A DAMP-Based Assay for Rapid and Affordable Diagnosis of Bacterial Meningitis Agents: Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae
Abstract
:1. Introduction
2. Results
2.1. DAMP Primers Design
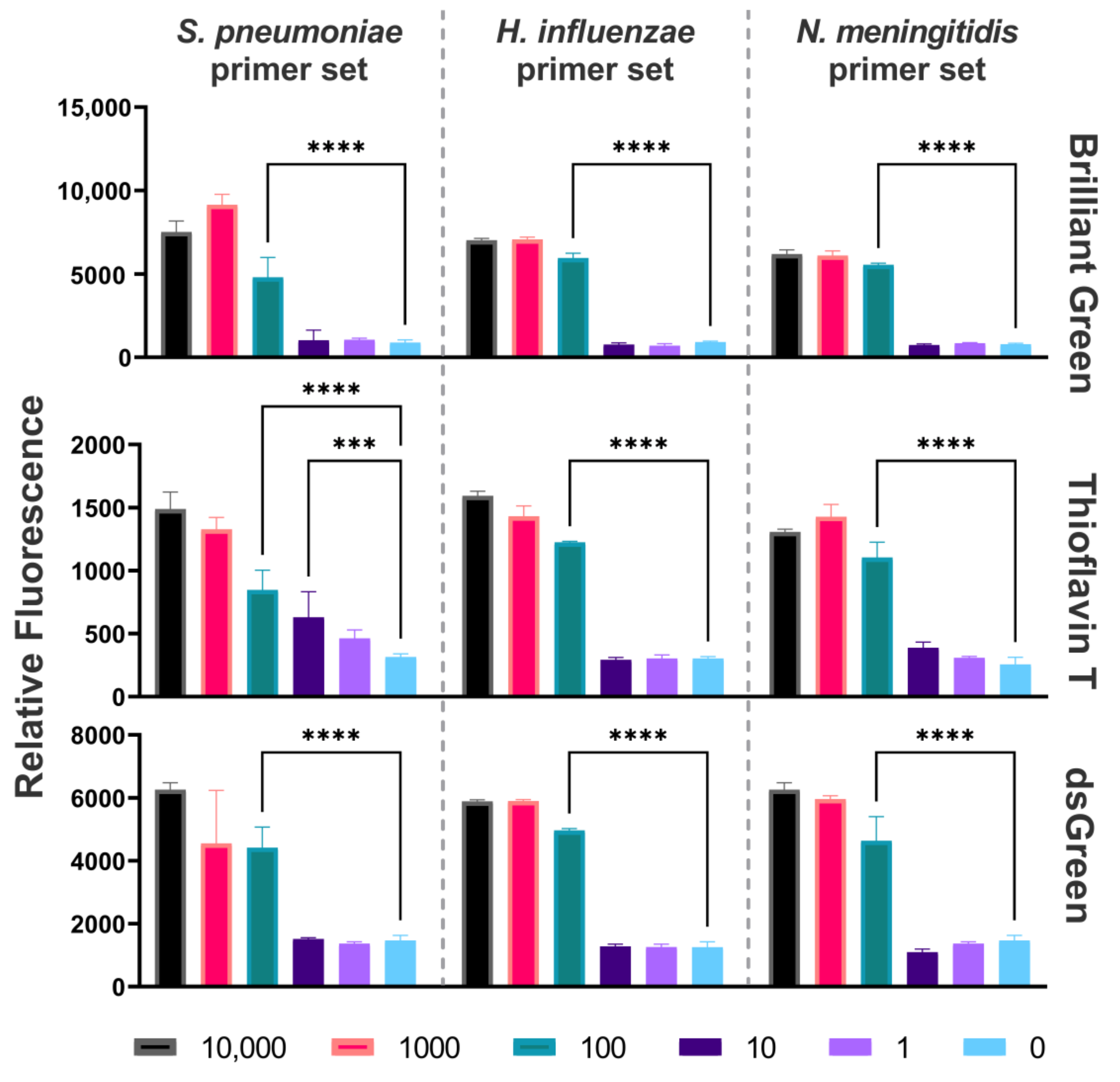
2.2. Limit of Detection
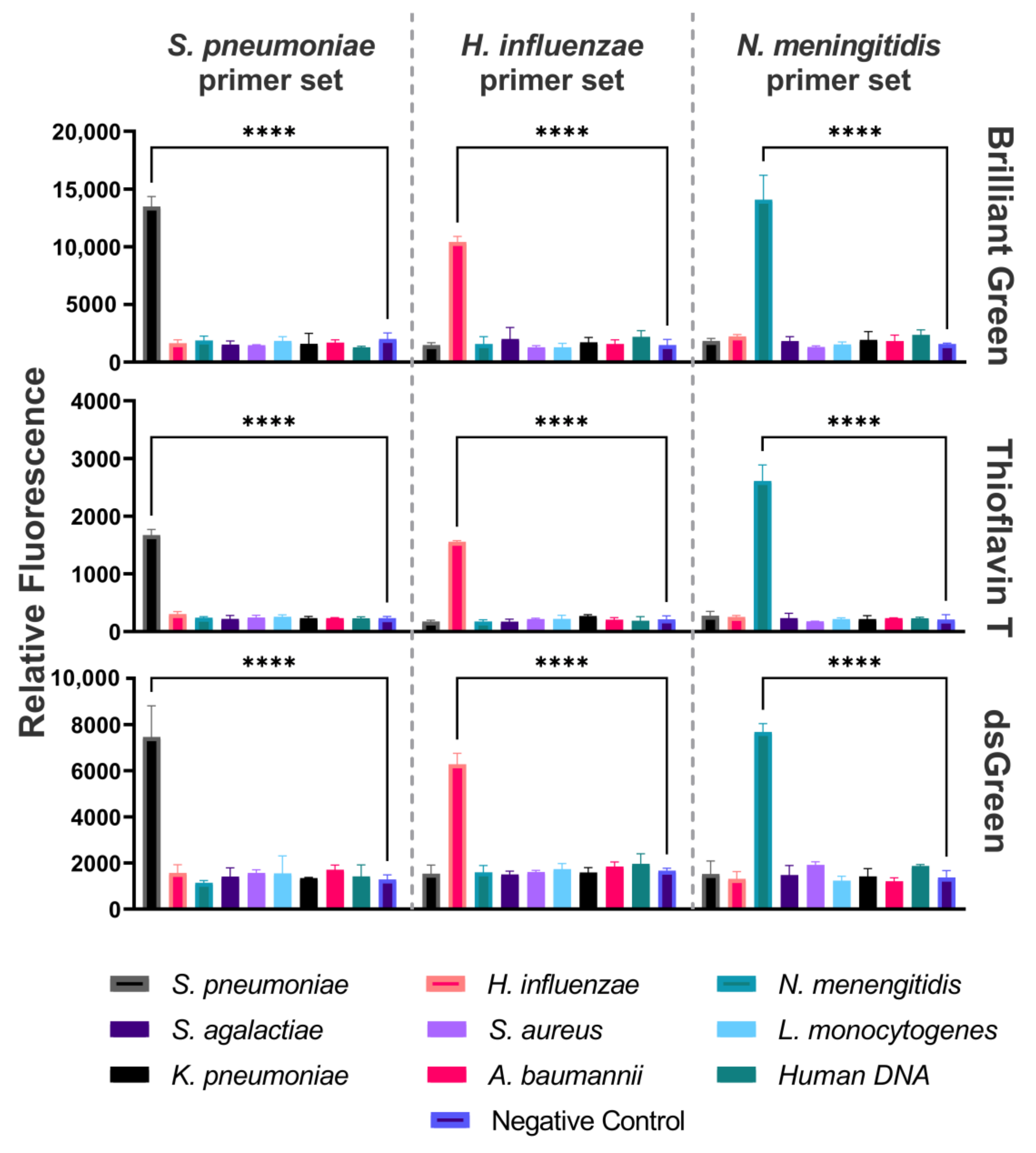
2.3. Specificity
2.4. Clinical Samples Testing
3. Discussion
4. Materials and Methods
4.1. Sample Collection and DNA Isolation
4.2. DAMP Primer Design
4.3. DAMP Reactions
4.4. DAMP Detection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.; Leib, S.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 2016, 22, S37–S62. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.D.; Lee, N.Y. Nucleic acid amplification-based microfluidic approaches for antimicrobial susceptibility testing. Analyst 2021, 146, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Suo, C.; Brown, T.; Wang, T.; Teichmann, S.A.; Bassett, A.R. INSIGHT: A population-scale COVID-19 testing strategy combining point-of-care diagnosis with centralized high-throughput sequencing. Sci. Adv. 2021, 7, eabe5054. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, L.; Herberg, J.; Sadarangani, M. Distinguishing community-acquired bacterial and viral meningitis: Microbes and biomarkers. J. Infect. 2024, 88, 106111. [Google Scholar] [CrossRef]
- Ghafari, S.; Namakin, K.; Khooban, A.R.; Askari, P.; Yousefi, M.; Ziaee, M. Comparison of Multiplex Quantitative Real-Time PCR and Culture Methods for the Diagnosis of Bacterial Meningitis in Patients with Suspected Meningitis. Infect. Epidemiol. Microbiol. 2023, 9, 219–228. [Google Scholar] [CrossRef]
- Michael, B.; Menezes, B.F.; Cunniffe, J.; Miller, A.; Kneen, R.; Francis, G.; Beeching, N.J.; Solomon, T. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg. Med. J. 2010, 27, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP Diagnostics at the Point-of-Care: Emerging Trends and Perspectives for the Developer Community. Expert Rev. Mol. Diagn. 2021, 21, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J. Advances in isothermal amplification: Novel strategies inspired by biological processes. Biosens. Bioelectron. 2015, 64, 196–211. [Google Scholar] [CrossRef]
- Gill, P.; Ghaemi, A. Nucleic Acid Isothermal Amplification Technologies—A Review. Nucleosides Nucleotides Nucleic Acids 2008, 27, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Asiello, P.J.; Baeumner, A.J. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 2011, 11, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-H.; Lin, Y.-C.; Teng, P.-H.; Chen, C.-L.; Lee, P.-Y. Real-time target-specific detection of loop-mediated isothermal amplification for white spot syndrome virus using fluorescence energy transfer-based probes. J. Virol. Methods 2011, 173, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Garafutdinov, R.R.; Kupova, O.Y.; Sakhabutdinova, A.R. Influence of Nucleotide Context on Non-Specific Amplification of DNA with Bst exo-DNA Polymerase. Biochemistry 2024, 89, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, S.-Y.; Kim, U.; Oh, S.-W. Diverse methods of reducing and confirming false-positive results of loop-mediated isothermal amplification assays: A review. Anal. Chim. Acta 2023, 1280, 341693. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Z.; Yin, K.; Sfeir, M.; Liu, C. Dual-Priming Isothermal Amplification (DAMP) for Highly Sensitive and Specific Molecular Detection with Ultralow Nonspecific Signals. Anal. Chem. 2019, 91, 12852–12858. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Yoshida, N.; Yamaguchi, S.; Hosaka, N.; Ota, Y.; Notomi, T.; Nakayama, T. A simple method for the detection of measles virus genome by loop-mediated isothermal amplification (LAMP). J. Med. Virol. 2005, 76, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Ravichandiran, V.; Ranjan, N. Beyond amyloid proteins: Thioflavin T in nucleic acid recognition. Biochimie 2021, 190, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B. Label-free fluorescent detection of DNA sequence based on interaction of brilliant green with double-stranded DNA. Microchim. Acta 2010, 171, 349–354. [Google Scholar] [CrossRef]
- Hoen, B.; Varon, E.; de Debroucker, T.; Fantin, B.; Grimprel, E.; Wolff, M.; Duval, X. Management of acute community-acquired bacterial meningitis (excluding newborns). Long version with arguments. Med. Mal. Infect. 2019, 49, 405–441. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.C.; Van Phan, T.; Nguyen, H.T.; Truong, K.H.; Do, V.C.; Pham, N.N.M.; Ho, T.V.; Phan, T.T.Q.; Hoang, T.A.; Soetewey, A.; et al. Childhood Bacterial Meningitis Surveillance in Southern Vietnam: Trends and Vaccination Implications from 2012 to 2021. Open Forum Infect. Dis. 2023, 10, ofad229. [Google Scholar] [CrossRef] [PubMed]
- Shkodenko, L.A.; Laushkina, V.O.; Rubel, M.S.; Sergeeva, E. Finger-Actuated Microfluidic Platform for Colorimetric Isothermal Diagnostics of Neisseria meningitidis and Herpes Simplex Virus. Russ. J. Bioorganic Chem. 2024, 50, 544–553. [Google Scholar] [CrossRef]
- Maltzeva, Y.I.; Gorbenko, D.A.; Nikitina, E.V.; Rubel, M.S.; Kolpashchikov, D.M. Visual Detection of Stem-Loop Primer Amplification (SPA) Products without Denaturation Using Peroxidase-like DNA Machines (PxDM). Int. J. Mol. Sci. 2023, 24, 7812. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, D.A.; Shkodenko, L.A.; Rubel, M.S.; Slita, A.V.; Nikitina, E.V.; Martens, E.A.; Kolpashchikov, D.M. DNA nanomachine for visual detection of structured RNA and double stranded DNA. Chem. Commun. 2022, 58, 5395–5398. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, D.A.; Shkodenko, L.V.; Nedorezova, D.D.; Rubel, M.D. Visual detection of bacterial and viral pathogens with peroxidase-like deoxyribozymes. In Molecular and Nano Machines III, Proceedings of the SPIE—The International Society for Optics and Photonics, San Diego, CA, USA, 21–25 August 2022; SPIE Digital Library: Bellingham, WA, USA, 2022; p. 1147709. [Google Scholar]
- Rubel, M.S.; Shkodenko, L.A.; Gorbenko, D.A.; Solyanikova, V.V.; Maltzeva, Y.I.; Rubel, A.A.; Koshel, E.I.; Kolpashchikov, D.M. Detection of Multiplex NASBA RNA Products Using Colorimetric Split G Quadruplex Probes. Methods Mol. Biol. 2023, 2709, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kovtunov, E.A.; Shkodenko, L.A.; Goncharova, E.A.; Nedorezova, D.D.; Sidorenko, S.V.; Koshel, E.I.; Kolpashchikov, D.M. Towards Point of Care Diagnostics: Visual Detection of Meningitis Pathogens Directly from Cerebrospinal Fluid. ChemistrySelect 2020, 5, 14572–14577. [Google Scholar] [CrossRef]
- de Filippis, I.; de Andrade, C.F.; Caldeira, N.; de Azevedo, A.C.; de Almeida, A.E. Comparison of PCR-based methods for the simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in clinical samples. Braz. J. Infect. Dis. 2016, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tzanakaki, G.; Tsopanomichalou, M.; Kesanopoulos, K.; Matzourani, R.; Sioumala, M.; Tabaki, A.; Kremastinou, J. Simultaneous single-tube PCR assay for the detection of Neisseria meningitidis, Haemophilus influenzae type b and Streptococcus pneumoniae. Clin. Microbiol. Infect. 2005, 11, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.E.; Guiver, M.; Borrow, R.; Edwards-Jones, V.; Fox, A.J.; Kaczmarski, E.B. Simultaneous Detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in Suspected Cases of Meningitis and Septicemia Using Real-Time PCR. J. Clin. Microbiol. 2001, 39, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-M.; Forsgren, A.; Janson, H. The Gene Encoding Protein D (Hpd) Is Highly Conserved among Haemophilus Influenzae Type b and Nontypeable Strains. Infect. Immun. 1995, 63, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Sirluck-Schroeder, I.; Al-Rawahi, G.N.; Gadkar, V.; Hoang, L.; Tsang, R.; Tilley, P. Limitation of ctrA as a Target for Neisseria meningitidis Identification and Potential Alternative Targets. J. Clin. Microbiol. 2022, 60, e0015222. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.d.G.S.; Tondella, M.L.; McCaustland, K.; Weidlich, L.; McGee, L.; Mayer, L.W.; Steigerwalt, A.; Whaley, M.; Facklam, R.R.; Fields, B.; et al. Evaluation and Improvement of Real-Time PCR Assays Targeting lytA, ply, and psaA Genes for Detection of Pneumococcal DNA. J. Clin. Microbiol. 2007, 45, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzi, G.; Kumbakumba, E.; Rasti, R.; Nanjebe, D.; Réu, P.; Nyehangane, D.; Mårtensson, A.; Nassejje, M.; Karlsson, J.; Mzee, J.; et al. Point-of-Care Approaches for Meningitis Diagnosis in a Low-Resource Setting (Southwestern Uganda): Observational Cohort Study Protocol of the “PI-POC” Trial. JMIR Res. Protoc. 2020, 9, e21430. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Thomas, M. Meningitis in adults: Diagnosis and management. Intern. Med. J. 2018, 48, 1294–1307. [Google Scholar] [CrossRef]
- Glimåker, M.; Johansson, B.; Grindborg, Ö.; Bottai, M.; Lindquist, L.; Sjölin, J. Adult Bacterial Meningitis: Earlier Treatment and Improved Outcome Following Guideline Revision Promoting Prompt Lumbar Puncture. Clin. Infect. Dis. 2015, 60, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Kaplan, S.; Kamboj, M.; Tang, Y.-W. Laboratory Diagnosis of Central Nervous System Infection. Curr. Infect. Dis. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; Thwaites, G.E.; Tunkel, A.R.; van de Beek, D. Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet 2012, 380, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, T.; Fairley, D.; Blackwood, B.; McKenna, J.; Shields, M.D. A systematic review of the diagnostic accuracy of Loop-mediated-isothermal AMPlification (LAMP) in the diagnosis of invasive meningococcal disease in children. BMC Pediatrics 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Bedarf, J.R.; Beraza, N.; Khazneh, H.; Özkurt, E.; Baker, D.; Borger, V.; Wüllner, U.; Hildebrand, F. Much ado about nothing? Off-target amplification can lead to false-positive bacterial brain microbiome detection in healthy and Parkinson’s disease individuals. Microbiome 2021, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Warmt, C.; Yaslanmaz, C.; Henkel, J. Investigation and validation of labelling loop mediated isothermal amplification (LAMP) products with different nucleotide modifications for various downstream analysis. Sci. Rep. 2022, 12, 7137. [Google Scholar] [CrossRef] [PubMed]
- Suleman, E.; Mtshali, M.S.; Lane, E. Investigation of false positives associated with loop-mediated isothermal amplification assays for detection of Toxoplasma gondii in archived tissue samples of captive felids. J. Vet.- Diagn. Investig. 2016, 28, 536–542. [Google Scholar] [CrossRef]
- Schneider, L.; Blakely, H.; Tripathi, A. Mathematical model to reduce loop mediated isothermal amplification (LAMP) false-positive diagnosis. Electrophoresis 2019, 40, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, D.; Deng, J.; Wang, Y.; Xu, J.; Ye, C. Loop-mediated isothermal amplification using self-avoiding molecular recognition systems and antarctic thermal sensitive uracil-DNA-glycosylase for detection of nucleic acid with prevention of carryover contamination. Anal. Chim. Acta 2017, 996, 74–87. [Google Scholar] [CrossRef]
- Hsieh, K.; Mage, P.L.; Csordas, A.T.; Eisenstein, M.; Soh, H.T. Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP). Chem. Commun. 2014, 50, 3747–3749. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, H.; Diao, Y. Advanced uracil DNA glycosylase-supplemented real-time reverse transcription loop-mediated isothermal amplification (UDG-rRT-LAMP) method for universal and specific detection of Tembusu virus. Sci. Rep. 2016, 6, 27605. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, E.J.; Kilgore, P.E.; Kim, S.A.; Takahashi, H.; Ohnishi, M.; Anh, D.D.; Dong, B.Q.; Kim, J.S.; Tomono, J.; et al. Clinical Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Neisseria meningitidis in Cerebrospinal Fluid. PLoS ONE 2015, 10, e0122922. [Google Scholar] [CrossRef]
- Kim, D.W.; Kilgore, P.E.; Kim, E.J.; Kim, S.A.; Anh, D.D.; Dong, B.Q.; Kim, J.S.; Seki, M. The Enhanced Pneumococcal LAMP Assay: A Clinical Tool for the Diagnosis of Meningitis Due to Streptococcus pneumoniae. PLoS ONE 2012, 7, e42954. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.J.; Tan, H.K.; Xu, Y.C.; Chen, Y.Z.; Xie, T.A.; Pan, Z.Y.; Yang, S.O.; Li, Q.; Li, X.Y.; Li, Z.X.; et al. A Pooled Analysis of the LAMP Assay for the Detection of Neisseria Meningitidis. BMC Infect. Dis. 2020, 20, 525. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Kilgore, P.E.; Kim, E.J.; Ohnishi, M.; Hayakawa, S.; Kim, D.W. Loop-Mediated Isothermal Amplifi-cation Methods for Diagnosis of Bacterial Meningitis. Front. Pediatr. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Antunes, W.; Moe, E.; Duarte, A.G.; Lima, L.M.P.; Santos, C.; Gomes, I.L.; Afonso, G.S.; Vieira, R.; Teles, H.S.S.; et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci. Rep. 2021, 11, 16430. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, Z.; Rashid, Z.; Ali, A.; Arif, A.; Ameen, F.; AlTami, M.S.; Yousaf, M.Z. Prospects of Microfluidic Technology in Nucleic Acid Detection Approaches. Biosensors 2023, 13, 584. [Google Scholar] [CrossRef]
- Mahalanabis, M.; Do, J.; AlMuayad, H.; Zhang, J.Y.; Klapperich, C.M. Erratum to: An Integrated Disposable Device for DNA Extraction and Helicase Dependent Amplification. Biomed. Microdevices 2010, 12, 353–359, reprinted in Biomed. Microdevices 2011, 13, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Toley, B.J.; Covelli, I.; Belousov, Y.; Ramachandran, S.; Kline, E.; Scarr, N.; Vermeulen, N.; Mahoney, W.; Lutz, B.R.; Yager, P. Isothermal strand displacement amplification (iSDA): A rapid and sensitive method of nucleic acid amplification for point-of-care diagnosis. Analyst 2015, 140, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent Advances in Recombinase Polymerase Amplification: Principle, Advantages, Disadvantages and Applications. Front. Cell. Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A. Phenol-Chloroform DNA Isolation Method. In DNA and RNA Isolation Techniques for Non-Experts; Springer International Publishing: Cham, Switzerland, 2022; pp. 33–39. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, S.; Wang, L.; Cao, J.; Liu, M.; Li, S.; Cao, X.; Guo, Y. A Rapid Detection of Haemophilus influenzae Using Multiple Cross Displacement Amplification Linked with Nanoparticle-Based Lateral Flow Biosensor. Front. Cell. Infect. Microbiol. 2021, 11, 721547. [Google Scholar] [CrossRef] [PubMed]
- McAvin, J.C.; Reilly, P.A.; Roudabush, R.M.; Barnes, W.J.; Salmen, A.; Jackson, G.W.; Beninga, K.K.; Astorga, A.; McCleskey, F.K.; Huff, W.B.; et al. Sensitive and Specific Method for Rapid Identification of Streptococcus pneumoniae Using Real-Time Fluorescence PCR. J. Clin. Microbiol. 2001, 39, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Qurbanalizadegan, M.; Ranjbar, R.; Ataee, R.; Hajia, M.; Goodarzi, Z.; Farshad, S.; Jafari, N.J.; Panahi, Y.; Kohanzad, H.; Rahbar, M.; et al. Specific PCR Assay for Rapid and Direct Detection of Neisseria meningitidis in Cerebrospinal Fluid Specimens. Iran. J. Public Health 2010, 39, 45–50. [Google Scholar] [PubMed]
- O’Steen, M.R.; Kolpashchikov, D.M. A self-assembling split aptamer multiplex assay for SARS-COVID19 and miniaturization of a malachite green DNA-based aptamer. Sens. Actuators Rep. 2022, 4, 100125. [Google Scholar] [CrossRef]

| Pathogen | Primer | Sequence (5′ ⮕ 3′) |
|---|---|---|
| Streptococcus pneumoniae | FO | ACAGGCTGGAAGAAAATCGC |
| RO | GCCATCTGGCTCTACTGTGA | |
| FI | TGGCGCCTTCTTTAGCGTCTAATTTTCAACGAAGAAGGTGCCATGA | |
| RI | ATCCAGTCAGCGGACGGAACATTTTGGCTTGTCTGCCAGTGTT | |
| FC | TGGCGCCTTCTTTAGCGTCTAA | |
| RC | ATCCAGTCAGCGGACGGAACA | |
| Haemophilus influenzae | FO | ACACTCTTCTGTGGACACTA |
| RO | ACGATGACGATTTGGGAATT | |
| FI | GCAAATGCAAGCGCTTTAGACTATTATCATTGCTCACCGTGG | |
| RI | AGCGATGACAAAAGATGGTCGTGCGACGTCAGTTAAACCG | |
| FC | GCAAATGCAAGCGCTTTAGACT | |
| RC | AGCGATGACAAAAGATGGTCGT | |
| Neisseria meningitidis | FO | AGTTGCCAGAGCAGTTGG |
| RO | CGCACACTATTCCCAGCAC | |
| FI | GGCGTTTTACCGACCACCGATTTTTGGCACGTGGTACGGTTTC | |
| RI | AGGCCGCCTGAAAAAAATGGCTTTTCGACACATTCGCCGCATTA | |
| FC | GGCGTTTTACCGACCACCGA | |
| RC | AGGCCGCCTGAAAAAAATGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkodenko, L.A.; Mohamed, A.-A.; Ateiah, M.; Rubel, M.S.; Koshel, E.I. A DAMP-Based Assay for Rapid and Affordable Diagnosis of Bacterial Meningitis Agents: Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. Int. J. Mol. Sci. 2024, 25, 8282. https://doi.org/10.3390/ijms25158282
Shkodenko LA, Mohamed A-A, Ateiah M, Rubel MS, Koshel EI. A DAMP-Based Assay for Rapid and Affordable Diagnosis of Bacterial Meningitis Agents: Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. International Journal of Molecular Sciences. 2024; 25(15):8282. https://doi.org/10.3390/ijms25158282
Chicago/Turabian StyleShkodenko, Liubov A., Al-Abbass Mohamed, Muhannad Ateiah, Maria S. Rubel, and Elena I. Koshel. 2024. "A DAMP-Based Assay for Rapid and Affordable Diagnosis of Bacterial Meningitis Agents: Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae" International Journal of Molecular Sciences 25, no. 15: 8282. https://doi.org/10.3390/ijms25158282







