Discovery of the Inhibitor Targeting the SLC7A11/xCT Axis through In Silico and In Vitro Experiments
Abstract
:1. Introduction
2. Results
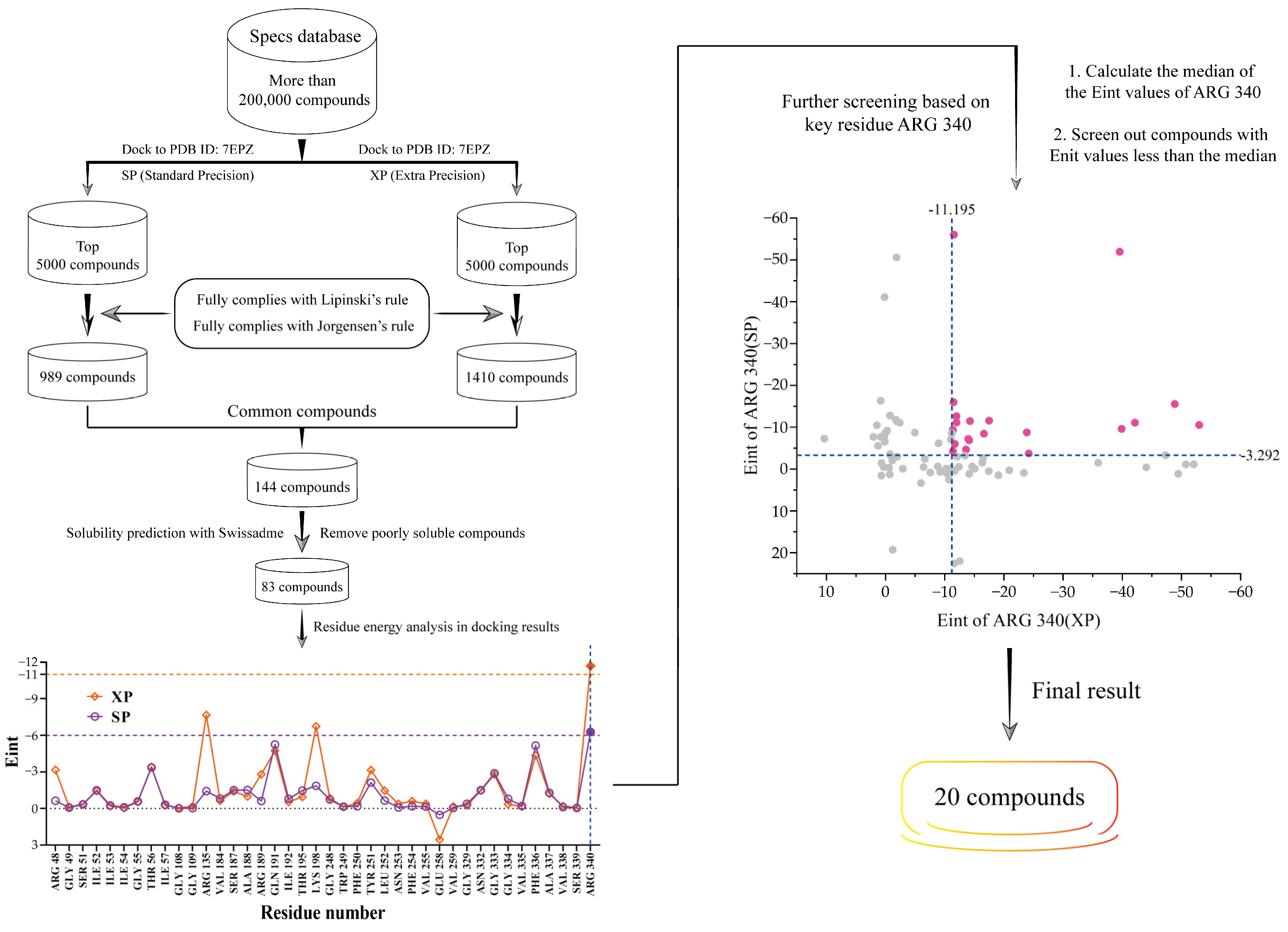
2.1. The Discovery of Potential SLC7A11/xCT Axis Inhibitors
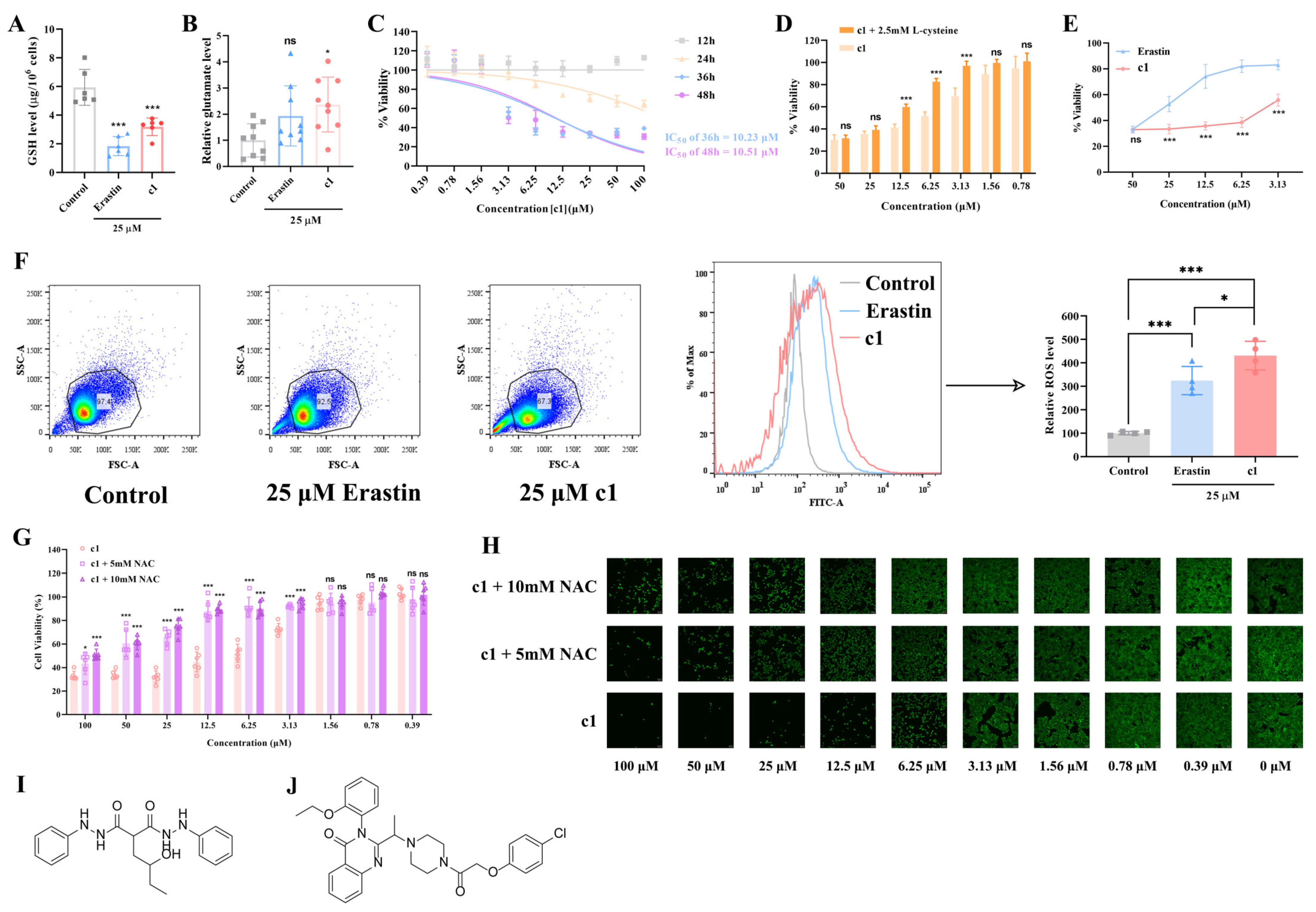
2.2. In Vitro Evaluation of the Effect Compound 1 Has on the Oxidative/Antioxidant Balance within HeLa Cells or Similar
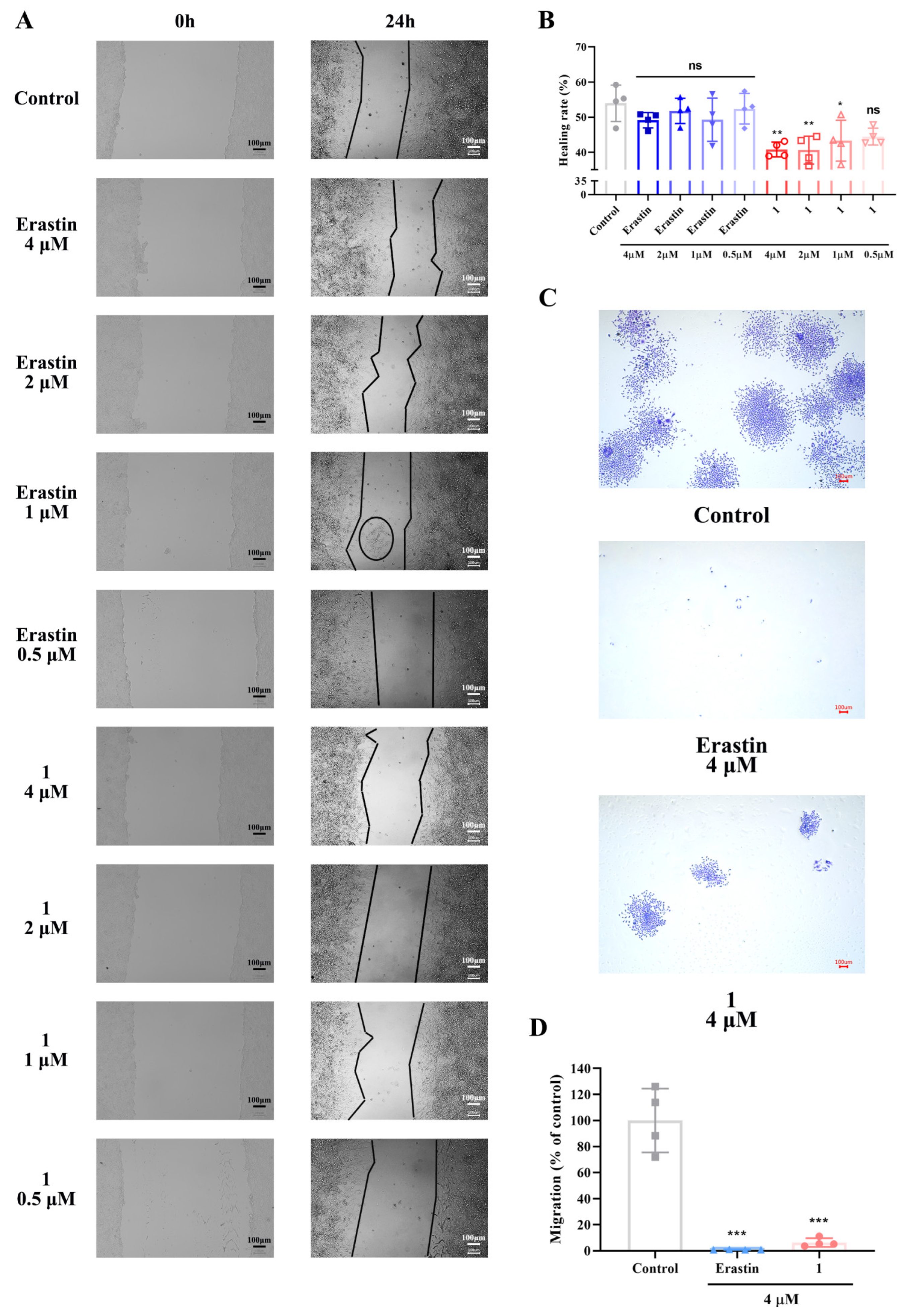
2.3. In Vitro Evaluation of the Inhibitory Effects of Compound 1 on HeLa Cell Migration at Low Concentrations
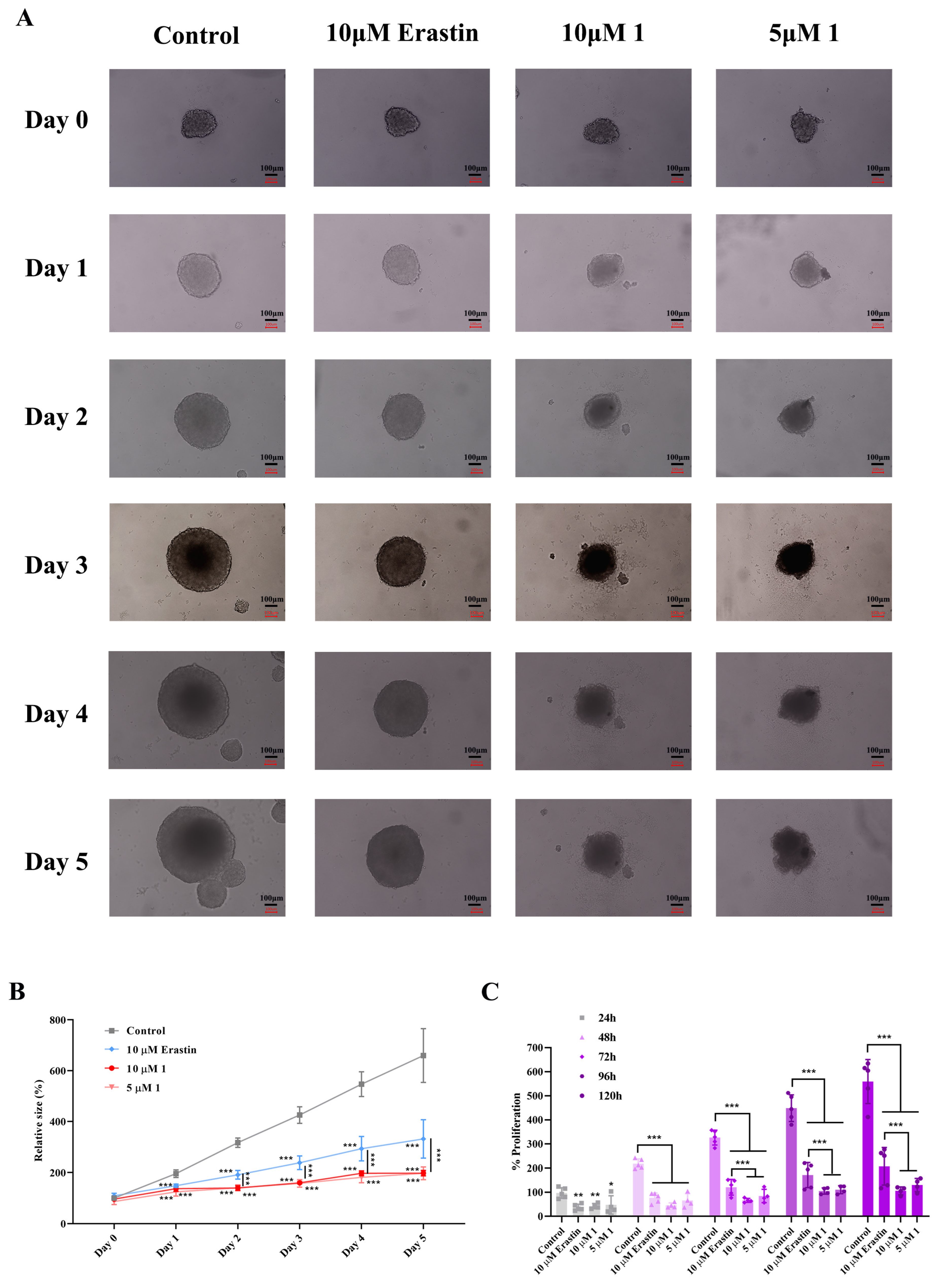
2.4. Assessment of the Toxic Effects of Compound 1 on HeLa Three-Dimensional Spheroids
2.5. Molecular Dynamics Simulations
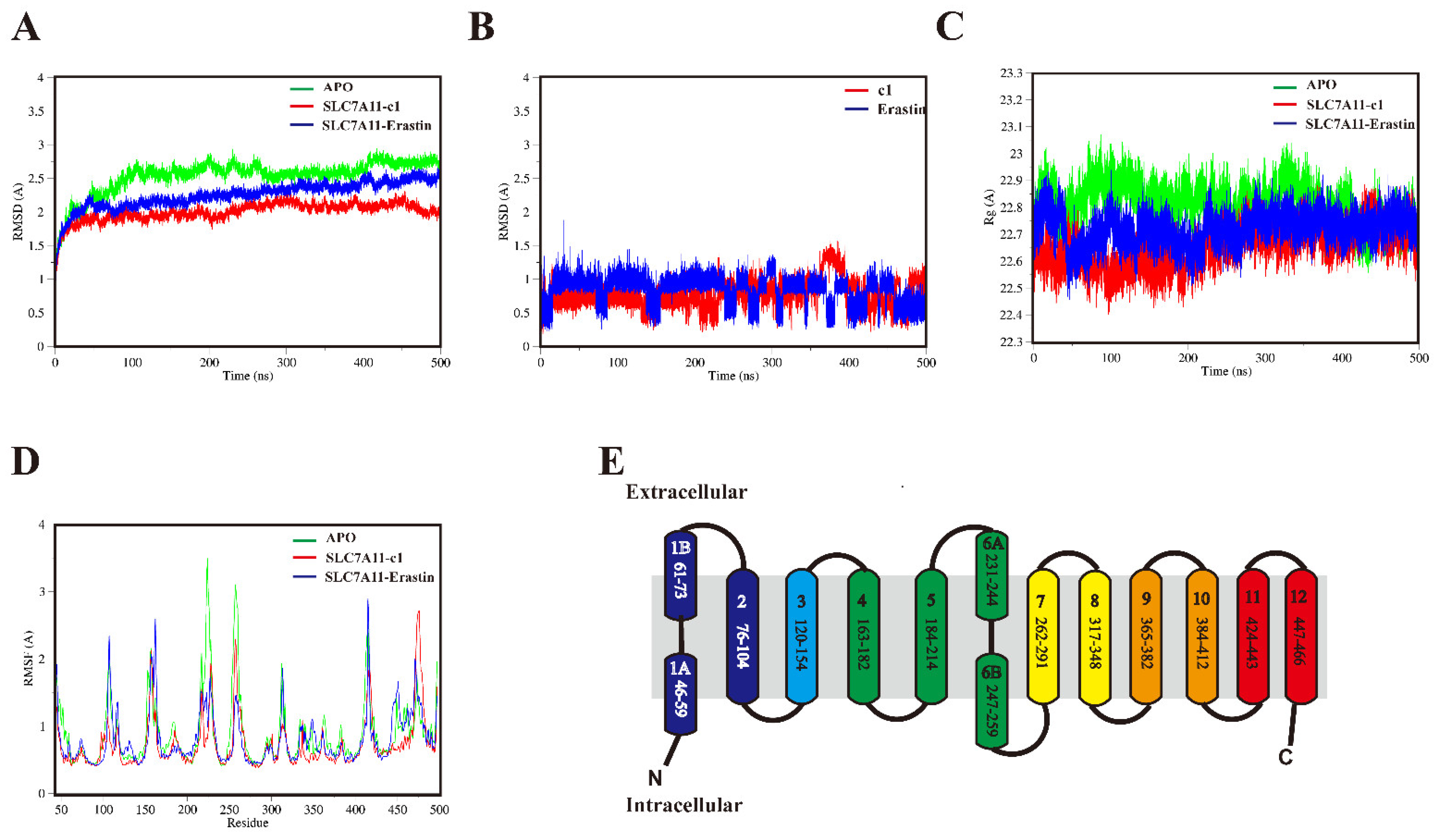
2.5.1. Structural Mobility and Compactness of SLC7A11 Systems
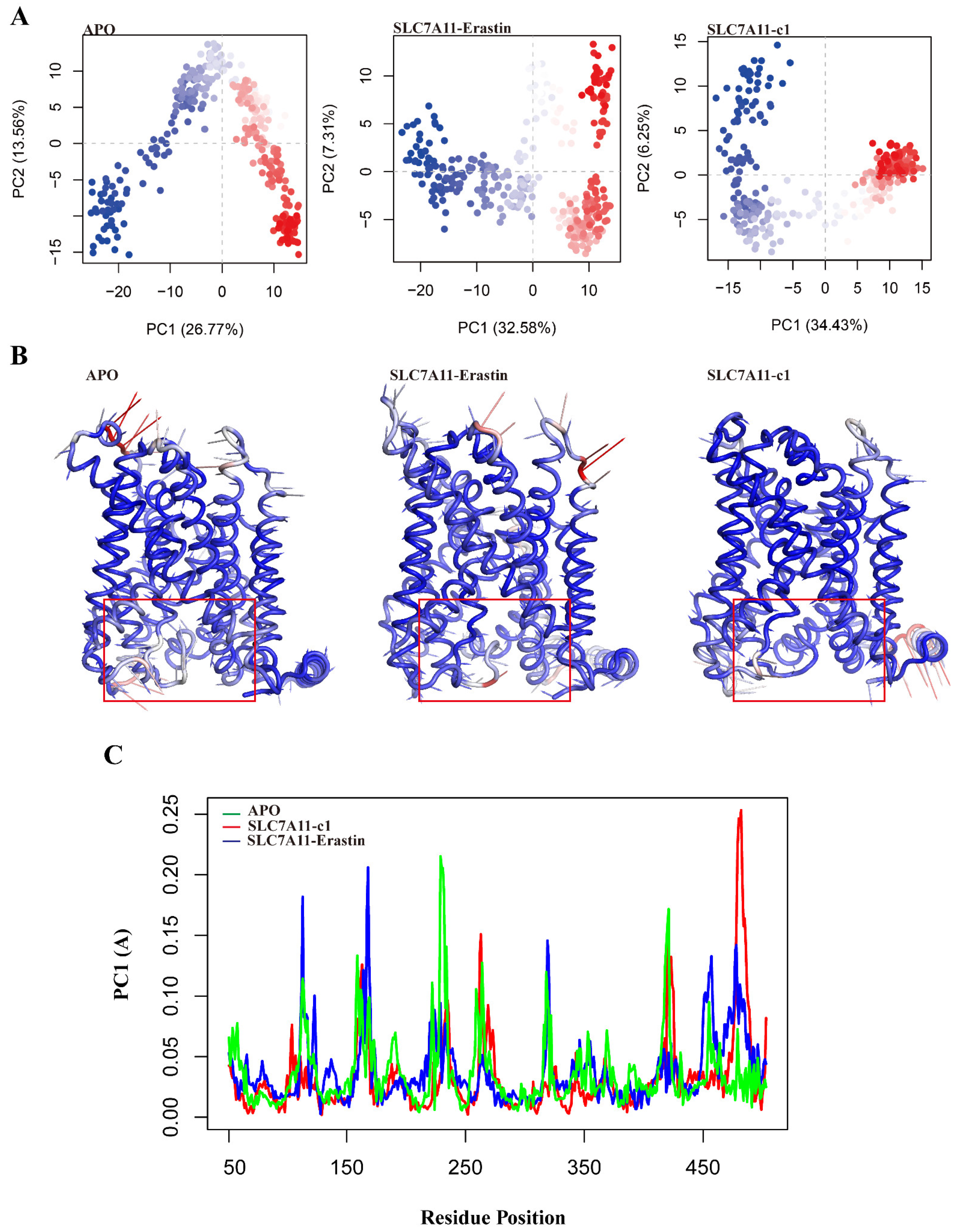
2.5.2. Collective Motions of SLC7A11 Systems
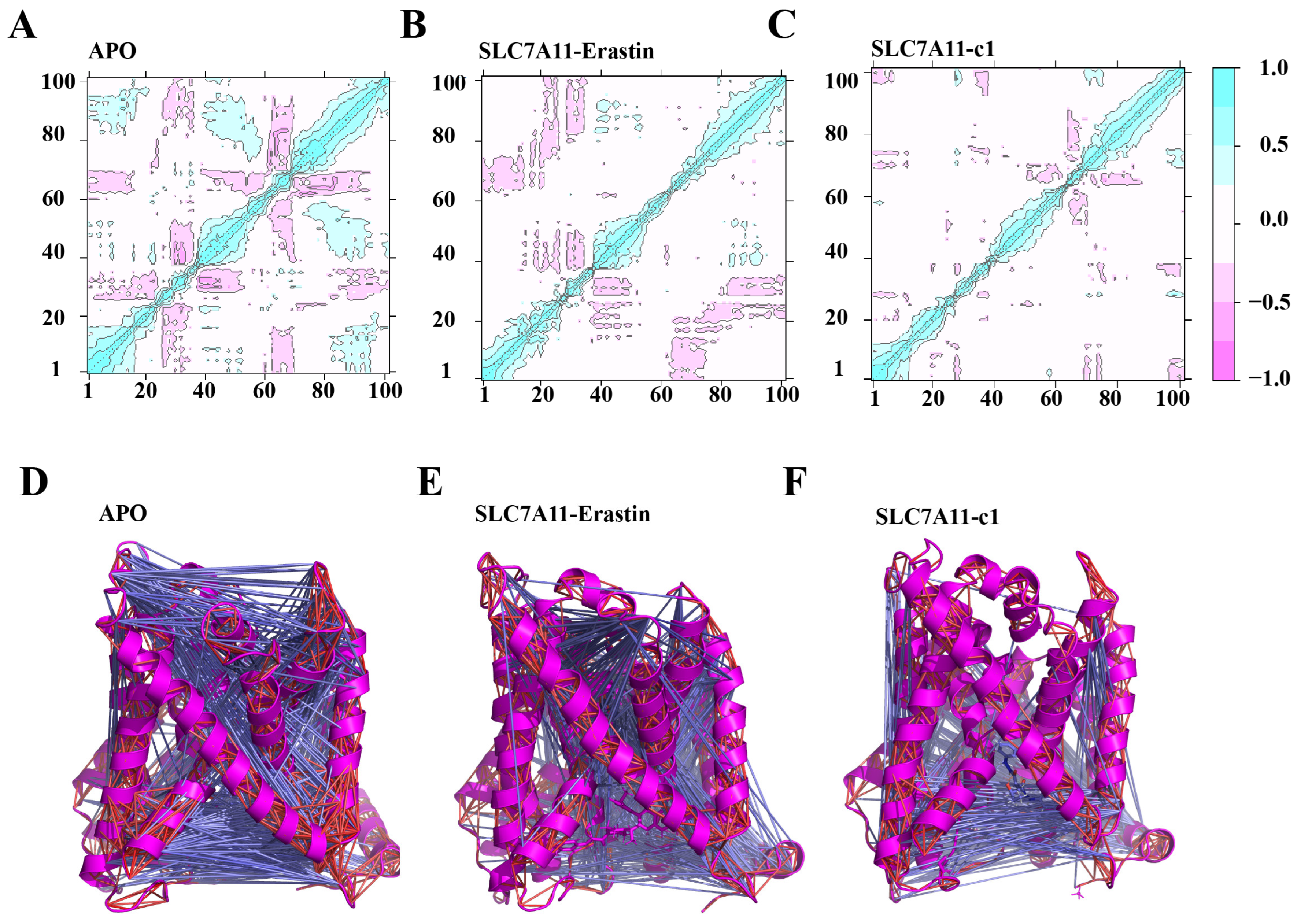
2.5.3. Local Correlation Motion Patterns
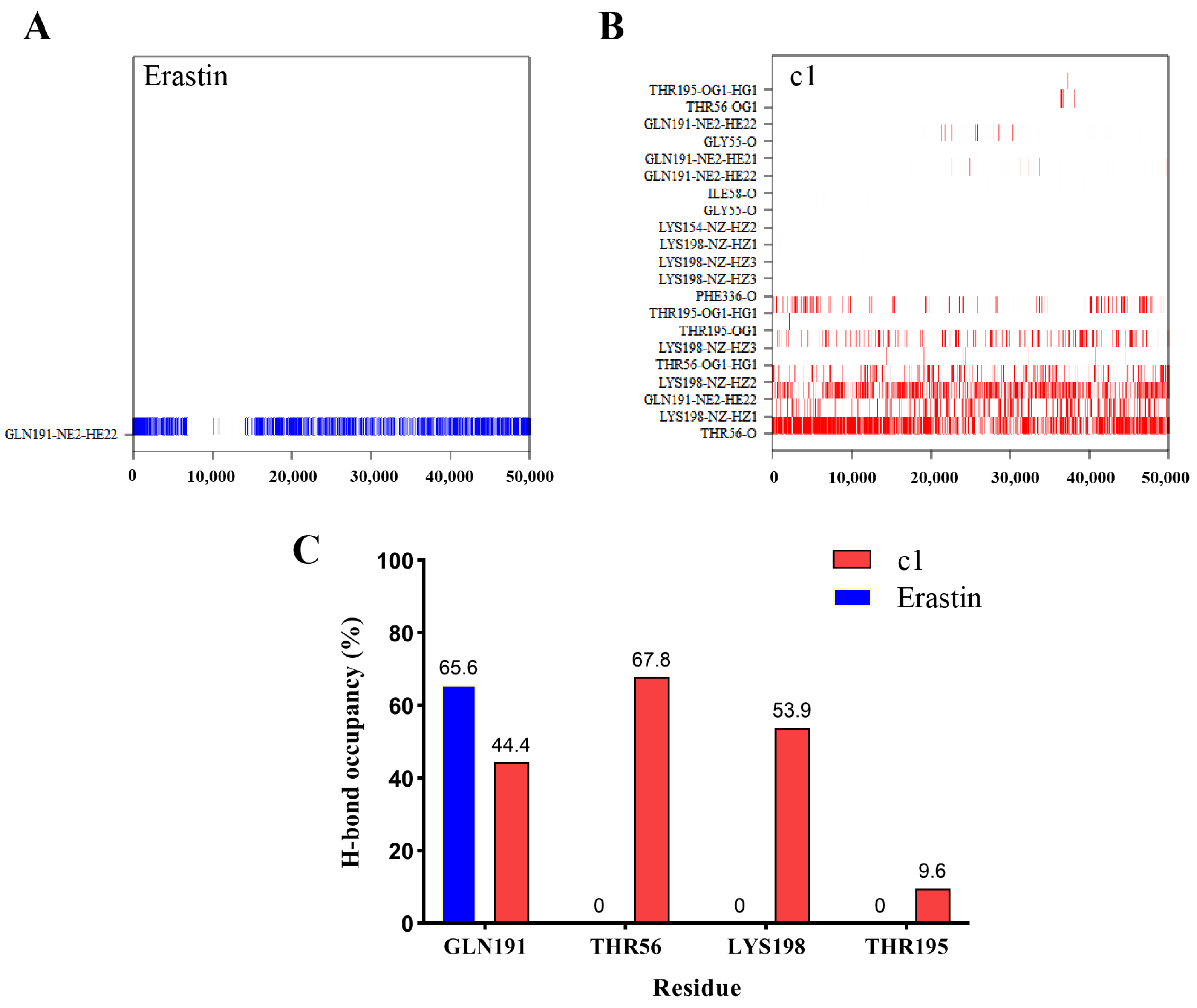
2.5.4. Hydrogen Bond Analysis
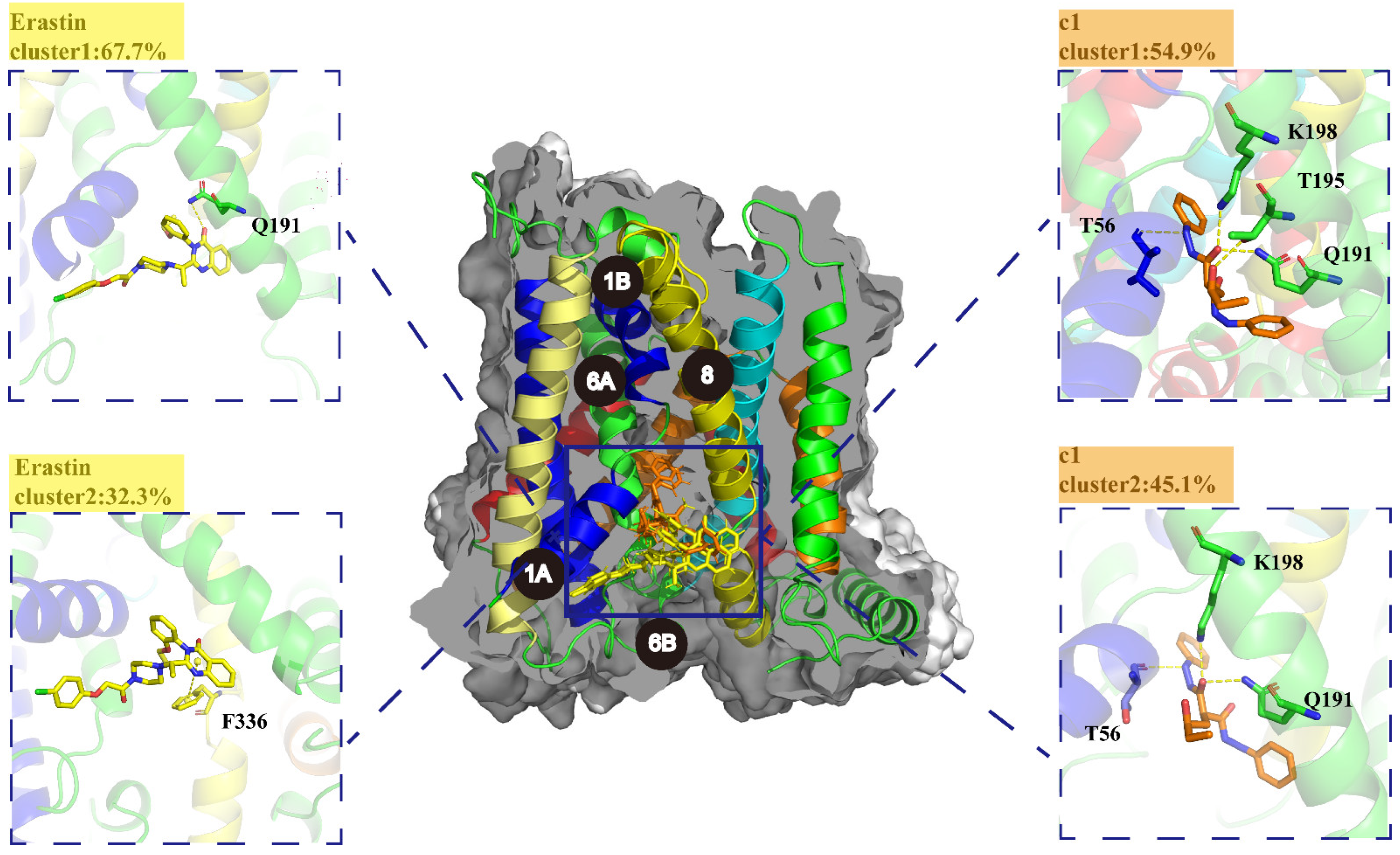
2.5.5. K-Means Clustering Analysis
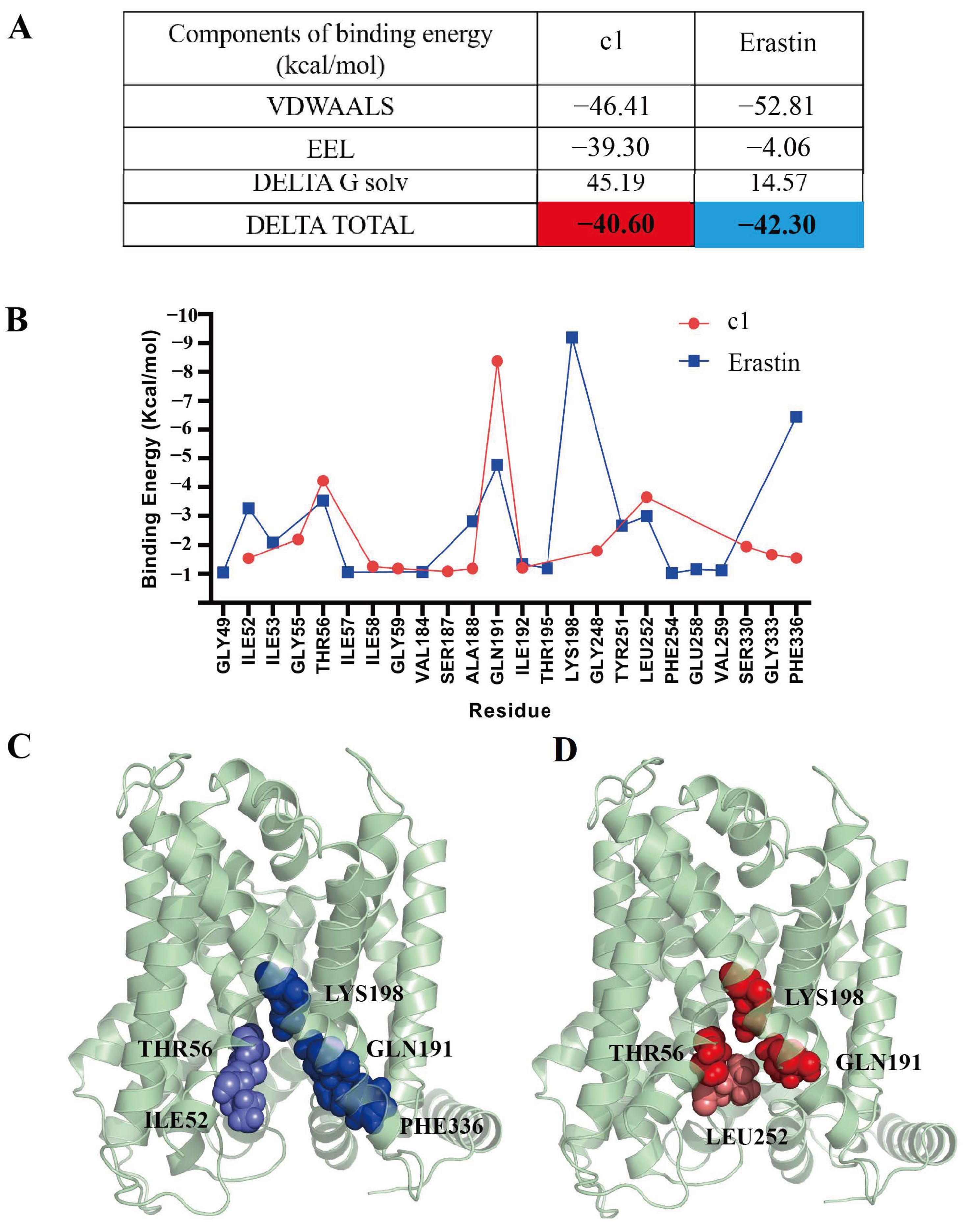
2.5.6. Free Energy Calculations
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Molecular Docking
4.3. Molecular Dynamics Simulations
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Cell Scratch Assay
4.7. Migration Assay
4.8. ROS Assay
4.9. Toxicity Assessment of 3D Tumor Spheroids
4.10. Calcein-AM Staining
4.11. Intracellular GSH Level Assay
4.12. Intracellular Glutamine Level Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zou, X.; Yan, Z.; Chen, C.; Chen, Y.; Fu, A. Preliminary Analysis of Cervical Cancer Immunotherapy. Am. J. Clin. Oncol. 2022, 45, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Ye, M.; Zhou, J.; Wang, Z.P.; Zhu, X. Recent Advances on the Molecular Mechanism of Cervical Carcinogenesis Based on Systems Biology Technologies. Comput. Struct. Biotechnol. J. 2019, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, O.A. Molecular pathogenesis of cervical cancer. Cancer Biol. Ther. 2011, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.F.; Nunes, R.A.L.; Morale, M.G.; Boccardo, E.; Aguayo, F.; Termini, L. Oxidative stress: Therapeutic approaches for cervical cancer treatment. Clinics 2018, 73 (Suppl. S1), e548s. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, C.; Cui, X.; Wu, J.; Cui, Z.; Shen, X. Trichosanthin inhibits cervical cancer by regulating oxidative stress-induced apoptosis. Bioengineered 2021, 12, 2779–2790. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A study of oxidative stress in cervical cancer- an institutional study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. Antioxidant Defenses: A Context-Specific Vulnerability of Cancer Cells. Cancers 2019, 11, 1208. [Google Scholar] [CrossRef]
- Hawk, M.A.; McCallister, C.; Schafer, Z.T. Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Cui, H.; Shen, D.H.; Wang, Y.; Chang, X.H.; Fu, T.Y.; Ye, X.; Yao, Y.Y. Up-regulation of mitochondrial antioxidation signals in ovarian cancer cells with aggressive biologic behavior. J. Zhejiang Univ. Sci. B 2011, 12, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, X.; Huang, P. xCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, T.; Chen, Z. Solute Carrier Family 7 Member 11 (SLC7A11) is a Potential Prognostic Biomarker in Uterine Corpus Endometrial Carcinoma. Int. J. Gen. Med. 2023, 16, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Xu, S.; Deng, Y.; Xu, B.; Yang, T.; Liu, W. Ferroptosis and Neurodegenerative Diseases: Insights into the Regulatory Roles of SLC7A11. Cell Mol. Neurobiol. 2023, 43, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Wu, Z.; Ma, Y.; Li, H. LncRNA FLVCR1-AS1 mediates miR-23a-5p/SLC7A11 axis to promote malignant behavior of cervical cancer cells. Bioengineered 2022, 13, 10454–10466. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Ke, D.; Li, Y.; Shi, J.; Wan, S.M.; Wang, A.J.; Zhao, M.N.; Gao, H. CNIH4 governs cervical cancer progression through reducing ferroptosis. Chem. Biol. Interact. 2023, 384, 110712. [Google Scholar] [CrossRef]
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Investig. 2020, 130, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Liu, F.; Wang, Z.; Gong, H.; Zhang, M.; Jia, Z.; Zhai, X. CircKIF4A enhances osteosarcoma proliferation and metastasis by sponging MiR-515-5p and upregulating SLC7A11. Mol. Biol. Rep. 2022, 49, 4525–4535. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadee, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Wang, L.; Leite de Oliveira, R.; Huijberts, S.; Bosdriesz, E.; Pencheva, N.; Brunen, D.; Bosma, A.; Song, J.Y.; Zevenhoven, J.; Los-de Vries, G.T.; et al. An Acquired Vulnerability of Drug-Resistant Melanoma with Therapeutic Potential. Cell 2018, 173, 1413–1425.e14. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Ling, V.; Wang, Y.Z.; Gout, P.W. The xc- cystine/glutamate antiporter: A mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer 2008, 99, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2021, 36, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Zhou, Z.; Qin, J.; Liu, W.; Wang, B.; Gu, Y. Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells. PLoS ONE 2016, 11, e0154605. [Google Scholar] [CrossRef]
- Nogara, P.A.; Saraiva Rde, A.; Caeran Bueno, D.; Lissner, L.J.; Lenz Dalla Corte, C.; Braga, M.M.; Rosemberg, D.B.; Rocha, J.B. Virtual screening of acetylcholinesterase inhibitors using the Lipinski’s rule of five and ZINC databank. Biomed Res. Int. 2015, 2015, 870389. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.B.; Ganesan, A.; Emery, F.S. Oral Administration of Peptide-Based Drugs: Beyond Lipinski’s Rule. ChemMedChem 2016, 11, 2245–2251. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Lifongo, L.L.; Judson, P.N.; Sippl, W.; Efange, S.M. How “drug-like” are naturally occurring anti-cancer compounds? J. Mol. Model. 2014, 20, 2069. [Google Scholar] [CrossRef]
- Nandikolla, A.; Mahadu Khetmalis, Y.; Mahalakshmi Naidu, K.; Karan Kumar, B.; Murugesan, S.; Chandra Sekhar, K.V.G. Discovery of potent antitubercular agents: Design, synthesis and biological evaluation of 4-(3-(4-substitutedpiperazin-1-yl)-quinoxalin-2-yl)-naphthalen-1-ol analogues. Toxicol Vitr. 2022, 82, 105370. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc(). Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Xie, E.; Li, Y.; Li, J.; Zhang, Y.; Chi, X.; Hu, X.; Xu, L.; Hou, T.; Stockwell, B.R.; et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022, 32, 687–690. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Kontoyianni, M. Docking and Virtual Screening in Drug Discovery. Methods Mol. Biol. 2017, 1647, 255–266. [Google Scholar] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grant, B.J.; Rodrigues, A.P.; ElSawy, K.M.; McCammon, J.A.; Caves, L.S. Bio3d: An R package for the comparative analysis of protein structures. Bioinformatics 2006, 22, 2695–2696. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Lee, G. Unveiling local and global conformational changes and allosteric communications in SOD1 systems using molecular dynamics simulation and network analyses. Comput. Biol. Med. 2024, 168, 107688. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Yin, Y.; Feng, X.; Xu, J.; Li, Y.; Li, T.; Liang, S.; He, X.; Liu, Z.; Wang, Y. Discovery of the Inhibitor Targeting the SLC7A11/xCT Axis through In Silico and In Vitro Experiments. Int. J. Mol. Sci. 2024, 25, 8284. https://doi.org/10.3390/ijms25158284
Yue J, Yin Y, Feng X, Xu J, Li Y, Li T, Liang S, He X, Liu Z, Wang Y. Discovery of the Inhibitor Targeting the SLC7A11/xCT Axis through In Silico and In Vitro Experiments. International Journal of Molecular Sciences. 2024; 25(15):8284. https://doi.org/10.3390/ijms25158284
Chicago/Turabian StyleYue, Jianda, Yekui Yin, Xujun Feng, Jiawei Xu, Yaqi Li, Tingting Li, Songping Liang, Xiao He, Zhonghua Liu, and Ying Wang. 2024. "Discovery of the Inhibitor Targeting the SLC7A11/xCT Axis through In Silico and In Vitro Experiments" International Journal of Molecular Sciences 25, no. 15: 8284. https://doi.org/10.3390/ijms25158284





