Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer
Abstract
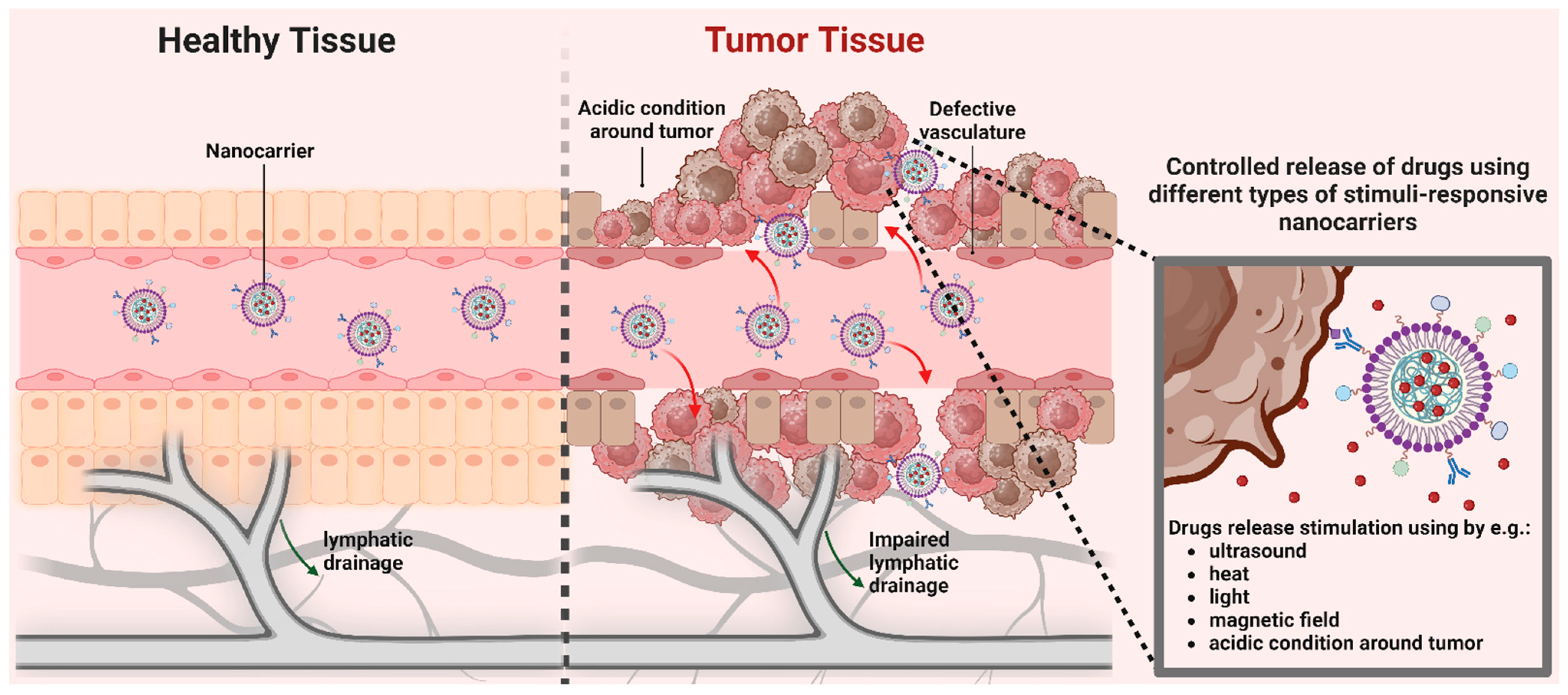
:1. Introduction
2. Types of Nanoparticles Employed for Ovarian Cancer Therapy
2.1. Plain Nanoparticles for Ovarian Cancer Treatment
| Name | Compounds | Type of Ovarian Cancer | Phase | Clinical Trial Number |
|---|---|---|---|---|
| PLD and SB-485232 | pegylated liposomal doxorubicin + interleukin 18 | Epithelial ovarian cancer | 1 | NCT00659178 |
| PLD and ATI-0918 | pegylated liposomal doxorubicin + liposomal formulation of doxorubicin hydrochloride | Ovarian cancer that has progressed or recurred after platinum-based chemotherapy | 1 | NCT01715168 |
| PLD and MK-4827 | pegylated liposomal doxorubicin + niraparib | Platinum-resistant/refractory HGSOC | 1 | NCT01227941 |
| PLD and Yondelis | pegylated liposomal doxorubicin + trabectedin | Platinum-sensitive advanced-relapsed epithelial ovarian cancer; advanced-relapsed ovarian cancer | 3 | NCT01846611 NCT00113607 NCT02394015 |
| PLD and Avastin | pegylated liposomal doxorubicin + bevacizumab | Platinum-resistant/refractory ovarian cancer; platinum-sensitive ovarian cancer | 1, 2 | NCT00846612 NCT00945139 NCT02163720 |
| PLD and Fludarabine | pegylated liposomal doxorubicin + fludarabine | Platinum-resistant or refractory ovarian cancer | 2 | NCT03335241 |
| PLD and BAY94–9343 | pegylated liposomal doxorubicin + anetumab ravtansine | Recurrent mesothelin-expressing platinum-resistant ovarian cancer | 1 | NCT02751918 |
| PLD and Hycamtin | pegylated liposomal doxorubicin + topotecan hydrochloride | Recurrent epithelial ovarian carcinoma | 3 | NCT01840943 |
| PLD and pazopanib | pegylated liposomal doxorubicin + pazopanib | Advanced relapsed platinum-sensitive or platinum-resistant ovarian cancer | 1/2 | NCT01035658 |
| PLD and EC-145 | pegylated liposomal doxorubicin + vintafolide (conjugate of desacetylvinblastine monohydrazide with folic acid | Platinum-resistant ovarian cancer | 2, 3 | NCT00722592 NCT01170650 |
| PLD and Ashwagandha | pegylated liposomal doxorubicin + withaferin A | Recurrent ovarian cancer | 1, 2 | NCT05610735 |
| PLD and Carboplatin | pegylated liposomal doxorubicin + carboplatin | Ovarian cancer recurrent within six to twelve months after initial carboplatin and paclitaxel chemotherapy | 2 | NCT00780039 |
| PLD and Telcyta | pegylated liposomal doxorubicin + TLK286 | Platinum refractory or resistant ovarian cancer | 1/2 | NCT00052065 |
| PLD or Hycamtin vs. Telcyta | pegylated liposomal doxorubicin, topotecan hydrochloride, TLK286 | Platinum refractory or resistant ovarian cancer | 3 | NCT00057720 |
| PLD and IMC-3 G3 | pegylated liposomal doxorubicin + olaratumab | Platinum refractory or resistant ovarian cancer | 2 | NCT00913835 |
| PLD and BMS-247550 | pegylated liposomal doxorubicin + ixabepilone | Advanced epithelial ovarian cancer, previously treated with platinum and a taxane | 1/2 | NCT00182767 |
| PLD vs. AZD2281 | pegylated liposomal doxorubicin, olaparib | BRCA1/2-positive advanced ovarian cancer patients who have failed previous platinum-based chemotherapy | 2 | NCT00628251 |
| PLD and AZD2281 | pegylated liposomal doxorubicin + olaparib | Platinum-resistant advanced ovarian cancer | 2 | NCT03161132 [46] |
| PLD and Vectibic | pegylated liposomal doxorubicin + panitumumab | Platinum-resistant epithelial ovarian cancer with KRAS wild-type | 2 | NCT00861120 |
| PLD and bortezomib | pegylated liposomal doxorubicin + bortezomib | BRCA wild-type platinum-resistant recurrent ovarian cancer | 2 | NCT03509246 [47] |
| PLD and BIBF 1120 | pegylated liposomal doxorubicin + nintedanib | Platinum-resistant ovarian cancer | 1 | NCT01485874 |
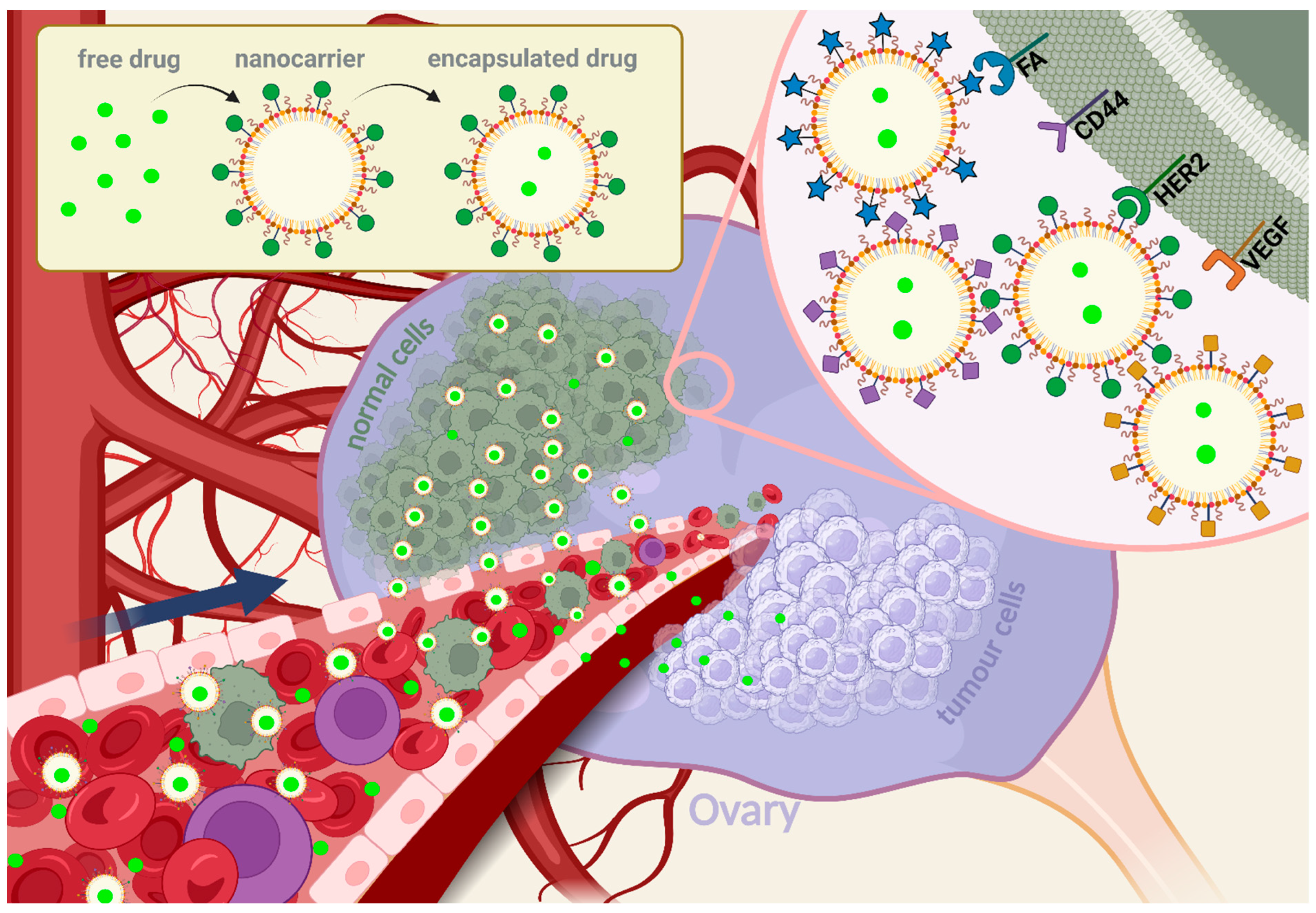
2.2. Therapeutic Strategies Based on Nanoparticles Modified with Active Targeting Ligands for Ovarian Cancer
2.2.1. Human Epidermal Growth Factor Receptor 2
2.2.2. Folic Acid Receptors
2.2.3. CD44, a Homing Cell Adhesion Molecule
2.2.4. Vascular Endothelial Growth Factor
3. PARP Inhibitor Nanoformulations
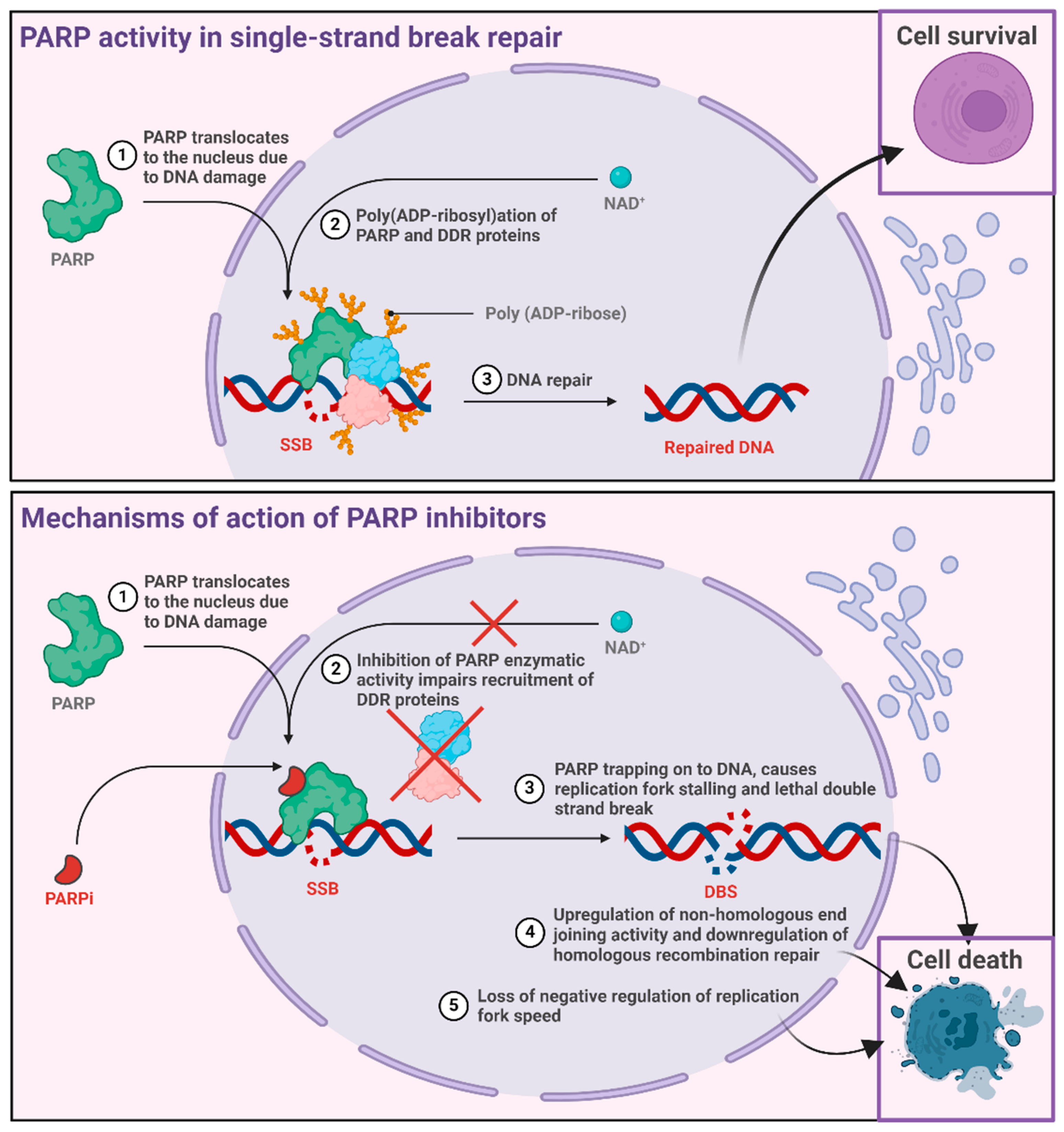
3.1. History and Mechanisms of Action
3.2. Encapsulated PARP Inhibitors for Ovarian Cancer Treatment
3.3. Targeted Therapy by Encapsulated PARP Inhibitors
4. Co-Encapsulation of Drugs as Combined Therapies for Ovarian Cancer
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saorin, A.; Saorin, G.; Duzagac, F.; Parisse, P.; Cao, N.; Corona, G.; Cavarzerani, E.; Rizzolio, F. Microfluidic production of amiodarone loaded nanoparticles and application in drug repositioning in ovarian cancer. Sci. Rep. 2024, 14, 6280. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, O.; Zeb, A.; Graham, S.; Szyperski, T.; Szender, J.B.; Odunsi, K.; Bahado-Singh, R. Metabolomics of biomarker discovery in ovarian cancer: A systematic review of the current literature. Metabolomics 2016, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.B.; Farquharson, M.; Mason, S.; Port, J.; Kruspig, B.; Dowson, S.; Stevenson, D.; Murphy, D.; Matzuk, M.; Kim, J.; et al. CRISPR/Cas9-derived models of ovarian high grade serous carcinoma targeting Brca1, Pten and Nf1, and correlation with platinum sensitivity. Sci. Rep. 2017, 7, 16827. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Leary, A.; Scott, C.L.; Serra, V.; Lord, C.J.; Bowtell, D.; Chang, D.K.; Garsed, D.W.; Jonkers, J.; Ledermann, J.A.; et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020, 31, 1606–1622. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Hockings, H.; Miller, R.E. The role of PARP inhibitor combination therapy in ovarian cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231173183. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef]
- Pathade, A.D.; Kommineni, N.; Bulbake, U.; Thummar, M.M.; Samanthula, G.; Khan, W. Preparation and Comparison of Oral Bioavailability for Different Nano-formulations of Olaparib. AAPS PharmSciTech 2019, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Singh, N.; Bhattacharya, S. Concepts on Smart Nano-Based Drug Delivery System. Recent. Pat. Nanotechnol. 2022, 16, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Seca, C.; Ferraresi, A.; Phadngam, S.; Vidoni, C.; Isidoro, C. Autophagy-dependent toxicity of amino-functionalized nanoparticles in ovarian cancer cells. J. Mater. Chem. B 2019, 7, 5376–5391. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf. B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef]
- Qiu, N.; Liu, X.; Zhong, Y.; Zhou, Z.; Piao, Y.; Miao, L.; Zhang, Q.; Tang, J.; Huang, L.; Shen, Y. Esterase-Activated Charge-Reversal Polymer for Fibroblast-Exempt Cancer Gene Therapy. Adv. Mater. 2016, 28, 10613–10622. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manouchehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Ríos, R.; Sánchez-Ramírez, D.R.; Ruiz-Saray, K.; Oceguera-Basurto, P.E.; Almada, M.; Juárez, J.; Zepeda-Moreno, A.; del Toro-Arreola, A.; Topete, A.; Daneri-Navarro, A. Cisplatin-loaded PLGA nanoparticles for HER2 targeted ovarian cancer therapy. Colloids Surf. B Biointerfaces 2019, 178, 199–207. [Google Scholar] [CrossRef]
- Shapira, A.; Livney, Y.D.; Broxterman, H.J.; Assaraf, Y.G. Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resist. Updates 2011, 14, 150–163. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265–286. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Targhotra, M.; Kumar, B.; Sahoo, P.K.; Chauhan, M.K. Polyplex: A Promising Gene Delivery System. Int. J. Pharm. Sci. Nanotechnol. 2019, 12, 4681–4686. [Google Scholar] [CrossRef]
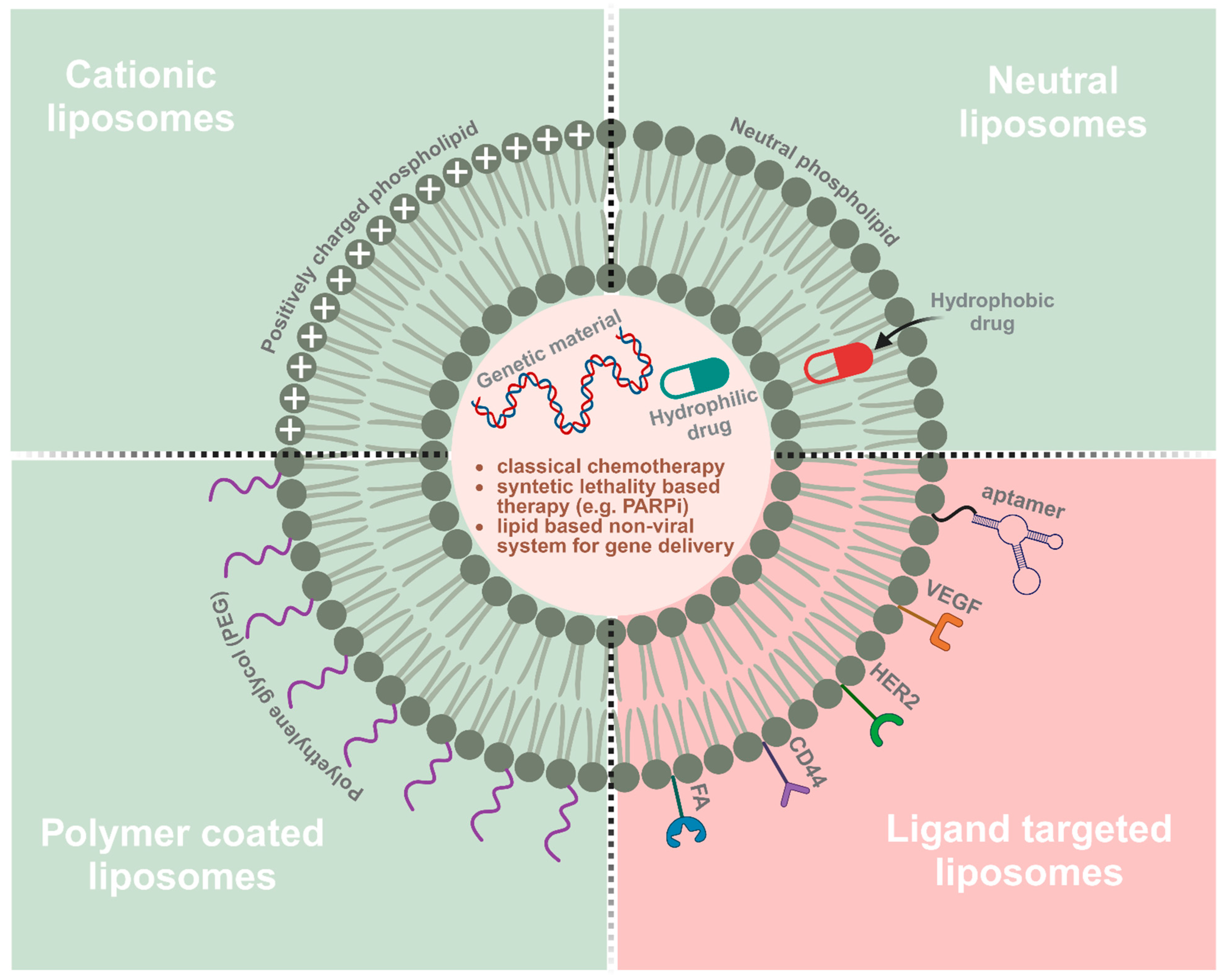
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Drakopoulou, E.; Anagnou, N.P.; Pappa, K.I. Gene Therapy for Malignant and Benign Gynaecological Disorders: A Systematic Review of an Emerging Success Story. Cancers 2022, 14, 3238. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, X.; Chen, W. The Current State of the Art in PARP Inhibitor-Based Delivery Nanosystems. Pharmaceutics 2022, 14, 1647. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Pisano, C.; Cecere, S.C.; Di Napoli, M.; Cavaliere, C.; Tambaro, R.; Facchini, G.; Scaffa, C.; Losito, S.; Pizzolorusso, A.; Pignata, S. Clinical Trials with Pegylated Liposomal Doxorubicin in the Treatment of Ovarian Cancer. J. Drug Deliv. 2013, 2013, 898146. [Google Scholar] [CrossRef]
- Green, A.E.; Rose, P.G. Pegylated liposomal doxorubicin in ovarian cancer. Int. J. Nanomed. 2006, 1, 229–239. [Google Scholar]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.-M.; Alzghari, S.; Ahn, C.; Trantham, H.; La-Beck, N.M. The Role of Pegylated Liposomal Doxorubicin in Ovarian Cancer: A Meta-Analysis of Randomized Clinical Trials. Oncologist 2013, 18, 1022–1031. [Google Scholar] [CrossRef]
- Tian, K.; Jia, X.; Zhao, X.; Liu, P. Biocompatible Reduction and pH Dual-Responsive Core Cross-Linked Micelles Based on Multifunctional Amphiphilic Linear–Hyperbranched Copolymer for Controlled Anticancer Drug Delivery. Mol. Pharm. 2017, 14, 799–807. [Google Scholar] [CrossRef]
- Keller, B.-L.; Lohmann, C.A.; Kyeremateng, S.O.; Fricker, G. Synthesis and Characterization of Biodegradable Poly(butyl cyanoacrylate) for Drug Delivery Applications. Polymers 2022, 14, 998. [Google Scholar] [CrossRef]
- Kanaani, L.; Ebrahimi Far, M.; Kazemi, S.M.; Choupani, E.; Mazloumi Tabrizi, M.; Ebrahimi Shahmabadi, H.; Akbarzadeh Khiyavi, A. General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.A.; Li, W.H.; Chen, P.H.; He, C.L.; Chang, Y.H.; Chuang, C.M. Intraperitoneal delivery of a novel liposome-encapsulated paclitaxel redirects metabolic reprogramming and effectively inhibits cancer stem cells in Taxol((R))-resistant ovarian cancer. Am. J. Transl. Res. 2015, 7, 841–855. [Google Scholar]
- Liu, Y.; Ng, Y.; Toh, M.R.; Chiu, G.N.C. Lipid-dendrimer hybrid nanosystem as a novel delivery system for paclitaxel to treat ovarian cancer. J. Control. Release 2015, 220, 438–446. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yokoyama, Y.; Osawa, Y.; Miura, R.; Mizunuma, H. Gene therapy for ovarian cancer using carbonyl reductase 1 DNA with a polyamidoamine dendrimer in mouse models. Cancer Gene Ther. 2015, 23, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Iglesias, M.; Bohn, U.; Calvo, E.; Garcia, Y.; Guerra, E.; Manso, L.; Santaballa, A.; Gonzalez-Martin, A. GEICO1601-ROLANDO: A multicentric single arm Phase II clinical trial to evaluate the combination of olaparib and pegylated liposomal doxorubicin for platinum-resistant ovarian cancer. Future Sci. OA 2019, 5, FSO370. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Nagler, A.; Sonneveld, P.; Blade, J.; Hajek, R.; Spencer, A.; San Miguel, J.; Robak, T.; Dmoszynska, A.; Horvath, N.; et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J. Clin. Oncol. 2007, 25, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, B.; Gerhards, R.; Strumberg, D.; Voigtmann, R. Combined detection of Her2/neu gene amplification and protein overexpression in effusions from patients with breast and ovarian cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1389–1400. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Symmans, W.F.; Pusztai, L.; Bloom, K.J. The HER-2/neu Gene and Protein in Breast Cancer 2003: Biomarker and Target of Therapy. Oncologist 2003, 8, 307–325. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Shah, D.; Osipo, C. Cancer stem cells and HER2 positive breast cancer: The story so far. Genes Dis. 2016, 3, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Révillion, F.; Bonneterre, J.; Peyrat, J.P. ERBB2 oncogene in human breast cancer and its clinical significance. Eur. J. Cancer 1998, 34, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, N.H.; Luebke, A.M.; Erbersdobler, A.; Knoefel, W.T.; Schraut, W.; Verde, P.E.; Stern, F.; Scheunemann, P.; Peiper, M.; Eisenberger, C.F.; et al. Copy Number of Chromosome 17 but Not HER2 Amplification Predicts Clinical Outcome of Patients with Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2004, 22, 4737–4745. [Google Scholar] [CrossRef] [PubMed]
- English, D.P.; Roque, D.M.; Santin, A.D. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol. Diagn. Ther. 2013, 17, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lassus, H. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol. Oncol. 2004, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Z.; Luo, H.; Xu, X.; Ye, M.; Sheng, B.; Zhu, X. The prognostic value of HER2 in ovarian cancer: A meta-analysis of observational studies. PLoS ONE 2018, 13, e0191972. [Google Scholar] [CrossRef]
- Meijer, S.L.; Wesseling, J.; Smit, V.T.; Nederlof, P.M.; Hooijer, G.K.; Ruijter, H.; Arends, J.W.; Kliffen, M.; van Gorp, J.M.; Sterk, L.; et al. HER2 gene amplification in patients with breast cancer with equivocal IHC results. J. Clin. Pathol. 2011, 64, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.S.; Matei, D.; Aghajanian, C.; Matulonis, U.A.; Brewer, M.; Fleming, G.F.; Hainsworth, J.D.; Garcia, A.A.; Pegram, M.D.; Schilder, R.J.; et al. Clinical activity of pertuzumab (rhuMAb 2 C4), a HER dimerization inhibitor, in advanced ovarian cancer: Potential predictive relationship with tumor HER2 activation status. J. Clin. Oncol. 2006, 24, 4324–4332. [Google Scholar] [CrossRef]
- Holm, J.; Hansen, S.I. Characterization of soluble folate receptors (folate binding proteins) in humans. Biological roles and clinical potentials in infection and malignancy. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140466. [Google Scholar] [CrossRef]
- Cheung, A.; Opzoomer, J.; Ilieva, K.M.; Gazinska, P.; Hoffmann, R.M.; Mirza, H.; Marlow, R.; Francesch-Domenech, E.; Fittall, M.; Dominguez Rodriguez, D.; et al. Anti-Folate Receptor Alpha-Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer. Clin. Cancer Res. 2018, 24, 5098–5111. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Boogerd, L.S.F.; Boonstra, M.C.; Beck, A.-J.; Charehbili, A.; Hoogstins, C.E.S.; Prevoo, H.A.J.M.; Singhal, S.; Low, P.S.; van de Velde, C.J.H.; Vahrmeijer, A.L. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget 2016, 7, 17442–17454. [Google Scholar] [CrossRef] [PubMed]
- O’Shannessy, D.J.; Somers, E.B.; Maltzman, J.; Smale, R.; Fu, Y.S. Folate receptor alpha (FRA) expression in breast cancer: Identification of a new molecular subtype and association with triple negative disease. Springerplus 2012, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Santaolalla, A.; Osborn, G.; Khiabany, A.; Grandits, M.; Lopez-Abente, J.; Palhares, L.; Chan Wah Hak, C.; et al. Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes. Br. J. Cancer 2023, 128, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, R.; Zhao, E.; Wang, Q.; Lian, W.; Xiong, J. Targeting CD44-positive ovarian cancers via engineered paclitaxel prodrug nanoparticles for enhanced chemotherapeutic efficacy. Biomed. Pharmacother. 2022, 154, 113655. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3 S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Gulia, M.; Nishal, S.; Maddiboyina, B.; Dutt, R.; Kumar Desu, P.; Wadhwa, R.; Jhawat, V. Physiological Pathway, diagnosis and nanotechnology based treatment strategies for ovarian Cancer: A review. Med. Omics 2023, 8, 100020. [Google Scholar] [CrossRef]
- Mei, C.; Gong, W.; Wang, X.; Lv, Y.; Zhang, Y.; Wu, S.; Zhu, C. Anti-angiogenic therapy in ovarian cancer: Current understandings and prospects of precision medicine. Front. Pharmacol. 2023, 14, 1147717. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-W.; Cheng, Y.-A.; Li, C.-C.; Ho, K.-W.; Chen, H.-J.; Chen, I.J.; Huang, B.-C.; Liu, H.-J.; Lu, Y.-C.; Cheng, C.-M.; et al. Enhancement of tumor tropism of mPEGylated nanoparticles by anti-mPEG bispecific antibody for ovarian cancer therapy. Sci. Rep. 2021, 11, 7598. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Chow, R.; Kim, H.; Lieu, T.; Xiao, M.; Kim, S.; Matuszewska, K.; Pereira, M.; Nguyen, D.L.; Petrik, J. Liposomal delivery of gene therapy for ovarian cancer: A systematic review. Reprod. Biol. Endocrinol. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Srinivasan, D.J.; Mukherjee, S.; Haroon, M.M.; Dar, G.H.; Venkatraman, U.; Patra, C.R.; Gopal, V. Engineered fusion protein-loaded gold nanocarriers for targeted co-delivery of doxorubicin and erbB2-siRNA in human epidermal growth factor receptor-2+ ovarian cancer. J. Mater. Chem. B 2017, 5, 7082–7098. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic Nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.; Graslund, T.; Eriksson Karlstrom, A.; Frejd, F.Y.; Nygren, P.A.; Lofblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Satpathy, M.; Wang, L.; Zielinski, R.; Qian, W.; Lipowska, M.; Capala, J.; Lee, G.Y.; Xu, H.; Wang, Y.A.; Mao, H.; et al. Active Targeting Using HER-2-Affibody-Conjugated Nanoparticles Enabled Sensitive and Specific Imaging of Orthotopic HER-2 Positive Ovarian Tumors. Small 2013, 10, 544–555. [Google Scholar] [CrossRef]
- Satpathy, M.; Wang, L.; Zielinski, R.J.; Qian, W.; Wang, Y.A.; Mohs, A.M.; Kairdolf, B.A.; Ji, X.; Capala, J.; Lipowska, M.; et al. Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles. Theranostics 2019, 9, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Maria, B.; Andrew, H.S.; Simon, P.L. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol. Med. 2018, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination with Carboplatin and Taxane in Patients with Ovarian Cancer in First Platinum-Sensitive Relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Vergote, I.; Oaknin, A.; Colombo, N.; Banerjee, S.; Oza, A.; Pautier, P.; Malek, K.; Birrer, M.J. FORWARD I: A Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol. 2018, 14, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Chuan, D.; Mu, M.; Hou, H.; Zhao, N.; Li, J.; Tong, A.; Zou, B.; Chen, H.; Han, B.; Guo, G. Folic acid-functionalized tea polyphenol as a tumor-targeting nano-drug delivery system. Mater. Des. 2021, 206, 109805. [Google Scholar] [CrossRef]
- Saifi, M.A.; Seal, S.; Godugu, C. Nanoceria, the versatile nanoparticles: Promising biomedical applications. J. Control. Release 2021, 338, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, M.; Das, S.; Mert, I.; Gupta, A.; Al-Wahab, Z.; Tebbe, C.; Dar, S.; Chhina, J.; Giri, S.; Munkarah, A.; et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 2016, 16, 220. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Naik, P.K.; Kazi, M.; Hussain, M.D. Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2022, 50, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Yilmaz, H.; Gunes, A.; Hamarat Sanlier, S. In vitro and in vivo evaluation of folate receptor-targeted a novel magnetic drug delivery system for ovarian cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–937. [Google Scholar] [CrossRef]
- Girija, A.R. Medical Applications of Polymer/Functionalized Nanoparticle Systems. In Polymer Composites with Functionalized Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 381–404. [Google Scholar]
- Zhu, D.; Liu, F.; Ma, L.; Liu, D.; Wang, Z. Nanoparticle-Based Systems for T1-Weighted Magnetic Resonance Imaging Contrast Agents. Int. J. Mol. Sci. 2013, 14, 10591–10607. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Hu, Y.; Shen, M.; Shi, X.; Zhang, G. Folic acid-targeted iron oxide nanoparticles as contrast agents for magnetic resonance imaging of human ovarian cancer. J. Ovarian Res. 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Tolcher, A.; Perez, C.A.; Orr, D.; Hamilton, E.; Zhao, Y.; Murciano-Goroff, Y.; Anders, C.; Adams, G.P.; Reddick, C.W.; et al. Abstract CT255: ELU-FRα-1: A study to evaluate ELU001 in patients with solid tumors that overexpress folate receptor alpha (FRα). Cancer Res. 2023, 83, CT255. [Google Scholar] [CrossRef]
- He, Z.Y.; Deng, F.; Wei, X.W.; Ma, C.C.; Luo, M.; Zhang, P.; Sang, Y.X.; Liang, X.; Liu, L.; Qin, H.X.; et al. Ovarian cancer treatment with a tumor-targeting and gene expression-controllable lipoplex. Sci. Rep. 2016, 6, 23764. [Google Scholar] [CrossRef]
- Guan, J.T.; Li, X.X.; Peng, D.W.; Zhang, W.M.; Qu, J.; Lu, F.; D’Amato, R.J.; Chi, Z.L. MicroRNA-18 a-5 p Administration Suppresses Retinal Neovascularization by Targeting FGF1 and HIF1 A. Front. Pharmacol. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Zhang, Y.G.; Yang, Y.H.; Ma, C.C.; Wang, P.; Du, W.; Li, L.; Xiang, R.; Song, X.R.; Zhao, X.; et al. In Vivo Ovarian Cancer Gene Therapy Using CRISPR-Cas9. Hum. Gene Ther. 2018, 29, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Zhang, X.; Guo, H.; Gao, H. Nanoparticles in precision medicine for ovarian cancer: From chemotherapy to immunotherapy. Int. J. Pharm. 2020, 591, 119986. [Google Scholar] [CrossRef] [PubMed]
- Deiss-Yehiely, E.; Brucks, S.D.; Boehnke, N.; Pickering, A.J.; Kiessling, L.L.; Hammond, P.T. Surface Presentation of Hyaluronic Acid Modulates Nanoparticle-Cell Association. Bioconjug. Chem. 2022, 33, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.; Assaraf, Y.G.; Slavkin, A.; Dolev, T.; Shahar, T.; Livney, Y.D. Developing Body-Components-Based Theranostic Nanoparticles for Targeting Ovarian Cancer. Pharmaceutics 2019, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.D.; Li, J.Q.; Chen, F.Y.; Dong, W.; Wen, L.J.; Fei, W.D.; Zhang, X.; Yang, P.L.; Zhang, X.M.; Zheng, C.H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef]
- Yang, X.; Iyer, A.K.; Singh, A.; Choy, E.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep. 2015, 5, 8509. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Taratula, O.; Garbuzenko, O.B.; Taratula, O.R.; Rodriguez-Rodriguez, L.; Minko, T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: An optimal delivery of siRNA and anticancer drug. Clin. Cancer Res. 2013, 19, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, J.; Zhang, J.; Wang, S.; Sun, Y.; Liu, Q.; Cheng, C. Dual Targeted Nanoparticles for the Codelivery of Doxorubicin and siRNA Cocktails to Overcome Ovarian Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 11575. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Kuhlmann, J.D.; Link, T.; Goeckenjan, M.; Hofbauer, L.C.; Gobel, A.; Rachner, T.D.; Wimberger, P. Clinical impact of soluble Neuropilin-1 in ovarian cancer patients and its association with its circulating ligands of the HGF/c-MET axis. Front. Oncol. 2022, 12, 974885. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Song, X.; Yi, T.; Li, S.; Deng, H.; Chen, X.; Li, Z.; Bai, Y.; Zhong, Q.; Wei, Y.; et al. Administration of PLGA nanoparticles carrying shRNA against focal adhesion kinase and CD44 results in enhanced antitumor effects against ovarian cancer. Cancer Gene Ther. 2013, 20, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obs. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhu, Y.; Yue, M.; Wang, F.; Li, Z.; Lin, M. The Therapeutic Effects of DDP/CD44-shRNA Nanoliposomes in AMF on Ovarian Cancer. Front. Oncol. 2022, 12, 811783. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Zhang, X.-L.; Li, D.-P.; Zhang, T.-T.; Gao, F.-F.; Liu, N.-F.; Sheng, X.-G. Antibody fragment-armed mesoporous silica nanoparticles for the targeted delivery of bevacizumab in ovarian cancer cells. Int. J. Pharm. 2015, 496, 1026–1033. [Google Scholar] [CrossRef]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, S.A.; Hempel, N.; Bergkvist, M. Development of Nanoscale Approaches for Ovarian Cancer Therapeutics and Diagnostics. Crit. Rev. Oncog. 2014, 19, 281–315. [Google Scholar] [CrossRef] [PubMed]
- Kohlberger, M.; Gadermaier, G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol. Appl. Biochem. 2021, 69, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, M.; Guo, Y.; Tu, K.; Wu, W.; Wang, J.; Tong, X.; Wu, W.; Qi, L.; Shi, D. Targeted chimera delivery to ovarian cancer cells by heterogeneous gold magnetic nanoparticle. Nanotechnology 2017, 28, 025101. [Google Scholar] [CrossRef] [PubMed]
- Green, L.S.; Jellinek, D.; Jenison, R.; Ostman, A.; Heldin, C.H.; Janjic, N. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry 1996, 35, 14413–14424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tan, W.; Zheng, J.; Su, Y.; Cui, M. Aptamer Nanomaterials for Ovarian Cancer Target Theranostics. Front. Bioeng. Biotechnol. 2022, 10, 884405. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Anjum, M.M.; Patel, K.K. Gemcitabine cationic polymeric nanoparticles against ovarian cancer: Formulation, characterization, and targeted drug delivery. Drug Deliv. 2022, 29, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Noorani, L.; Stenzel, M.; Liang, R.; Pourgholami, M.H.; Morris, D.L. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnol. 2015, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Green, A.; Brown, N.; Robinson, A.; Senat, M.; Testino, B.; Dinulescu, D.M.; Sridhar, S. Sustained delivery of PARP inhibitor Talazoparib for the treatment of BRCA-deficient ovarian cancer. Front. Oncol. 2023, 13, 1175617. [Google Scholar] [CrossRef]
- Kim, G.; Ison, G.; McKee, A.E.; Zhang, H.; Tang, S.; Gwise, T.; Sridhara, R.; Lee, E.; Tzou, A.; Philip, R.; et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin. Cancer Res. 2015, 21, 4257–4261. [Google Scholar] [CrossRef]
- Montemorano, L.; Lightfoot, M.; Bixel, K. Role of Olaparib as Maintenance Treatment for Ovarian Cancer: The Evidence to Date. OncoTargets Ther. 2019, 12, 11497–11506. [Google Scholar] [CrossRef]
- Arora, S.; Balasubramaniam, S.; Zhang, H.; Berman, T.; Narayan, P.; Suzman, D.; Bloomquist, E.; Tang, S.; Gong, Y.; Sridhara, R.; et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist 2021, 26, e164–e172. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Colombo, I.; Lheureux, S.; Oza, A.M. Rucaparib: A novel PARP inhibitor for BRCA advanced ovarian cancer. Drug Des. Dev. Ther. 2018, 12, 605–617. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Anscher, M.S.; Chang, E.; Gao, X.; Gong, Y.; Weinstock, C.; Bloomquist, E.; Adeniyi, O.; Charlab, R.; Zimmerman, S.; Serlemitsos-Day, M.; et al. FDA Approval Summary: Rucaparib for the Treatment of Patients with Deleterious BRCA-Mutated Metastatic Castrate-Resistant Prostate Cancer. Oncologist 2021, 26, 139–146. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Krivak, T.C.; Kabil, N.; Munley, J.; Moore, K.N. PARP Inhibitors in Ovarian Cancer: A Review. Target. Oncol. 2023, 18, 471–503. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ji, F.; Helleday, T.; Ying, S. Mechanisms for stalled replication fork stabilization: New targets for synthetic lethality strategies in cancer treatments. EMBO Rep. 2018, 19, e46263. [Google Scholar] [CrossRef]
- Patel, M.; Nowsheen, S.; Maraboyina, S.; Xia, F. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: A review. Cell Biosci. 2020, 10, 35. [Google Scholar] [CrossRef]
- Hanzlikova, H.; Kalasova, I.; Demin, A.A.; Pennicott, L.E.; Cihlarova, Z.; Caldecott, K.W. The Importance of Poly(ADP-Ribose) Polymerase as a Sensor of Unligated Okazaki Fragments during DNA Replication. Mol. Cell 2018, 71, 319–331.e3. [Google Scholar] [CrossRef]
- Maya-Mendoza, A.; Moudry, P.; Merchut-Maya, J.M.; Lee, M.; Strauss, R.; Bartek, J. High speed of fork progression induces DNA replication stress and genomic instability. Nature 2018, 559, 279–284. [Google Scholar] [CrossRef]
- Baldwin, P.; Ohman, A.W.; Tangutoori, S.; Dinulescu, D.M.; Sridhar, S. Intraperitoneal delivery of NanoOlaparib for disseminated late-stage cancer treatment. Int. J. Nanomed. 2018, 13, 8063–8074. [Google Scholar] [CrossRef]
- Corradetti, B.; Freile, P.; Pells, S.; Bagnaninchi, P.; Park, J.; Fahmy, T.M.; de Sousa, P.A. Paracrine signalling events in embryonic stem cell renewal mediated by affinity targeted nanoparticles. Biomaterials 2012, 33, 6634–6643. [Google Scholar] [CrossRef]
- Magalhaes, J.A.; Arruda, D.C.; Baptista, M.S.; Tada, D.B. Co-Encapsulation of Methylene Blue and PARP-Inhibitor into Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Enhanced PDT of Cancer. Nanomaterials 2021, 11, 1514. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhou, Z.; Chang, Y.; Liu, Y.; Shen, Y.; Li, Q.; Zhang, L. Ribonucleotide reductase M2 (RRM2): Regulation, function and targeting strategy in human cancer. Genes Dis. 2024, 11, 218–233. [Google Scholar] [CrossRef]
- Li, Y.; Cen, Y.; Fang, Y.; Tang, S.; Li, S.; Ren, Y.; Zhang, H.; Lu, W.; Xu, J. Breaking the Iron Homeostasis: A “Trojan Horse” Self-Assembled Nanodrug Sensitizes Homologous Recombination Proficient Ovarian Cancer Cells to PARP Inhibition. ACS Nano 2022, 16, 12786–12800. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, F.; Wu, M.; Tian, L.; Gong, F.; Zhong, X.; Chen, M.; Liu, Z.; Liu, B. Bioorthogonal Coordination Polymer Nanoparticles with Aggregation-Induced Emission for Deep Tumor-Penetrating Radio- and Radiodynamic Therapy. Adv. Mater. 2021, 33, e2007888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Guan, X.; Wu, Q.; Zhang, M.; Cui, P.; Wang, C.; Chen, X.; Meng, X.; Ma, T. Reversal of Cisplatin Resistance in Ovarian Cancer by the Multitargeted Nanodrug Delivery System Tf-Mn-MOF@Nira@CDDP. ACS Appl. Mater. Interfaces 2023, 15, 26484–26495. [Google Scholar] [CrossRef]
- Neufeld, M.J.; DuRoss, A.N.; Landry, M.R.; Winter, H.; Goforth, A.M.; Sun, C. Co-delivery of PARP and PI3 K inhibitors by nanoscale metal-organic frameworks for enhanced tumor chemoradiation. Nano Res. 2019, 12, 3003–3017. [Google Scholar] [CrossRef]
- Wang, L.; Evans, J.C.; Ahmed, L.; Allen, C. Folate receptor targeted nanoparticles containing niraparib and doxorubicin as a potential candidate for the treatment of high grade serous ovarian cancer. Sci. Rep. 2023, 13, 3226. [Google Scholar] [CrossRef] [PubMed]
- Dasa, S.S.K.; Diakova, G.; Suzuki, R.; Mills, A.M.; Gutknecht, M.F.; Klibanov, A.L.; Slack-Davis, J.K.; Kelly, K.A. Plectin-targeted liposomes enhance the therapeutic efficacy of a PARP inhibitor in the treatment of ovarian cancer. Theranostics 2018, 8, 2782–2798. [Google Scholar] [CrossRef]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J.; et al. Layer-by-layer nanoparticles for novel delivery of cisplatin and PARP inhibitors for platinum-based drug resistance therapy in ovarian cancer. Bioeng. Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef]
- Juan, A.; Noblejas-López, M.d.M.; Bravo, I.; Arenas-Moreira, M.; Blasco-Navarro, C.; Clemente-Casares, P.; Lara-Sánchez, A.; Pandiella, A.; Alonso-Moreno, C.; Ocaña, A. Enhanced Antitumoral Activity of Encapsulated BET Inhibitors When Combined with PARP Inhibitors for the Treatment of Triple-Negative Breast and Ovarian Cancers. Cancers 2022, 14, 4474. [Google Scholar] [CrossRef]
- Balmaña, J.; Tung, N.M.; Isakoff, S.J.; Graña, B.; Ryan, P.D.; Saura, C.; Lowe, E.S.; Frewer, P.; Winer, E.; Baselga, J.; et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014, 25, 1656–1663. [Google Scholar] [CrossRef]
- Lee, J.-M.; Hays, J.L.; Chiou, V.L.; Annunziata, C.M.; Swisher, E.M.; Harrell, M.I.; Yu, M.; Gordon, N.; Sissung, T.M.; Ji, J.; et al. Phase I/Ib study of olaparib and carboplatin in women with triple negative breast cancer. Oncotarget 2017, 8, 79175–79187. [Google Scholar] [CrossRef]
- Novohradsky, V.; Zajac, J.; Vrana, O.; Kasparkova, J.; Brabec, V. Simultaneous delivery of olaparib and carboplatin in PEGylated liposomes imparts this drug combination hypersensitivity and selectivity for breast tumor cells. Oncotarget 2018, 9, 28456–28473. [Google Scholar] [CrossRef]
- Li, Y.; Cen, Y.; Tu, M.; Xiang, Z.; Tang, S.; Lu, W.; Zhang, H.; Xu, J. Nanoengineered Gallium Ion Incorporated Formulation for Safe and Efficient Reversal of PARP Inhibition and Platinum Resistance in Ovarian Cancer. Research 2023, 6, 0070. [Google Scholar] [CrossRef]
- Geenen, J.J.J.; Schellens, J.H.M. Molecular Pathways: Targeting the Protein Kinase Wee1 in Cancer. Clin. Cancer Res. 2017, 23, 4540–4544. [Google Scholar] [CrossRef]
- Fang, Y.; McGrail, D.J.; Sun, C.; Labrie, M.; Chen, X.; Zhang, D.; Ju, Z.; Vellano, C.P.; Lu, Y.; Li, Y.; et al. Sequential Therapy with PARP and WEE1 Inhibitors Minimizes Toxicity while Maintaining Efficacy. Cancer Cell 2019, 35, 851–867.e7. [Google Scholar] [CrossRef]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2018, 16, 81–104. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Y.; Hu, X.; Lu, F.; Qin, T.; Zhang, L.; Guo, E.; Yang, B.; Fu, Y.; Hu, D.; et al. Codelivery of adavosertib and olaparib by tumor-targeting nanoparticles for augmented efficacy and reduced toxicity. Acta Biomater. 2023, 157, 428–441. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Z.; Xu, C.; Guo, B.; Guo, P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J. Control. Release 2019, 311–312, 43–49. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Cortes, A.; Guerra, E.; Garcia, Y.; Iglesias, M.; Bohn Sarmiento, U.; Calvo Garcia, E.; Manso Sanchez, L.; Santaballa, A.; Oaknin, A.; et al. Olaparib in combination with pegylated liposomal doxorubicin for platinum-resistant ovarian cancer regardless of BRCA status: A GEICO phase II trial (ROLANDO study). ESMO Open 2021, 6, 100212. [Google Scholar] [CrossRef]
- Duska, L.R.; Krasner, C.N.; O’Malley, D.M.; Hays, J.L.; Modesitt, S.C.; Mathews, C.A.; Moore, K.N.; Thaker, P.H.; Miller, A.; Purdy, C.; et al. A phase Ib/II and pharmacokinetic study of EP0057 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Gynecol. Oncol. 2021, 160, 688–695. [Google Scholar] [CrossRef]
- Boere, I.; Vergote, I.; Hanssen, R.; Jalving, M.; Gennigens, C.; Ottevanger, P.; van de Wouw, Y.J.; Rijcken, C.J.F.; Mathijssen, R.H.J.; Ledermann, J. CINOVA: A phase II study of CPC634 (nanoparticulate docetaxel) in patients with platinum resistant recurrent ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 1247–1252. [Google Scholar] [CrossRef]

| Compound | Nanoparticle | Type of Ovarian Cancer | Phase | Clinical Trial Number |
|---|---|---|---|---|
| Lurtotecan | liposome | Advanced or recurrent epithelial OC | 2 | NCT00010179 NCT00046800 |
| Topotecan | liposome | Advanced solid tumours that have relapsed, are refractory to standard therapy, or for whom there is no standard therapy available | 1 | NCT00765973 |
| Irinotecan and bevacizumab | liposomal irinotecan as sucrosofate | Platinum-resistant, recurrent or refractory OC | 2 | NCT04753216 |
| Mitoxantrone hydrochloride | liposome | Platinum-resistant or platinum refractory relapsed OC | 1 | NCT04718376 |
| Abraxane and carboplatin | paclitaxel formulated as an albumin-bound nanoparticle | Platinum-sensitive OC | 2 | NCT00466986 |
| Abraxane and bevacizumab | paclitaxel formulated as an albumin-bound nanoparticle | Recurrent, platinum-resistant epithelial OC | 2 | NCT00407563 |
| EP0057 (CRLX101) and bevacizumab | CPT formulated as a cyclodextrin-based nanoparticle | Recurrent, platinum-resistant OC | 2 | NCT01652079 |
| EP0057 (CRLX101) and paclitaxel | CPT formulated as a cyclodextrin-based nanoparticle | Recurrent or persistent epithelial OC | 1/2 | NCT02389985 [159] |
| EP0057 (CRLX101) and olaparib | CPT formulated as a cyclodextrin-based nanoparticle | Platinum-resistant OC or advanced OC who have received at least one prior line of platinum-based chemotherapy followed by a PARP inhibitor | 2 | NCT04669002 |
| EGEN-001 | PEG-PEI-cholesterol Lipopolymer-encased IL-12 DNA Plasmid Vector GEN-1 | Recurrent epithelial OC | 2 | NCT01118052 |
| ELU- FRα-1 | C’Dot drug conjugate (CDC), consisting of payloads (exatecans) and targeting moieties (folic acid analogues) covalently bound by linkers to the C’Dot particle carrier | Tumours overexpressing FRα | 1/2 | NCT05001282 |
| CPC634 | Docetaxel entrapped in CriPec nanoparticles | Platinum-resistant OC | 2 | NCT03742713 [160] |
| Apatinib and paclitaxel | Apatinib, an antiangiogenic agent targeting vascular endothelial growth factor receptor (VEGFR2) and albumin-bound paclitaxel | Recurrent, platinum-resistant OC | 2 | NCT03942068 |
| Paclitaxel | Paclitaxel formulated as an albumin-stabilised nanoparticle | Recurrent or persistent platinum-resistant OC | 2 | NCT00499252 |
| Paclical | Paclitaxel (micellar) nanoparticles | Epithelial ovarian cancer | 3 | NCT00989131 |
| Rapamycin | nanoparticle albumin-bound rapamycin | Recurrent OC, stage III/IV | 1 | NCT02646319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gralewska, P.; Gajek, A.; Marczak, A.; Rogalska, A. Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 8304. https://doi.org/10.3390/ijms25158304
Gralewska P, Gajek A, Marczak A, Rogalska A. Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer. International Journal of Molecular Sciences. 2024; 25(15):8304. https://doi.org/10.3390/ijms25158304
Chicago/Turabian StyleGralewska, Patrycja, Arkadiusz Gajek, Agnieszka Marczak, and Aneta Rogalska. 2024. "Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer" International Journal of Molecular Sciences 25, no. 15: 8304. https://doi.org/10.3390/ijms25158304







