Caffeine Protects Keratinocytes from Trichophyton mentagrophytes Infection and Behaves as an Antidermatophytic Agent
Abstract
:1. Introduction
2. Results
2.1. Antidermatophytic Activity of Caffeine
2.2. Modulation of Fungal Cell Wall β-1,3-Glucan and Chitin in Response to Caffeine
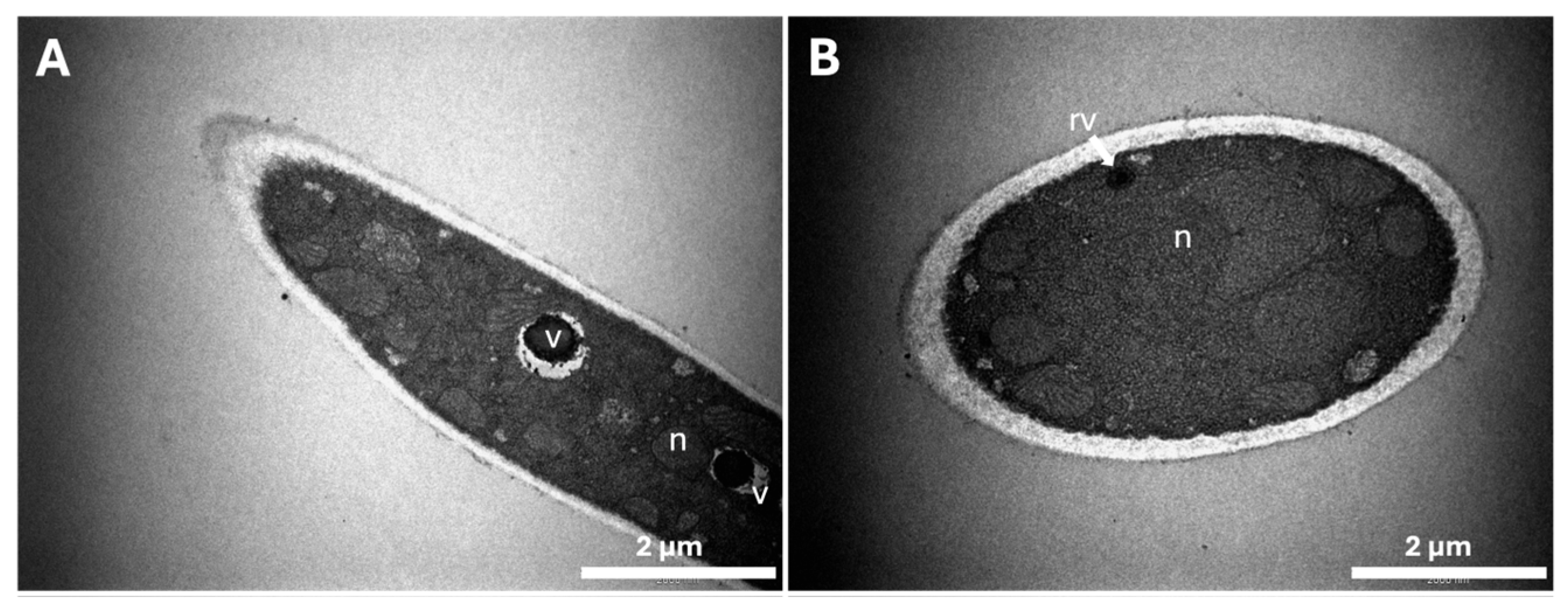
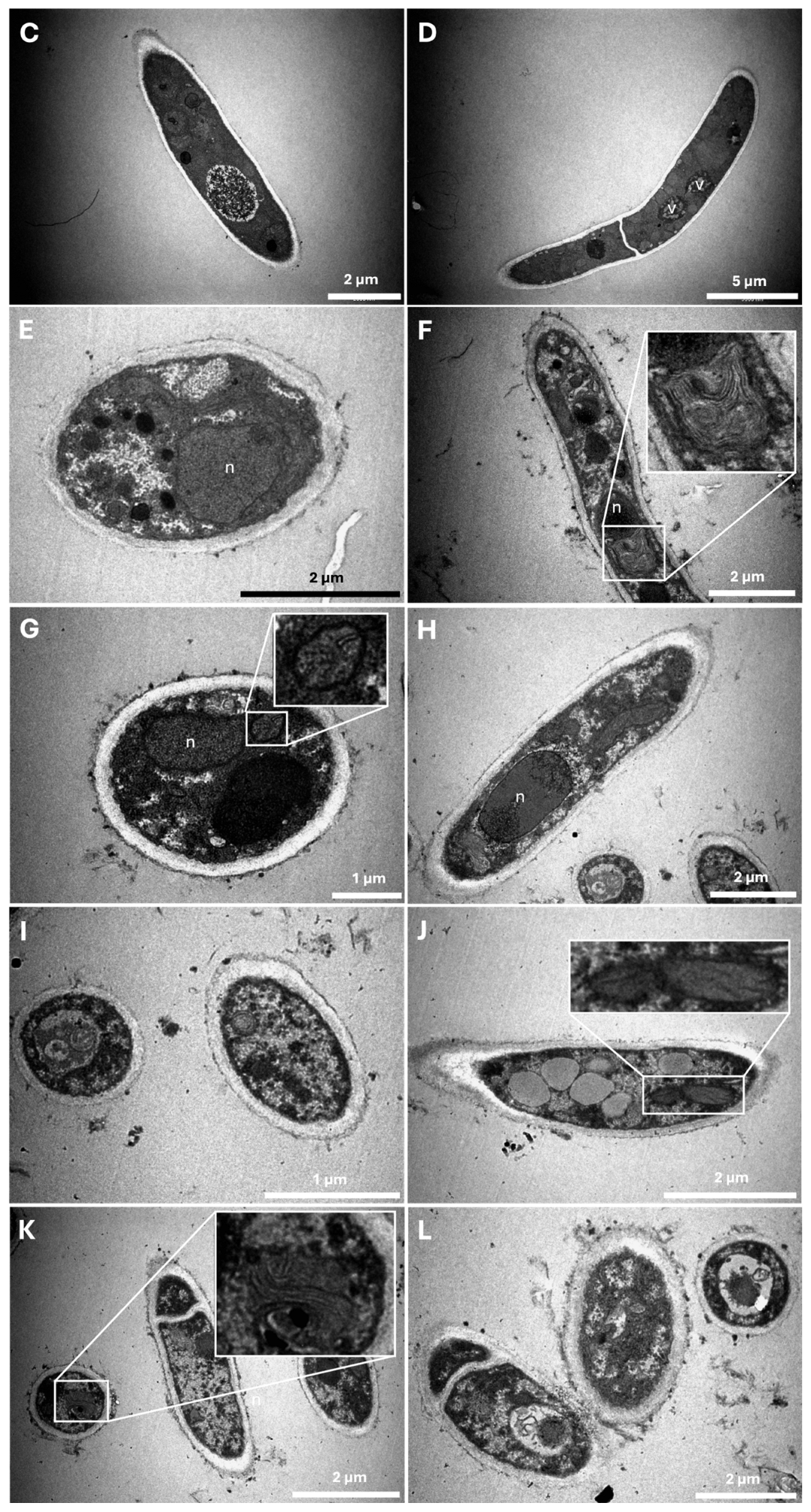
2.3. Characterization of Ultrastructural Changes
2.4. Antifungal Properties of Caffeine during Keratinocyte Infections by T. mentagrophytes Spores
2.4.1. Viability of Keratinocytes upon Infection with T. mentagrophytes Spores
2.4.2. Ungermination of Microconidia during HaCaT Infection
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Antidermatophytic Activity of Caffeine
4.3. Fungal Cell Wall β-1,3-Glucan and Chitin Quantifications
4.4. Characterization of Morphological and Ultrastructural Changes
4.5. Infection Assays
4.6. Keratinocyte Viability Assay
4.7. Ungerminated Microconidia Assays
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Han, W.; Ming, M.; He, Y.Y. Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J. Biol. Chem. 2011, 286, 22825–22832. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of caffeine effects on human health and emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef] [PubMed]
- Ősz, B.E.; Jîtcă, G.; Ștefănescu, R.E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and its antioxidant properties-it is all about dose and source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.L.; Israeli, A.F.; Madan, R. Caffeine in skincare: Its Role in skin Cancer, sun protection, and cosmetics. Indian J. Dermatol. 2023, 68, 546–550. [Google Scholar]
- Herman, A.; Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Ski. Pharmacol. Physiol. 2012, 26, 8–14. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of caffeine, 1,3,7-trimethylxanthine, to control Escherichia coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Sledz, W.; Los, E.; Paczek, A.; Rischka, J.; Motyka, A.; Zoledowska, S.; Piosik, J.; Lojkowska, E. Antibacterial activity of caffeine against plant pathogenic bacteria. Acta Biochim. Pol. 2015, 62, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Ohlan, D. In vitro studies on antifungal activity of tea (Camellia sinensis) and coffee (Coffea arabica) against wood-rotting fungi. J. Basic Microbiol. 1997, 37, 159–165. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Krychowiak-Masnicka, M.; Gołunski, G.; Łosiewska, A.; Borowik, A.; Wyrzykowski, D.; Piosik, J. New life of an old drug: Caffeine as a modulator of antibacterial activity of commonly used antibiotics. Pharmaceuticals 2022, 15, 872. [Google Scholar] [CrossRef]
- Rathi, B.; Gupta, S.; Kumar, P.; Kesarwani, V.; Dhanda, R.S.; Kushwaha, S.K.; Yadav, M. Anti-biofilm activity of caffeine against uropathogenic E. coli is mediated by curli biogenesis. Sci. Rep. 2022, 12, 18903. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M. Review: Cell wall assembly in yeast. Yeast 1994, 10, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Kuranda, K.; Leberre, V.; Sokol, S.; Palamarczyk, G.; François, J. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 2006, 61, 1147–1166. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.A.; Selvaggini, S.; Brujin, I.D.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.P.; Gow, N.A.R. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2009, 63, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Torres-Garcia, S.; Yaseen, I.; Shukla, M.; Audergon, P.N.C.B.; White, S.A.; Pidoux, A.L.; Allshire, R.C. Epigenetic gene silencing by heterochromatin primes fungal resistance. Nature 2020, 585, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R.; Fernandes, C.; Gonçalves, T. Antifungal activity of spent coffee ground extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef]
- Jartarkar, S.R.; Patil, A.; Goldust, Y.; Cockerell, C.J.; Schwartz, R.A.; Grabbe, S.; Goldust, M. Pathogenesis, immunology and management of dermatophytosis. J. Fungi 2021, 8, 39. [Google Scholar] [CrossRef]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Emerging trends in the use of topical antifungal-corticosteroid combinations. J. Fungi 2022, 8, 812. [Google Scholar] [CrossRef]
- Raut, J.S.; Chauhan, N.M.; Shinde, R.B.; Karuppayil, S.M. Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. J. Med. Plants Res. 2013, 7, 777–782. [Google Scholar]
- Nasrollahi, Z.; Yadegari, M.H. Antifungal activity of caffeine in combination with fluconazole against Candida albicans. Infect. Epidemiol. Med. 2016, 2, 18–21. [Google Scholar] [CrossRef]
- AlEraky, D.M.; Abuohashish, H.M.; Gad, M.M.; Alshuyukh, M.H.; Bugshan, A.S.; Almulhim, K.S.; Mahmoud, M.M. The Antifungal and antibiofilm activities of caffeine against Candida albicans on polymethyl methacrylate denture base material. Biomedicines 2022, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Sano, C.M.; Yazaki, K.; Sano, H. Caffeine fostering of mycoparasitic fungi against phytopathogens. Plant Signal. Behav. 2016, 11, e1113362. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.J.; Bain, J.M.; Liddle, C.; Leaves, I.; Hacker, C.; da Silva, R.P.; Yuecel, R.; Bebes, A.; Stead, D.; Childers, D.S.; et al. Nature of β-1,3-glucan-exposing features on Candida albicans cell wall and their modulation. mBio 2022, 13, e02605-22. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Anjos, J.; Walker, L.A.; Silva, B.M.A.; Cortes, L.; Mota, M.; Munro, C.A.; Gow, N.A.R.; Gonçalves, T. Modulation of Alternaria infectoria cell wall chitin and glucan synthesis by cell wall synthase inhibitors. Antimicrob. Agents Chemother. 2014, 58, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; MacCallum, D.M.; Jacobsen, M.D.; Walker, L.A.; Odds, F.C.; Gow, N.A.R.; Munro, C.A. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob. Agents Chemother. 2012, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Munro, C.A.; De Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A.R. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- Walker, L.A.; Gow, N.A.R.; Munro, C.A. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 2013, 57, 146–154. [Google Scholar] [CrossRef]
- Fernandes, C.; Sousa-Baptista, J.; Lenha-Silva, A.F.; Calheiros, D.; Correia, E.; Figueirinha, A.; Salgueiro, L.; Gonçalves, T. Azorean black tea (Camellia sinensis) antidermatophytic and fungicidal properties. Molecules 2023, 28, 7775. [Google Scholar] [CrossRef] [PubMed]
- Correia, E.E.M.; Figueirinha, A.; Rodrigues, L.; Pinela, J.; Calhelha, R.C.; Barros, L.; Fernandes, C.; Salgueiro, L.; Gonçalves, T. The chemical profile, and antidermatophytic, anti-Inflammatory, antioxidant and antitumor activities of Withania chevalieri A.E. Gonç. ethanolic extract. Plants 2023, 12, 2502. [Google Scholar] [CrossRef]
- Martin, H.; Rodriguez-Pachon, J.M.; Ruiz, C.; Nombela, C.; Molina, M. Regulatory mechanism for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Prados-Rosales, R.; Silva, B.M.A.; Nakouzi-Naranjo, A.; Zuzarte, M.; Chatterjee, S.; Stark, R.E.; Casadevall, A.; Gonçalves, T. Activation of melanin synthesis in Alternaria infectoria by antifungal drugs. Antimicrob. Agents Chemother. 2016, 60, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Enyart, D.S.; Crocker, C.L.; Stansell, J.R.; Cutrone, M.; Dintino, M.M.; Kinsey, S.T.; Brown, S.L.; Baumgarner, B.L. Low-dose caffeine administration increases fatty acid utilization and mitochondrial turnover in C2C12 skeletal myotubes. Physiol. Rep. 2020, 8, e14340. [Google Scholar] [CrossRef]
- Merk, D.; Greulich, J.; Vierkant, A.; Cox, F.; Eckermann, O.; von Ameln, F.; Dyballa-Rukes, N.; Altschmied, J.; Ale-Agha, N.; Jakobs, P.; et al. Caffeine Inhibits Oxidative Stress- and Low Dose Endotoxemia-Induced Senescence—Role of Thioredoxin-1. Antioxidants 2023, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ale-Agha, N.; Goy, C.; Jakobs, P.; Spyridopoulos, I.; Gonnissen, S.; Dyballa-Rukes, N.; Aufenvenne, K.; von Ameln, F.; Zurek, M.; Spannbrucker, T.; et al. CDKN1B/p27 is localized in mitochondria and improves respiration-dependent processes in the cardiovascular system-New mode of action for caffeine. PLoS Biol. 2018, 16, e2004408. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S.; Gallagher, C.M.; Walter, P. ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J. Cell Sci. 2014, 127, 4078–4088. [Google Scholar] [CrossRef]
- Weber, R.W.S. Vacuoles and the fungal lifestyle. Mycologist 2002, 16, 10–20. [Google Scholar] [CrossRef]
- Pivarcsi, A.; Nagy, I.; Kemeny, L. Innate immunity in the skin: How keratinocytes fight against pathogens. Curr. Immunol. Rev. 2005, 1, 29–42. [Google Scholar] [CrossRef]
- Brasch, J. Current knowledge of host response in human tinea. Mycoses 2009, 52, 304–312. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair: A showcase for cell plasticity and migration. Curr. Opin. Cell Biol. 2016, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ojeh, N.; Stojadinovic, O.; Pastar, I.; Sawaya, A.; Yin, N.; Tomic-Canic, M. The effects of caffeine on wound healing. Int. Wound J. 2014, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Satti, N.K.; Dutt, P.; Prasad, R.; Khan, I.A. Hydroxychavicol: A phytochemical targeting cutaneous fungal infections. Sci. Rep. 2016, 6, 37867. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural products: An alternative to conventional therapy for dermatophytosis? Mycopathologia 2017, 182, 143–167. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Fernandes, C.; Mota, M.; Barros, L.; Dias, M.I.; Ferreira, I.C.F.R.; Piedade, A.P.; Casadevall, A.; Gonçalves, T. Pyomelanin synthesis in Alternaria alternata inhibits DHN-melanin synthesis and decreases cell wall chitin content and thickness. Front. Microbiol. 2021, 12, 691433. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca, D.M.; Rodrigues, L.; Sousa-Baptista, J.; Marcos-Tejedor, F.; Mota, M.; Cunha, R.A.; Fernandes, C.; Gonçalves, T. Caffeine Protects Keratinocytes from Trichophyton mentagrophytes Infection and Behaves as an Antidermatophytic Agent. Int. J. Mol. Sci. 2024, 25, 8303. https://doi.org/10.3390/ijms25158303
da Fonseca DM, Rodrigues L, Sousa-Baptista J, Marcos-Tejedor F, Mota M, Cunha RA, Fernandes C, Gonçalves T. Caffeine Protects Keratinocytes from Trichophyton mentagrophytes Infection and Behaves as an Antidermatophytic Agent. International Journal of Molecular Sciences. 2024; 25(15):8303. https://doi.org/10.3390/ijms25158303
Chicago/Turabian Styleda Fonseca, Diogo M., Lisa Rodrigues, José Sousa-Baptista, Félix Marcos-Tejedor, Marta Mota, Rodrigo A. Cunha, Chantal Fernandes, and Teresa Gonçalves. 2024. "Caffeine Protects Keratinocytes from Trichophyton mentagrophytes Infection and Behaves as an Antidermatophytic Agent" International Journal of Molecular Sciences 25, no. 15: 8303. https://doi.org/10.3390/ijms25158303






