M.G. Plasmacytoid dendritic cells: Origin matters. Nat. Immunol. 2018, 19, 652–654. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [PubMed]
- Klarquist, J.; Zhou, Z.; Shen, N.; Janssen, E.M. Dendritic Cells in Systemic Lupus Erythematosus: From Pathogenic Players to Therapeutic Tools. Mediat. Inflamm. 2016, 2016, 5045248. [Google Scholar] [CrossRef] [PubMed]


| HC CoV-2(−) (n = 13) | LN CoV-2(+) (n = 17) | LN CoV-2(−) (n = 10) | p Value | |
|---|---|---|---|---|
| Age [years] | 38.77 ± 9.16 | 48.82 ± 14.46 | 41.5 ± 12.37 | 0.0864 |
| SLE duration [years] | n.a. | 16 (2, 37) | 14.5 (4, 22) | 0.6043 |
| SLICC [score] | n.a. | 0 (0, 4) | 0.5 (0, 3) | 0.9019 |
| SLEDAI [score] | n.a. | 6 (2, 16) | 10 (0, 26) | 0.6750 |
| SLAM-R [score] | n.a. | 6 (2, 17) | 5 (1, 13) | 0.4433 |
| Symptoms [organ affected] | n.a. | |||
| Joints | 16 (94.12%) | 9 (90%) | ||
| Skin | 12 (70.59%) | 9 (90%) | ||
| Neurologic | 2 (11.76%) | 0 (0%) | ||
| Hematologic | 15 (88.24%) | 8 (80%) | ||
| Renal | 17 (100%) | 10 (100%) | ||
| LN duration [years] | n.a. | 10 (1, 39) | 17.5 (6, 38) | 0.0929 |
| Treatment | n.a. | |||
| Glucocorticoids | 15 (88.24%) | 9 (90%) | ||
| Immunosuppressants | 11 (54.71%) | 8 (80%) | ||
| COVID-19 infection | n.a. | n.a. | ||
| Fever or chills | 12 (70.59%) | |||
| Cough | 11 (54.71%) | |||
| Shortness of breath or difficulty breathing | 2 (11.76%) | |||
| Fatigue | 16 (94.12%) | |||
| Muscle or body aches | 12 (70.59%) | |||
| New loss of taste or smell | 2 (11.76%) | |||
| Sore throat | 7 (41.18%) | |||
| Congestion or runny nose | 10 (58.82%) | |||
| Nausea or vomiting | 2 (11.76%) | |||
| Diarrhea | 1 (5.88%) | |||
| Hospitalization | 3 (17.65%) | |||
| ESR [mm/h] | n.a. | 10.5 (2, 29) | 10 (0, 42) | 0.0864 |
| eGFR [mL/min/1.73 m2] | n.a. | 84 (13, 90) | 90 (74, 90) | 0.0929 |
| Creatinine [mg/dL] | n.a. | 0.82 (0.54, 4.17) | 0.78 (0.53, 0.85) | 0.2231 |
| Anti-dsDNA [IU/mL] | n.a. | 212.34 (0, 800) | 258.45 (0, 746.73) | 0.9794 |
| HC CoV-2(−) | LN CoV-2(+) | LN CoV-2(−) | p Value | |
|---|---|---|---|---|
| CD3+ T cells [%] | 66.2 (40.9, 86.1) | 76.2 (53.2, 90.2) | 68.1 (31.3, 92.8) | 0.3155 |
| CD3+CD4+ (Th) cells [%] | 45 (37.7, 70.2) | 31.7 (12, 50.2) * | 27.4 (5.7, 77.3) * | 0.0091 |
| CD3+CD8+ (Tc) cells [%] | 40.9 (26, 47.3) | 52.5 (32.4, 79) * | 56.1 (14.9, 80.1) * | 0.0069 |
| CD4+/CD8+ ratio | 1.11 (0.8, 2.7) | 0.63 (0.18, 1.55) * | 0.51 (0.08, 5.19) * | 0.0119 |
| CD4+CD28+ cells [%] | 99.5 (95.1, 100) | 95.6 (84.7, 100) | 94.95 (51.7, 99.5) * | 0.0202 |
| CD4+CD69+ cells [%] | 3.75 (0.59, 9.36) | 1.42 (0.44, 8.02) | 5.37 (0.51, 18.1) | 0.0606 |
| CD4+HLA-DR+ cells [%] | 3.37 (1.69, 6.12) | 7.27 (2.58, 13.3) * | 6.81 (3.57, 18.8) * | 0.0012 |
| CD8+CD28+ cells [%] | 81.2 (45.6, 96.2) | 63.5 (42.8, 95) | 49.15 (15.6, 90.2) | 0.0639 |
| CD8+CD69+ cells [%] | 2.59 (0.65, 11.4) | 2.31 (0.71, 11.7) | 3.4 (1.23, 10) | 0.4331 |
| CD8+HLA-DR+ cells [%] | 6.42 (3.78, 31) | 19.6 (2.78, 41.5) * | 21.2 (5.31, 48.7) | 0.0200 |
| Naive CD4+ T cells [%] | 51.5 (7.51, 83) | 55.8 (37.6, 77.5) | 44 (18, 81.9) | 0.3053 |
| CD4+ Tcm cells [%] | 18.9 (6.4, 75) | 17.2 (6.51, 29.2) | 15.5 (8.52, 24.7) | 0.5662 |
| CD4+Tem cells [%] | 15.2 (2.79, 30.8) | 14.5 (5.62, 31.9) | 22.7 (5.46, 40.4) | 0.1333 |
| CD4+ Temra cells [%] | 5.98 (1.46, 21.7) | 10.8 (7.32, 18.4) | 13.2 (4.08, 48) | 0.0726 |
| Naive CD8+ T cells [%] | 43.4 (16.3, 68.9) | 39.1 (11.8, 89.3) | 30.55 (14.8, 79.5) | 0.6818 |
| CD8+ Tcm cells [%] | 2.46 (0.49, 6.1) | 1.78 (0.54, 5.45) | 0.7 (0.1, 3.38) * | 0.0226 |
| CD8+ Tem cells [%] | 5.22 (1.41, 12.5) | 3.65 (0.7, 13.7) | 2.89 (0.6, 6.65) | 0.1186 |
| CD8+ Temra cells [%] | 43.4 (26.7, 74.8) | 56 (9.07, 76.3) | 64.6 (13.7, 82.7) | 0.4083 |
| Tregs [%] | 6.2 (4.81, 9.07) | 8.68 (5.67, 20.2) * | 8.12 (2.22, 16.5) | 0.0031 |
| CD19+ B cells [%] | 8.01 (3.49, 15.1) | 5.34 (2.82, 13) | 7.14 (1.06, 15.6) | 0.2611 |
| TBs [%] | 1.72 (0.03, 3.58) | 0.67 (0, 24) | 0.44 (0, 8.14) | 0.2927 |
| PBs [%] | 0.07 (0, 1.03) | 0.29 (0, 3.53) * | 0.18 (0, 0.6) | 0.0152 |
| Naive B cells [%] | 64.6 (51.8, 82.4) | 74.6 (20, 87.6) | 57.5 (4.38, 83.6) | 0.2452 |
| DNM B cells [%] | 9.25 (4.13, 16.1) | 12.3 (3.06, 65.6) | 8.43 (4.46, 32.7) | 0.7416 |
| NSM B cells [%] | 15.4 (4.3, 29.5) | 2.65 (1.71, 21) ** | 13.9 (2.89, 80.6) | 0.0003 |
| SM B cells [%] | 7.87 (1.79, 22) | 9.74 (1.98, 29.6) | 7.29 (2.47, 20.2) | 0.7412 |
| HC CoV-2(−) | LN CoV-2(+) | LN CoV-2(−) | p Value | |
|---|---|---|---|---|
| CD14+ cells [%] | 80.6 (75.7, 89.8) | 87.1 (80.3, 95.5) * | 91.85 (78.7, 97) * | 0.0010 |
| Classical monocytes [%] | 69.7 (56,83.4) | 79.8 (70.2, 84.5) ** | 65.35 (45.3, 87.5) | 0.0074 |
| TL2+ cells [%] | 99.9 (99.4, 100) | 99.8 (12, 100) | 99.6 (99.3, 100) * | 0.0295 |
| TL4+ cells [%] | 99.6 (99, 100) | 99.9 (96.8, 100) * | 99.7 (99, 100) | 0.0341 |
| Intermediate monocytes [%] | 3.97 (2.4, 6.62) | 5.77 (2.03, 8.74) * | 5.44 (3.19, 9.44) | 0.0144 |
| TL2+ cells [%] | 98.7 (79.7, 100) | 92.4 (5.26, 99.5) * | 94.1 (79.2, 97.5) | 0.0202 |
| TL4+ cells [%] | 100 (98.9, 100) | 100 (99, 100) | 99.95 (99.2, 100) | 0.5787 |
| Non-classical monocytes [%] | 7.43 (3.5, 14.7) | 5.57 (0.47, 10.7) | 4.64 (0.76, 13.8) | 0.1839 |
| TL2+ cells [%] | 66.8 (1.85, 94.5) | 24.4 (0.73, 90.1) | 55.75 (0.87, 79.5) | 0.1057 |
| TL4+ cells [%] | 99.5 (98.3, 100) | 99.8 (90.6, 100) | 98.3 (99.7, 100) | 0.9063 |
| HC CoV-2(−) | LN CoV-2(+) | LN CoV-2(−) | p Value | |
|---|---|---|---|---|
| cDC1 [%] | 1.92 (0.33, 8.79) | 5.78 (0.72, 24.4) | 3.73 (2, 25) | 0.0585 |
| cDC2 [%] | 0.27 (0.1, 5.91) | 1.19 (0.14, 16.8) | 0.52 (0, 1.88) | 0.0982 |
| DC2 [%] | 6.67 (0, 12.5) | 5.3 (0, 10.5) | 0 (0, 11.1) | 0.1340 |
| DC3 [%] | 62.5 (0, 81.3) | 40 (6.67, 93.6) | 20.3 (0, 76.4) | 0.0497 |
| infl DC3 [%] | 40 (0, 86.7) | 23.1 (0, 66.7) | 13.95 (0, 84.3) | 0.1179 |
| CD11c+CD80+ cells [%] | 23.8 (11.6, 39.8) | 34.3 (5.2, 63.7) | 20.35 (3.61, 78.9) | 0.5005 |
| CD11c+CD86+ cells [%] | 97.8 (62.8, 99.6) | 97.7 (71.3, 99.4) | 95.6 (86.2, 99.7) | 0.9127 |
| HC CoV-2(−) | LN CoV-2(+) | LN CoV-2(−) | p Value | |
|---|---|---|---|---|
| IFN-γ [pg/mL] | 3.94 (2.56, 14.33) | 3.74 (2.89, 5.31) | 4.03 (2.56, 6.17) | 0.5066 |
| IL-2 [pg/mL] | 3.65 (2.34, 4.88) | 3.65 (2.70, 5.60) | 4.07 (0, 6.12) | 0.5966 |
| IL-4 [pg/mL] | 3.53 (1.78, 5.72) | 3.40 (2.28, 5.48) | 3.68 (1.45, 5.26) | 0.8949 |
| IL-6 [pg/mL] | 5.35 (1.91, 7.29) | 7.58 (3.55, 36.01) * | 7.61 (2.54, 15.37) | 0.0206 |
| IL-10 [pg/mL] | 1.95 (0.00, 3.22) | 2.62 (0, 11.59) | 3.50 (0, 10.87) * | 0.0309 |
| IL-17A [pg/mL] | 0 (0, 78.79) | 8.27 (0, 66.08) | 6.99 (0, 48.05) | 0.2179 |
| TNF [pg/mL] | 5.55 (0, 6.68) | 4.37 (2.48, 42.43) | 5.55 (0, 8.93) | 0.7973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska, K.A.; Ciesielska-Figlon, K.; Komorniczak, M.; Bułło-Piontecka, B.; Dębska-Ślizień, A.; Wardowska, A. Peripheral Blood Mononuclear Cells and Serum Cytokines in Patients with Lupus Nephritis after COVID-19. Int. J. Mol. Sci. 2024, 25, 8278. https://doi.org/10.3390/ijms25158278
Lisowska KA, Ciesielska-Figlon K, Komorniczak M, Bułło-Piontecka B, Dębska-Ślizień A, Wardowska A. Peripheral Blood Mononuclear Cells and Serum Cytokines in Patients with Lupus Nephritis after COVID-19. International Journal of Molecular Sciences. 2024; 25(15):8278. https://doi.org/10.3390/ijms25158278
Chicago/Turabian StyleLisowska, Katarzyna A., Klaudia Ciesielska-Figlon, Michał Komorniczak, Barbara Bułło-Piontecka, Alicja Dębska-Ślizień, and Anna Wardowska. 2024. "Peripheral Blood Mononuclear Cells and Serum Cytokines in Patients with Lupus Nephritis after COVID-19" International Journal of Molecular Sciences 25, no. 15: 8278. https://doi.org/10.3390/ijms25158278





