Molecular Basis of Cardiomyopathies in Type 2 Diabetes
Abstract
1. Introduction
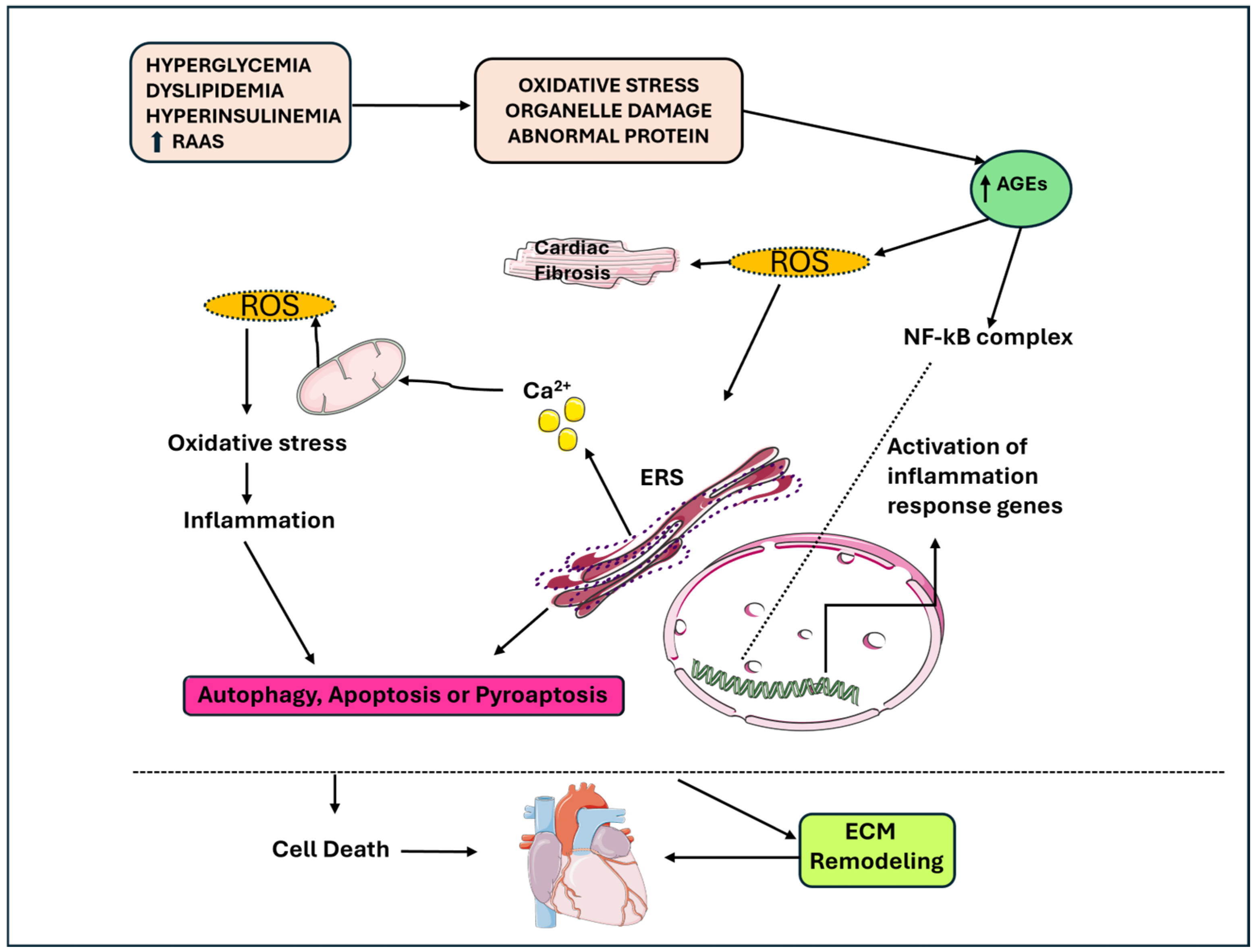
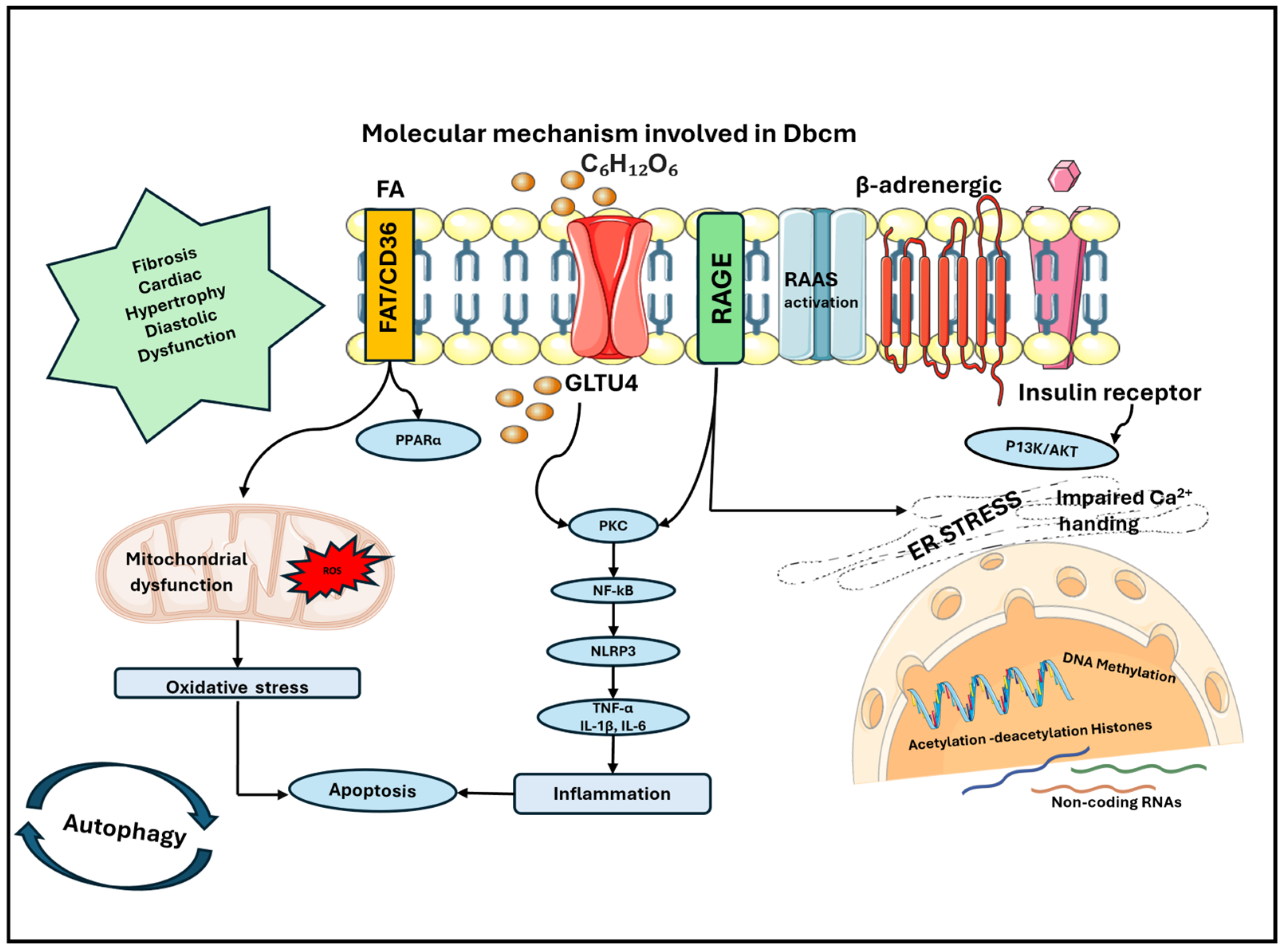
2. Inflammation
3. Mitochondrial Dysfunction
4. Molecular Mechanisms Determining Cardiac Remodeling
5. Myocardial Calcium Handling
6. Epigenetic Changes
6.1. DNA Methylation/Demethylation
6.2. Histone Modifications
6.3. Non-Coding RNAs
| Main Mechanism | Ref. | In Vitro/In Vivo/Ex Vivo | Molecular Evidence |
|---|---|---|---|
| Oxidative stress | [20] | In vivo, STZ-induced diabetic rats | ↑ ROS, TNF-α, RAGE and NFkB-p65 |
| Inflammation, oxidative stress, cardiac hypertrophy and fibrosis | [23] | In vivo, STZ-induced diabetic mice In vitro, AC16 human myocardial cells treated with high glucose | In STZ-induced diabetic mice: ↑ cardiomyocyte area ↑ collagen deposition ↑ IL-1β, IL-6, IL-18 and TNFα ↑ activation of NLRP3 In AC16 cells: ↑ phosphorylation levels of NF-κB p65 and IκB ↑ ROS |
| Inflammation, oxidative stress, apoptosis, pyroptosis, cardiac remodeling and ventricular dysfunction | [24] | In vivo, STZ-induced diabetic mice In vitro, high-glucose-treated H9c2 cardiomyocytes | ↑ NLRP3, ASC, caspase-1 and IL-1β NLRP3 gene silencing reduces left ventricular dysfunction in DbCM and reversed myocardial remodeling In H9c2 cells: ↑ ROS and phosphorylation of NF-kB p65 In cells pretreated with NAC: ↓ ROS activity, NF-kB, NLRP3, ASC, pro-caspaspe-1, activated caspase-1, pro- IL-1β and mature IL-1β |
| Inflammation and pyroptosis | [31] | In vitro, H9c2 rat cardiomyoblast cell line treated with high glucose | ↑ LDH, IL-1β, IL-18, NLRP3, GSDMD-N, caspase-1 and IL-1β ↑ miR-223-3p |
| Inflammation and mitochondrial dysfunction | [32] | In vivo, mice overexpressing IGFII in pancreatic beta cells in hyperlipidemic background with T2DM fed with high-fat diet for 12 weeks with induced MI | Impaired mitophagy in peri-infarct regions of LV, ↑ mtDNA, activation of Aim2, NLRC4 inflammasome, caspase-I, IL-18 and cardiomyocyte death |
| Interstitial fibrosis | [33] | In vivo, STZ-induced diabetic mice | LV systolic functional impairment and ↑ interstitial fibrosis |
| Inflammation | [42] | In vivo, STZ-induced diabetic mice | Release of mtDNA and activation of cGAS-STING signaling pathway leading to the activation of NLRP3 and ↑ TNF-α, IFN-β, IL-1β and IL-18 |
| Mitochondrial dysfunction | [45] | In vivo, T2DM human patients | ↓ myocardial glucose utilization rate |
| Mitochondrial dysfunction | [48] | In vivo, STZ-induced diabetic mice | ↑ PPAR alpha, ACO and M-CPTI |
| Mitochondrial dysfunction | [53] | In vivo, STZ-induced diabetic mice In vitro, HL-1 murine cardiomyocytes | In STZ-induced diabetic mice: ↓ HIF-1α after ischemia In HL-1 cardiomyocytes: failure of hypoxia-mediated metabolic adaptation with increased lactate efflux and glucose consumption |
| Mitochondrial dysfunction and oxidative stress | [55] | In vivo, STZ-induced diabetic mice | ↑ ROS, catalase activity, SOD, TFAM, PGC-1α and CO1 ↓ electron transport chain complexes and SIRT3 activity ↑ acetylation of MnSOD |
| Mitochondrial dysfunction | [67] | In vivo, T1D mice | ↑ 8-OHdG, O-GlcNAcylation of Ogg1 activity and mtDNA damage |
| Mitochondrial dysfunction | [68] | In vivo, STZ-induced diabetic mice | ↑ mitochondrial OGT, ↓ mito-specific O-GlcNAcase (OGA), OGT is mislocalized and ↓ interaction of OGT and complex IV |
| Cardiac hypertrophy | [69] | Ex vivo, cardiomyocytes isolated from type 2 diabetic db/db mice | ↑ HBP and O-GlcNAc levels |
| Cardiac hypertrophy | [70] | In vitro, endothelial vein cells of T2DM patients | ↑ O-GlcNAc levels |
| Cardiac dysfunction | [73] | In vivo, polygenic T2DM mice In vitro, isolated coronary endothelial cells from mice | ↑ coronary endothelial cells apoptosis, ↓ coronary flow velocity reserve, ↓ cardiac contractility and ↑ p53 protein levels |
| Cardiac fibrosis and inflammation | [77] | In vivo, STZ-induced diabetic mice | LV dysfunction, diastolic stiffness; ↑ TGFβ, IL1β, and fibrosis; ↓ MMP-2 activity |
| RAS, apoptosis and cardiac fibrosis | [80] | In vivo, STZ-induced diabetic mice | ↑ intracellular ATII, angiotensinogen and renin in cardiac myocytes Superoxide production, myocyte apoptosis and cardiac fibrosis were inhibited by RAS inhibitors |
| Cardiac fibrosis | [82] | In vitro, differentiated murine mesangial cells exposed to D-glucose | ↑ incorporation of 3[H]proline ↑ stimulation of collagen types I and IV by high D-glucose |
| RAS and cardiac fibrosis | [83] | In vitro, neonatal rat ventricular fibroblasts exposed to high glucose | ↑ intracellular ATII and TGFβ, ↑ collagen-1 synthesis by cardiac fibroblasts that is inhibited by RAS inhibitors |
| Myocyte death and cardiac hypertrophy | [87] | In vivo, STZ-induced diabetic mice | ↑ myocyte apoptosis, AT, AT II, renin and AT1 |
| Myocyte death | [88] | In vitro, neonatal rat ventricular cardiomyocytes cultured with glucose | ↓ number of lysosomes with acidic pH ↑ Galectin3-RFP puncta and leakage of CTSD |
| Inflammation and myocyte death | [89] | In vivo, T2DM patients | ↓ ratios of CD4(+)CD25(hi) Treg/Th17 cells and CD4(+)CD25(hi) Treg/Th1 cells |
| Alterations of myocardial calcium handling | [95] | In vivo, sedentary db/db mice | ↑ diastolic SR-Ca(2+) leak ↓ synchrony of Ca(2+) release, transverse-tubule density, peak systolic and diastolic Ca(2+), caffeine-induced Ca(2+) release and SR Ca(2+) ATPase-mediated Ca(2+) uptake during diastole rate |
| Alterations of myocardial calcium handling and oxidative stress | [96] | In vitro, neonatal rat cardiomyocytes treated with AGEs | ↓ calcium transient amplitude and sarcoplasmic reticulum calcium content ↑ ROS, NADPH oxidase activity, activation of p38 kinase and nuclear translocation of NF-κB with the subsequent induction of inducible NO synthase expression |
| Hypertrophy, apoptosis and alterations of myocardial calcium handling | [97] | In vitro, pluripotent stem cells generated from urine epithelial cells of T2DM patients | Irregular Ca(2+) transient waveforms, decreased transient amplitude, shorter transient duration, shorter decay, slower maximal rising rate and slower maximal decay rate ↑ apoptosis, cellular hypertrophy and lipid accumulation |
| Alterations of myocardial calcium handling | [98] | In vivo, STZ-induced diabetic mice | ↑ Ca(2+) transient ↓ Na(+)/Ca(2+) exchanger current |
| Alterations of myocardial calcium handling | [99] | In vivo, T2DM patients | ↓ left ventricular ERG, KCNH2 and KCNJ3 gene expression ↑ NCX1, KCNJ2, KCNJ5 and SLC8A1 gene expression ↑ QT interval |
| AGEs and alterations of myocardial calcium handling | [101] | In vivo, STZ-induced diabetic mice | AGEs formation on intracellular RyR2 |
| Alterations of myocardial calcium handling | [102] | In vitro, HEK-293 cells | ↑ Ca(2+) uptake velocity for expressed SERCA2 by exposure to CaM kinase I1 |
| AGEs and alterations of myocardial calcium handling | [103] | In vivo, STZ-induced diabetic mice | ↓ heart relaxation ↓ SERCA2a expression ↑ PLB |
| Epigenetic changes | [107] | In vivo, STZ-induced diabetic mice In vivo, formalin-fixed and paraffin-embedded human myocardial archived tissues In vitro, neonatal rat cardiomyocytes exposed to glucose | Absence of methylation in the promoter regions of the DUSP-1 |
| Epigenetic changes | [111] | In vivo, nondiabetic women | Positive association between the methylation levels of the CpG site 783 with insulin sensitivity |
| Epigenetic changes | [112] | In vitro, HL-1 cardiomyocytes | ↓ SERCA2a RNA and protein expression ↑ methylation in the SERCA2a promoter region ↑ DNA methyltransferase 1 expression |
| Epigenetic changes | [116] | In vitro, vascular smooth muscle cells | 12(S)-HETE activated Src, focal adhesion kinase, Akt, p38MAPK, CREB, expression of monocyte chemoattractant protein-1, IL-6 genes and histone H3-Lys-9/14 acetylation on their promoters |
| Epigenetic changes | [117] | In vitro, neonatal rat cardiomyocytes exposed to glucose In vivo, STZ-induced diabetic rats | Cellular hypertrophy and ↑ mRNA expression of ANP, BNP, ANG, MEF2A, MEF2C and transcriptional coactivator p300 In STZ-induced diabetic rats: ↑ ANP, BNP, ANG, mRNA, p300, MEF2A, and MEF2C expression |
| Epigenetic changes | [119] | In vivo, STZ-induced diabetic mice | Dysregulation of 316 out of 1008 total miRNAs implicated in myocardial signalling networks that trigger apoptosis, fibrosis, hypertrophic growth, autophagy oxidative stress and heart failure |
| Epigenetic changes | [121] | In vivo, STZ-induced diabetic mice | Upregulation of miR-203 inhibited the activation of PI3K/Akt signaling pathway and ↓ PIK3CA, PI3K, Akt, CoI I, CoI III, ANP, MDA and ROS in the myocardial tissues |
| Epigenetic changes | [123] | In vivo and ex vivo, STZ-induced diabetic mice In vitro, cultured cardiomyocytes | In STZ-induced diabetic mice: ↓ miR-144 in heart tissues In cultured cardiomyocytes: high glucose exposure induced ↓ miR-144 |
| Epigenetic changes | [124] | In vivo, STZ-induced diabetic mice | miR-451 knockdown attenuated cardiac fibrosis, improved cardiac function and suppressed endothelial-to-mesenchymal transition |
| Epigenetic changes | [126] | In vivo, mice treated with anti-miR-199a | Upregulation of genes related to cytoplasmic translation and mitochondrial respiratory chain complex assembly |
| Epigenetic changes | [127] | In vitro, H9C2 cells exposed to high glucose | ↑ miR-1 expression level ↓ mitochondrial membrane potential ↑ cytochrome-c release and apoptosis |
| Epigenetic changes | [128] | In vivo, STZ-induced diabetic mice In vitro, cardiac muscle cell line HL-1 | In STZ-induced diabetic mice: ↓ GAS5 ↑ NLRP3, caspase-1, Pro-caspase-1, IL-1β and IL-18 GAS5 overexpression improved cardiac function and myocardial hypertrophy In cardiac muscle cell line HL-1: ↑ NLRP3, caspase-1, Pro-caspase-1, IL-1β and IL-18, |
| Epigenetic changes | [129] | In vivo, STZ-induced diabetic mice In vitro, H9C2 cells exposed to high glucose | STZ-induced diabetic mice: ↑ p53 and p21 ↓ miR-30c and miR-181a H9C2 cells exposed to high glucose: ↑ p53 and p21 ↓ miR-181a |
| Epigenetic changes | [130] | In vivo, STZ-induced diabetic mice | ↑ miR-195 expression |
| Epigenetic changes | [131] | In vivo, mice with obesity and diabetes induced by high-fat diet In vitro, neonatal rat cardiac myocytes stimulated with palmitic acid | In neonatal rat cardiac myocytes: ↑ miR-451 expression in a dose- and time-dependent manner In cardiomyocyte-specific miR-451 knockout mice: cardiac hypertrophy and contractile reserves were ameliorated, ↑ Cab39 and phosphorylated AMPK, and ↓ mTOR |
| Epigenetic changes | [133] | In vivo, T2DM human patients undergoing coronary artery bypass graft surgery In vivo, type-2 diabetic mice In vitro, human ventricular cardiomyocytes treated with high glucose | ↑ miR-532 expression in the right atrial appendage tissue, which was associated with downregulation of ARC In human ventricular cardiomyocytes (AC16) treated with high glucose: inhibition of miR-532 activity in high-glucose-cultured human cardiomyocytes prevented the downregulation of ARC and attenuated apoptotic cell death |
7. Prospective Treatments
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Armocida, B.; Monasta, L.; Sawyer, S.M.; Bustreo, F.; Onder, G.; Castelpietra, G.; Pricci, F.; Minardi, V.; Giacomozzi, C.; Abbafati, C.; et al. The Burden of Type 1 and Type 2 Diabetes among Adolescents and Young Adults in 24 Western European Countries, 1990–2019: Results from the Global Burden of Disease Study 2019. Int. J. Public Health 2024, 68, 1606491. [Google Scholar] [CrossRef] [PubMed]
- Valaiyapathi, B.; Gower, B.; Ashraf, P.A. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [PubMed]
- Pastore, I.; Bolla, A.M.; Montefusco, L.; Lunati, M.E.; Rossi, A.; Assi, E.; Zuccotti, G.V.; Fiorina, P. The impact of diabetes mellitus on cardiovascular risk onset in children and adolescents. Int. J. Mol. Sci. 2020, 21, 4928. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Faienza, M.F.; Scicchitano, P.; Cortese, F.; Gesualdo, M.; Zito, A.; Basile, M.; Recchia, P.; Leogrande, D.; Viola, D.; et al. Insulin resistance and endothelial function in children and adolescents. Int. J. Cardiol. 2014, 174, 343–347. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Delvecchio, M.; Zito, A.; De Palma, F.; Cortese, F.; Nitti, A.; Massari, E.; Gesualdo, M.; Ricci, G.; et al. Vascular Function and Myocardial Performance Indices in Children Born Small for Gestational Age. Circ. J. 2016, 80, 958–963. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Zhang, J.; Zhang, R.; Zhao, L.; Ren, H.; Zou, Y.; Wang, T.; Wang, J.; Zhao, Y.; et al. Early-onset of type 2 diabetes mellitus is a risk factor for diabetic nephropathy progression: A biopsy-based study. Aging 2021, 13, 8146–8154. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, N.; Curtis, A.J.; Heritier, S.; Gadowski, A.M.; Pavkov, M.E.; Kenealy, T.; Owens, D.R.; Thomas, R.L.; Song, S.; Wong, J. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: Systematic review and meta-analyses. Diabetologia 2021, 64, 275–287. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, X.; Ji, L.; Yang, W.; Lu, J.; Weng, J.; Jia, W.; Shan, Z.; Liu, J.; Tian, H. The characteristics of newly diagnosed adult early-onset diabetes: A population-based cross-sectional study. Sci. Rep. 2017, 7, 46534. [Google Scholar] [CrossRef]
- Ke, C.; Shah, B.R.; Thiruchelvam, D.; Echouffo-Tcheugui, J.B. Association between Age at Diagnosis of Type 2 Diabetes and Hospitalization for Heart Failure: A Population-Based Study. J. Am. Heart Assoc. 2024, 13, e030683. [Google Scholar] [CrossRef]
- Lindberg, L.; Danielsson, P.; Persson, M.; Marcus, C.; Hagman, E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med. 2020, 17, e1003078. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Patel, N.T.; Villarreal-Gonzalez, M.; Taub, P.R. Prevalence of diabetic cardiomyopathy in patients with type 2 diabetes in a large academic medical center. BMC Med. 2024, 22, 195. [Google Scholar] [CrossRef]
- NHS Digital. National Diabetes Audit, 2017–2018. Available online: https://files.digital.nhs.uk/91/084B1D/National%20Diabetes%20Audit%2C%202017-18%2C%20Report%202a.pdf (accessed on 13 December 2019).
- Huo, J.-L.; Feng, Q.; Pan, S.; Fu, W.-J.; Liu, Z.; Liu, Z. Diabetic cardiomyopathy: Early diagnostic biomarkers, pathogenetic mechanisms, and therapeutic interventions. Cell Death Discov. 2023, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Sudo, S.Z.; Montagnoli, T.L.; Rocha, B.D.S.; Santos, A.D.; de Sá, M.P.; Zapata-Sudo, G. Diabetes-induced cardiac autonomic neuropathy: Impact on heart function and prognosis. Biomedicines 2022, 10, 3258. [Google Scholar] [CrossRef]
- Ramesh, P.; Yeo, J.L.; Brady, E.M.; McCann, G.P. Role of inflammation in diabetic cardiomyopathy. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221083530. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Frati, G.; Schirone, L.; Chimenti, I.; Yee, D.; Biondi-Zoccai, G.; Volpe, M.; Sciarretta, S. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc. Res. 2017, 113, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Aragno, M.; Mastrocola, R.; Medana, C.; Catalano, M.G.; Vercellinatto, I.; Danni, O.; Boccuzzi, G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology 2006, 147, 5967–5974. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, R.; Tan, H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int. J. Biol. Sci. 2019, 15, 1345. [Google Scholar] [CrossRef]
- Qu, X.-F.; Zhai, B.-Z.; Hu, W.-L.; Lou, M.-H.; Chen, Y.-H.; Liu, Y.-F.; Chen, J.-G.; Mei, S.; You, Z.-Q.; Liu, Z. Pyrroloquinoline quinone ameliorates diabetic cardiomyopathy by inhibiting the pyroptosis signaling pathway in C57BL/6 mice and AC16 cells. Eur. J. Nutr. 2022, 61, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, M.; Zhang, Y.; An, F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE 2014, 9, e104771. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-M.; Liu, Y.; White, D.; Su, Y.; Drew, B.G.; Bruce, C.R.; Kiriazis, H.; Xu, Q.; Jennings, N.; Bobik, A.; et al. Deletion of macrophage migration inhibitory factor protects the heart from severe ischemia–reperfusion injury: A predominant role of anti-inflammation. J. Mol. Cell. Cardiol. 2011, 50, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Seropian, I.M.; Toldo, S.; Mezzaroma, E.; Abbate, A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm. Res. 2013, 62, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.J.; van den Pangaart, P.S.; Aerts, J.M.; Boon, L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. J. Endocrinol. 2007, 192, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, H.; Liu, P.; Wang, H.; Liu, J.; Gao, C.; Liu, Y.; Lian, K.; Yang, L.; Sun, L. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 2013, 108, 329. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Faienza, M.F.; Altomare, M.; Nacci, C.; Montagnani, M.; Valente, F.; Cortese, F.; Gesualdo, M.; Zito, A.; Mancarella, R.; et al. Endothelial and Metabolic Function Interactions in Overweight/Obese Children. J. Atheroscler. Thromb. 2016, 23, 950–959. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, Y.; Qin, J.; Xu, H.; Liu, L.; Wan, H.; Zhang, C.; Fan, W.; Qu, S. MicroRNA-223–3p promotes pyroptosis of cardiomyocyte and release of inflammasome factors via downregulating the expression level of SPI1 (PU. 1). Toxicology 2022, 476, 153252. [Google Scholar] [CrossRef]
- Devi, T.D.; Babu, M.; Mäkinen, P.; Kaikkonen, M.U.; Heinaniemi, M.; Laakso, H.; Ylä-Herttuala, E.; Rieppo, L.; Liimatainen, T.; Naumenko, N. Aggravated postinfarct heart failure in type 2 diabetes is associated with impaired mitophagy and exaggerated inflammasome activation. Am. J. Pathol. 2017, 187, 2659–2673. [Google Scholar] [CrossRef]
- Lother, A.; Bondareva, O.; Saadatmand, A.R.; Pollmeier, L.; Härdtner, C.; Hilgendorf, I.; Weichenhan, D.; Eckstein, V.; Plass, C.; Bode, C. Diabetes changes gene expression but not DNA methylation in cardiac cells. J. Mol. Cell. Cardiol. 2021, 151, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Kesherwani, V.; Shahshahan, H.R.; Mishra, P.K. Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS ONE 2017, 12, e0182828. [Google Scholar] [CrossRef]
- Henson, S.M.; Aksentijevic, D. Senescence and type 2 diabetic cardiomyopathy: How young can you die of old age? Front. Pharmacol. 2021, 12, 716517. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006, 55 (Suppl. 2), S9–S15. [Google Scholar] [CrossRef]
- Gollmer, J.; Zirlik, A.; Bugger, H. Mitochondrial mechanisms in diabetic cardiomyopathy. Diabetes Metab. J. 2020, 44, 33. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. The role of mitochondrial abnormalities in diabetic cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 7863. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2021, 178, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Y.; Liu, Y.; Gao, J.; Feng, L.; Zhang, Y.; Shi, L.; Zhang, M.; Guo, D.; Qi, B. Mitochondrial quality control in the maintenance of cardiovascular homeostasis: The roles and interregulation of UPS, mitochondrial dynamics and mitophagy. Oxidative Med. Cell. Longev. 2021, 2021, 3960773. [Google Scholar] [CrossRef]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef]
- Barth, E.; Stämmler, G.; Speiser, B.; Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992, 24, 669–681. [Google Scholar] [CrossRef]
- Opie, L.H. Heart Physiology: From Cell to Circulation; Lippincott Williams Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Ohtake, T.; Yokoyama, I.; Watanabe, T.; Momose, T.; Serezawa, T.; Nishikawa, J.; Sasaki, Y. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1995, 36, 456–463. [Google Scholar]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef] [PubMed]
- Barger, P.M.; Kelly, D.P. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc. Med. 2000, 10, 238–245. [Google Scholar] [CrossRef]
- Finck, B.N.; Lehman, J.J.; Leone, T.C.; Welch, M.J.; Bennett, M.J.; Kovacs, A.; Han, X.; Gross, R.W.; Kozak, R.; Lopaschuk, G.D. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 2002, 109, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zlobine, I.; Gopal, K.; Ussher, J.R. Lipotoxicity in obesity and diabetes-related cardiac dysfunctioniochim. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 1555–1568. [Google Scholar]
- Brindley, D.N.; Kok, B.P.; Kienesberger, P.C.; Lehner, R.; Dyck, J.R. Shedding light on the enigma of myocardial lipotoxicity: The involvement of known and putative regulators of fatty acid storage and mobilization. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E897–E908. [Google Scholar] [CrossRef] [PubMed]
- Sletten, A.C.; Peterson, L.R.; Schaffer, J.E. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J. Intern. Med. 2018, 284, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, J.; Zirlik, A.; Bugger, H. Established and Emerging Mechanisms of Diabetic Cardiomyopathy. J. Lipid Atheroscler. 2019, 8, 26–47. [Google Scholar] [CrossRef]
- Dodd, M.S.; Sousa Fialho, M.D.L.; Montes Aparicio, C.N.; Kerr, M.; Timm, K.N.; Griffin, J.L.; Luiken, J.; Glatz, J.F.C.; Tyler, D.J.; Heather, L.C. Fatty Acids Prevent Hypoxia-Inducible Factor-1α Signaling through Decreased Succinate in Diabetes. JACC Basic Transl. Sci. 2018, 3, 485–498. [Google Scholar] [CrossRef]
- Sturza, A.; Duicu, O.M.; Vaduva, A.; Dănilă, M.D.; Noveanu, L.; Varró, A.; Muntean, D.M. Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.R.; Bagul, P.K.; Katare, P.B.; Anwar Mohammed, S.; Padiya, R.; Banerjee, S.K. Garlic activates SIRT-3 to prevent cardiac oxidative stress and mitochondrial dysfunction in diabetes. Life Sci. 2016, 164, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cater, M.; Krizancic Bombek, L. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Spinelli, S.; Guida, L.; Passalacqua, M.; Magnone, M.; Cossu, V.; Sambuceti, G.; Marini, C.; Sturla, L.; Zocchi, E. Abscisic Acid and Its Receptors LANCL1 and LANCL2 Control Cardiomyocyte Mitochondrial Function, Expression of Contractile, Cytoskeletal and Ion Channel Proteins and Cell Proliferation via ERRalpha. Antioxidants 2023, 12, 1692. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Horvath, T.L. The role of mitochondrial uncoupling proteins in lifespan. Pflugers Arch. Eur. J. Physiol. 2010, 459, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Garikipati, V.N.S.; Kishore, R. Mitochondrial dysfunction and its impact on diabetic heart. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, C.; Zhu, P.; Li, Y. Novel Insights Into Molecular Mechanism of Mitochondria in Diabetic Cardiomyopathy. Front. Physiol. 2020, 11, 609157. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Dihoum, A.; Mordi, I.R.; Choy, A.M.; Rena, G.; Lang, C.C. Left Ventricular Hypertrophy in Diabetic Cardiomyopathy: A Target for Intervention. Front. Cardiovasc. Med. 2021, 8, 746382. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.L.; Hsu, Y.C.; Chang, S.T.; Chung, C.M.; Lin, C.L. The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. Int. J. Mol. Sci. 2023, 24, 8604. [Google Scholar] [CrossRef]
- Sundgren, N.C.; Giraud, G.D.; Schultz, J.M.; Lasarev, M.R.; Stork, P.J.; Thornburg, K.L. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1481–R1489. [Google Scholar] [CrossRef]
- Hu, X.; Bai, T.; Xu, Z.; Liu, Q.; Zheng, Y.; Cai, L. Pathophysiological Fundamentals of Diabetic Cardiomyopathy. Compr. Physiol. 2017, 7, 693–711. [Google Scholar] [PubMed]
- Cairns, M.; Joseph, D.; Essop, M.F. The dual role of the hexosamine biosynthetic pathway in cardiac physiology and pathophysiology. Front. Endocrinol. 2022, 13, 984342. [Google Scholar] [CrossRef] [PubMed]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Cividini, F.; Scott, B.T.; Dai, A.; Han, W.; Suarez, J.; Diaz-Juarez, J.; Diemer, T.; Casteel, D.E.; Dillmann, W.H. O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J. Biol. Chem. 2016, 291, 26515–26528. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Ma, J.; Hart, G.W. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, 6050–6055. [Google Scholar] [CrossRef]
- Marsh, S.A.; Dell’Italia, L.J.; Chatham, J.C. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 2011, 40, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Masaki, N.; Feng, B.; Bretón-Romero, R.; Inagaki, E.; Weisbrod, R.M.; Fetterman, J.L.; Hamburg, N.M. O-GlcNAcylation Mediates Glucose-Induced Alterations in Endothelial Cell Phenotype in Human Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e014046. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L.; Xue, R.Q.; Xi, H.; Ming, Z.; Yu, X.J.; Liu, L.Z.; Wu, Q.; Si, Y.; Li, D.L.; Zang, W.J. Cholinergic drugs ameliorate endothelial dysfunction by decreasing O-GlcNAcylation via M3 AChR-AMPK-ER stress signaling. Life Sci. 2019, 222, 1–12. [Google Scholar] [CrossRef]
- Karimi, M.; Pavlov, V.I.; Ziegler, O.; Sriram, N.; Yoon, S.Y.; Agbortoko, V.; Alexandrova, S.; Asara, J.; Sellke, F.W.; Sturek, M.; et al. Robust effect of metabolic syndrome on major metabolic pathways in the myocardium. PLoS ONE 2019, 14, e0225857. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Q.; Tsuji-Hosokawa, A.; Watanabe, M.; Willson, C.; Lai, N.; Wang, J.; Dai, A.; Scott, B.T.; Dillmann, W.H.; et al. Overexpression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovasc. Res. 2020, 116, 1186–1198. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Matsushima, S.; Ikeda, S.; Ikeda, M.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; et al. DPP (Dipeptidyl Peptidase)-4 Inhibitor Attenuates Ang II (Angiotensin II)-Induced Cardiac Hypertrophy via GLP (Glucagon-Like Peptide)-1-Dependent Suppression of Nox (Nicotinamide Adenine Dinucleotide Phosphate Oxidase) 4-HDAC (Histone Deacetylase) 4 Pathway. Hypertension 2020, 75, 991–1001. [Google Scholar]
- Zhao, Q.D.; Viswanadhapalli, S.; Williams, P.; Shi, Q.; Tan, C.; Yi, X.; Bhandari, B.; Abboud, H.E. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 2015, 131, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Rutschow, S.; Jäger, S.; Linderer, A.; Anker, S.; Riad, A.; Unger, T.; Schultheiss, H.P.; Pauschinger, M.; Tschöpe, C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: The role of angiotensin type 1 receptor antagonism. Diabetes 2007, 56, 641–646. [Google Scholar] [CrossRef]
- Jia, G.; Habibi, J.; DeMarco, V.G.; Martinez-Lemus, L.A.; Ma, L.; Whaley-Connell, A.T.; Aroor, A.R.; Domeier, T.L.; Zhu, Y.; Meininger, G.A.; et al. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension 2015, 66, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Le, B.; Khode, R.; Baker, K.M.; Kumar, R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 2008, 57, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.K.; Murohara, T. Diabetes-related heart failure. Circ. J. 2014, 78, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Ziyadeh, F.N.; Sharma, K.; Ericksen, M.; Wolf, G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J. Clin. Investig. 1994, 93, 536–542. [Google Scholar] [CrossRef]
- Singh, V.P.; Baker, K.M.; Kumar, R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: Role in extracellular matrix production. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1675–H1684. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Frangogiannis, N.G. Diabetic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166044. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Li, B.; Latini, R.; Sonnenblick, E.H.; Anversa, P.; Leri, A.; Kajstura, J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II—Dependent. Lab. Investig. 2000, 80, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Zhao, F.; Kobayashi, T.; Hagiwara, M.; Kaminaris, A.; Li, C.; Cai, F.; Huang, Y.; Liang, Q. Hyperglycemia-induced cardiomyocyte death is mediated by lysosomal membrane injury and aberrant expression of cathepsin D. Biochem. Biophys. Res. Commun. 2020, 523, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Shi, X.; Zhang, B.; Liu, H.; Zhang, L.; Ding, W.; Zhao, Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: Relationship with metabolic factors and complications. J. Mol. Med. 2012, 90, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Rice, G.E. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J. Endocrinol. 2007, 193, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Piek, A.; de Boer, R.A.; Sillje, H.H. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016, 21, 199–211. [Google Scholar] [CrossRef]
- Luo, M.; Anderson, M.E. Mechanisms of altered Ca2⁺ handling in heart failure. Circ. Res. 2013, 113, 690–708. [Google Scholar] [CrossRef]
- Dattani, A.; Singh, A.; McCann, G.P.; Gulsin, G.S. Myocardial Calcium Handling in Type 2 Diabetes: A Novel Therapeutic Target. J. Cardiovasc. Dev. Dis. 2023, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Stølen, T.O.; Høydal, M.A.; Kemi, O.J.; Catalucci, D.; Ceci, M.; Aasum, E.; Larsen, T.; Rolim, N.; Condorelli, G.; Smith, G.L.; et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ. Res. 2009, 105, 527–536. [Google Scholar] [CrossRef]
- Hegab, Z.; Mohamed, T.M.A.; Stafford, N.; Mamas, M.; Cartwright, E.J.; Oceandy, D. Advanced glycation end products reduce the calcium transient in cardiomyocytes by increasing production of reactive oxygen species and nitric oxide. FEBS Open Bio 2017, 7, 1672–1685. [Google Scholar] [CrossRef]
- Tang, L.; Wang, H.; Dai, B.; Wang, X.; Zhou, D.; Shen, J.; Guo, F.; Wang, J.; Zhou, J.; Wang, H.; et al. Human induced pluripotent stem cell-derived cardiomyocytes reveal abnormal TGFβ signaling in type 2 diabetes mellitus. J. Mol. Cell. Cardiol. 2020, 142, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Al Kury, L.T.; Sydorenko, V.; Smail, M.M.; Qureshi, M.A.; Shmygol, A.; Papandreou, D.; Singh, J.; Howarth, F.C. Calcium signaling in endocardial and epicardial ventricular myocytes from streptozotocin-induced diabetic rats. J. Diabetes Investig. 2021, 12, 493–500. [Google Scholar] [CrossRef]
- Ashrafi, R.; Modi, P.; Oo, A.Y.; Pullan, D.M.; Jian, K.; Zhang, H.; Gerges, J.Y.; Hart, G.; Boyett, M.R.; Davis, G.K.; et al. Arrhythmogenic gene remodelling in elderly patients with type 2 diabetes with aortic stenosis and normal left ventricular ejection fraction. Exp. Physiol. 2017, 102, 1424–1434. [Google Scholar] [CrossRef]
- Walker, C.A.; Spinale, F.G. The structure and function of the cardiac myocyte: A review of fundamental concepts. J. Thorac. Cardiovasc. Surg. 1999, 118, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bidasee, K.R.; Nallani, K.; Yu, Y.; Cocklin, R.R.; Zhang, Y.; Wang, M.; Dincer, U.D.; Besch, H.R., Jr. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 2003, 52, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Curotto Kurzydlowski, K.; Narayanan, N.; MacLennan, D.H. Identification of Ser38 as the site in cardiac sarcoplasmic reticulum Ca(2+)-ATPase that is phosphorylated by Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1994, 269, 26492–26496. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Groop, L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes 2009, 58, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Khullar, M.; Cheema, B.S.; Raut, S.K. Emerging Evidence of Epigenetic Modifications in Vascular Complication of Diabetes. Front. Endocrinol. 2017, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Khanna, S.; Raut, S.K.; Sharma, S.; Sharma, R.; Khullar, M. DUSP-1 gene expression is not regulated by promoter methylation in diabetes-associated cardiac hypertrophy. Ther. Adv. Cardiovasc. Dis. 2017, 11, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Suo, W.; Zhang, X.; Yang, Y.; Zhao, W.; Li, H.; Ni, Q. Targeting epigenetics in diabetic cardiomyopathy: Therapeutic potential of flavonoids. Biomed. Pharmacother. 2023, 157, 114025. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Cao, Y.; Qin, J.; Song, X.; Zhang, Q.; Shi, Y.; Cao, L. DNA methylation, its mediators and genome integrity. Int. J. Biol. Sci. 2015, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.; Tabaee, S.S. DNA methylation abnormalities in atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Krause, B.J.; Cataldo, L.R.; Vega, J.; Salas-Pérez, F.; Mennickent, P.; Gallegos, R.; Milagro, F.I.; Prieto-Hontoria, P.; Riezu-Boj, J.I.; et al. PPARGC1A Gene Promoter Methylation as a Biomarker of Insulin Secretion and Sensitivity in Response to Glucose Challenges. Nutrients 2020, 12, 2790. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chen, Y.C.; Cheng, C.C.; Lee, T.I.; Chen, Y.J.; Chen, S.A. Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit. Care Med. 2010, 38, 217–222. [Google Scholar] [CrossRef]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef]
- Stillman, B. Histone Modifications: Insights into Their Influence on Gene Expression. Cell 2018, 175, 6–9. [Google Scholar] [CrossRef]
- Weeks, K.L. HDAC inhibitors and cardioprotection: Homing in on a mechanism of action. eBioMedicine 2019, 40, 21–22. [Google Scholar] [CrossRef]
- Reddy, M.A.; Sahar, S.; Villeneuve, L.M.; Lanting, L.; Natarajan, R. Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 387–393. [Google Scholar] [CrossRef]
- Feng, B.; Chen, S.; Chiu, J.; George, B.; Chakrabarti, S. Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E1119–E1126. [Google Scholar] [CrossRef] [PubMed]
- Marzano, F.; Faienza, M.F.; Caratozzolo, M.F.; Brunetti, G.; Chiara, M.; Horner, D.S.; Annese, A.; D’Erchia, A.M.; Consiglio, A.; Pesole, G.; et al. Pilot study on circulating miRNA signature in children with obesity born small for gestational age and appropriate for gestational age. Pediatr. Obes. 2018, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Lüscher, T.F.; Cosentino, F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur. Heart J. 2016, 37, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Lin, Q.; Xu, Q. Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene 2019, 715, 143995. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Zhang, B.; Shi, Y.; Cui, L.; Zhao, X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc. Pathol. 2015, 24, 375–381. [Google Scholar] [CrossRef]
- Liang, C.; Gao, L.; Liu, Y.; Liu, Y.; Yao, R.; Li, Y.; Xiao, L.; Wu, L.; Du, B.; Huang, Z.; et al. MiR-451 antagonist protects against cardiac fibrosis in streptozotocin-induced diabetic mouse heart. Life Sci. 2019, 224, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30 (Suppl. 1), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Zhu, X.; Huang, J.; Li, Y.; Zhou, K.; Hua, Y.; Yan, F.; Wang, D.Z.; Luo, Y. Adeno-associated virus-mediated delivery of anti-miR-199a tough decoys attenuates cardiac hypertrophy by targeting PGC-1alpha. Mol. Ther. Nucleic Acids 2021, 23, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Song, Y.H.; Geng, Y.J.; Lin, Q.X.; Shan, Z.X.; Lin, S.G.; Li, Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem. Biophys. Res. Commun. 2008, 376, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, H.; Xu, Q.; Xu, C.; Yang, L.; Huang, C. LncRNA GAS5 inhibits NLRP3 inflammasome activation-mediated pyroptosis in diabetic cardiomyopathy by targeting miR-34b-3p/AHR. Cell Cycle 2020, 19, 3054–3065. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.K.; Singh, G.B.; Rastogi, B.; Saikia, U.N.; Mittal, A.; Dogra, N.; Singh, S.; Prasad, R.; Khullar, M. miR-30c and miR-181a synergistically modulate p53-p21 pathway in diabetes induced cardiac hypertrophy. Mol. Cell. Biochem. 2016, 417, 191–203. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, J.; Yu, Y.; Li, M.; Ni, R.; Wang, G.; Chen, R.; Li, J.; Fan, G.C.; Lacefield, J.C.; et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015, 58, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Gaetano, C.; Martelli, F. Oxidative stress and microRNAs in vascular diseases. Int. J. Mol. Sci. 2013, 14, 17319–17346. [Google Scholar] [CrossRef]
- Chandrasekera, D.N.K.; Neale, J.P.H.; van Hout, I.; Rawal, S.; Coffey, S.; Jones, G.T.; Bunton, R.; Sugunesegran, R.; Parry, D.; Davis, P.; et al. Upregulation of microRNA-532 enhances cardiomyocyte apoptosis in the diabetic heart. Apoptosis 2020, 25, 388–399. [Google Scholar] [CrossRef]
- Huang, J.H.; Chen, Y.C.; Lee, T.I.; Kao, Y.H.; Chazo, T.F.; Chen, S.A.; Chen, Y.J. Glucagon-like peptide-1 regulates calcium homeostasis and electrophysiological activities of HL-1 cardiomyocytes. Peptides 2016, 78, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Li, Y.G.; Wang, G.Y.; Bi, Y.G.; Zhao, Y.; Yan, M.L.; Liu, X.; Wei, M.; Wan, L.L.; Zhang, Q.Y. Metformin protects high glucose-cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol. Med. Rep. 2020, 22, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin Protects H9C2 Cardiomyocytes from High-Glucose and Hypoxia/Reoxygenation Injury via Inhibition of Reactive Oxygen Species Generation and Inflammatory Responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, S.; Bhansali, A.; Dutta, P.; Walia, R.; Dhawan, V. Metformin upregulates mitophagy in patients with T2DM: A randomized placebo-controlled study. J. Cell. Mol. Med. 2020, 24, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Dia, M.; Leon, C.; Chanon, S.; Bendridi, N.; Gomez, L.; Rieusset, J.; Thibault, H.; Paillard, M. Effect of Metformin on T2D-Induced MAM Ca(2+) Uncoupling and Contractile Dysfunction in an Early Mouse Model of Diabetic HFpEF. Int. J. Mol. Sci. 2022, 23, 3569. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, D.; Yu, J.; Jiang, W.; Liao, X.; Zhao, Q. Potential diabetic cardiomyopathy therapies targeting pyroptosis: A mini review. Front. Cardiovasc. Med. 2022, 9, 985020. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Pillai, K.K.; Hassan, M.Q.; Dhyani, N.; Ismail, M.V.; Najmi, A.K. Levosimendan reduces myocardial damage and improves cardiodynamics in streptozotocin induced diabetic cardiomyopathy via SERCA2a/NCX1 pathway. Life Sci. 2016, 153, 55–65. [Google Scholar] [CrossRef]
- Torre, E.; Arici, M.; Lodrini, A.M.; Ferrandi, M.; Barassi, P.; Hsu, S.C.; Chang, G.J.; Boz, E.; Sala, E.; Vagni, S.; et al. SERCA2a stimulation by istaroxime improves intracellular Ca2+ handling and diastolic dysfunction in a model of diabetic cardiomyopathy. Cardiovasc. Res. 2022, 118, 1020–1032. [Google Scholar] [CrossRef]
- Chen, Y.; Du, J.; Zhao, Y.T.; Zhang, L.; Lv, G.; Zhuang, S.; Qin, G.; Zhao, T.C. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc. Diabetol. 2015, 14, 99. [Google Scholar] [CrossRef]
- Xu, Z.; Tong, Q.; Zhang, Z.; Wang, S.; Zheng, Y.; Liu, Q.; Qian, L.B.; Chen, S.Y.; Sun, J.; Cai, L. Inhibition of HDAC3 prevents diabetic cardiomyopathy in OVE26 mice via epigenetic regulation of DUSP5-ERK1/2 pathway. Clin. Sci. 2017, 131, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Dhingra, N.K.; Butler, J.; Anker, S.D.; Ferreira, J.P.; Filippatos, G.; Januzzi, J.L.; Lam, C.S.P.; Sattar, N.; Peil, B.; et al. Empagliflozin in the treatment of heart failure with reduced ejection fraction in addition to background therapies and therapeutic combinations (EMPEROR-Reduced): A post-hoc analysis of a randomised, double-blind trial. Lancet Diabetes Endocrinol. 2022, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bajaj, M.; Yang, H.C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef]
- Lee, T.I.; Chen, Y.C.; Lin, Y.K.; Chung, C.C.; Lu, Y.Y.; Kao, Y.H.; Chen, Y.J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef]
- Cortese, F.; Scicchitano, P.; Cortese, A.M.; Meliota, G.; Andriani, A.; Truncellito, L.; Calculli, G.; Giordano, P.; Ciccone, M.M. Uric Acid in Metabolic and Cerebrovascular Disorders: A Review. Curr. Vasc. Pharmacol. 2020, 18, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Giordano, P.; Scicchitano, P.; Faienza, M.F.; De Pergola, G.; Calculli, G.; Meliota, G.; Ciccone, M.M. Uric acid: From a biological advantage to a potential danger. A focus on cardiovascular effects. Vasc. Pharmacol. 2019, 120, 106565. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.N.; Sanders, P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc. Res. 2014, 102, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.K.; Mistry, N.; Puar, P.; Verma, R.; Anker, S.; Mazer, C.D.; Verma, S. SGLT2 inhibitors and cardiac remodelling: A systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021, 8, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Chang, N.C.; Lin, S.Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Kang, S.; Verma, S.; Hassanabad, A.F.; Teng, G.; Belke, D.D.; Dundas, J.A.; Guzzardi, D.G.; Svystonyuk, D.A.; Pattar, S.S.; Park, D.S.J.; et al. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can. J. Cardiol. 2020, 36, 543–553. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Janardhan, A.; Chen, J.; Crawford, P.A. Altered systemic ketone body metabolism in advanced heart failure. Tex. Heart Inst. J. 2011, 38, 533–538. [Google Scholar]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Empagliflozin’s Fuel Hypothesis: Not so Soon. Cell Metab. 2016, 24, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef] [PubMed]
| Primary Effect | Primary Indication | Pleiotropic Effect in DdCM | |
|---|---|---|---|
| GLP-1 receptor agonists | Inhibits glucagon release | T2DM | Ameliorates calcium handling |
| Stimulates insulin incretion | Anti-inflammatory effect | ||
| Slows gastric emptying | Reduces oxidative stress | ||
| Metformin (AMPK activator) | Inhibits the RC in the liver | T2DM | Enhances antioxidant activity |
| Lowers glucose production | Decreases mitochondrial ROS production | ||
| Increases insulin sensitivity | Upregulates mitophagy | ||
| Normalizes calcium handling | |||
| PDE5 inhibitors | Vasodilation (smooth muscle cell relaxation) | Pulmonary hypertension | Inhibits NLRP3 inflammasome-mediated pyroptosis |
| Erectile disfunction | |||
| Phenofibrate (PPARα activator) | Increases lipolysis | Mixed dyslipidemia | Inhibits NLRP3 inflammasome-mediated pyroptosis |
| Reduces apoprotein C-III | |||
| Levosimendan (Calcium sensitizer) | Inotropic agent | Acute heart failure | Stabilizes calcium-troponin C complex |
| Istaroxime | Inotropic agent | Acute heart failure | Increases expression of SERCA-2 and sodium-calcium exchanger-1 |
| SGLT-2 inhibitors | Increases urinary glucose excretion | T2DM | Lowers uric acid levels (oxidative stress) |
| Lowers plasma glucose | Heart failure | Reduces epicardial adipose tissue and activin-A levels, which lowers inflammation and fibrosis | |
| Reduces left ventricular mass and fibrosis | |||
| Increases oxygen release (hemoconcentration) | |||
| Shifts toward free fatty acids oxidation | |||
| Promotes ketogenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giardinelli, S.; Meliota, G.; Mentino, D.; D’Amato, G.; Faienza, M.F. Molecular Basis of Cardiomyopathies in Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 8280. https://doi.org/10.3390/ijms25158280
Giardinelli S, Meliota G, Mentino D, D’Amato G, Faienza MF. Molecular Basis of Cardiomyopathies in Type 2 Diabetes. International Journal of Molecular Sciences. 2024; 25(15):8280. https://doi.org/10.3390/ijms25158280
Chicago/Turabian StyleGiardinelli, Silvia, Giovanni Meliota, Donatella Mentino, Gabriele D’Amato, and Maria Felicia Faienza. 2024. "Molecular Basis of Cardiomyopathies in Type 2 Diabetes" International Journal of Molecular Sciences 25, no. 15: 8280. https://doi.org/10.3390/ijms25158280
APA StyleGiardinelli, S., Meliota, G., Mentino, D., D’Amato, G., & Faienza, M. F. (2024). Molecular Basis of Cardiomyopathies in Type 2 Diabetes. International Journal of Molecular Sciences, 25(15), 8280. https://doi.org/10.3390/ijms25158280







