Persistence of Infectious Canine Distemper Virus in Murine Xenotransplants of Canine Histiocytic Sarcoma Cells after Intratumoral Application
Abstract
1. Introduction
2. Results
2.1. Relative Tumor Growth
2.2. Histological and Immunohistological Results
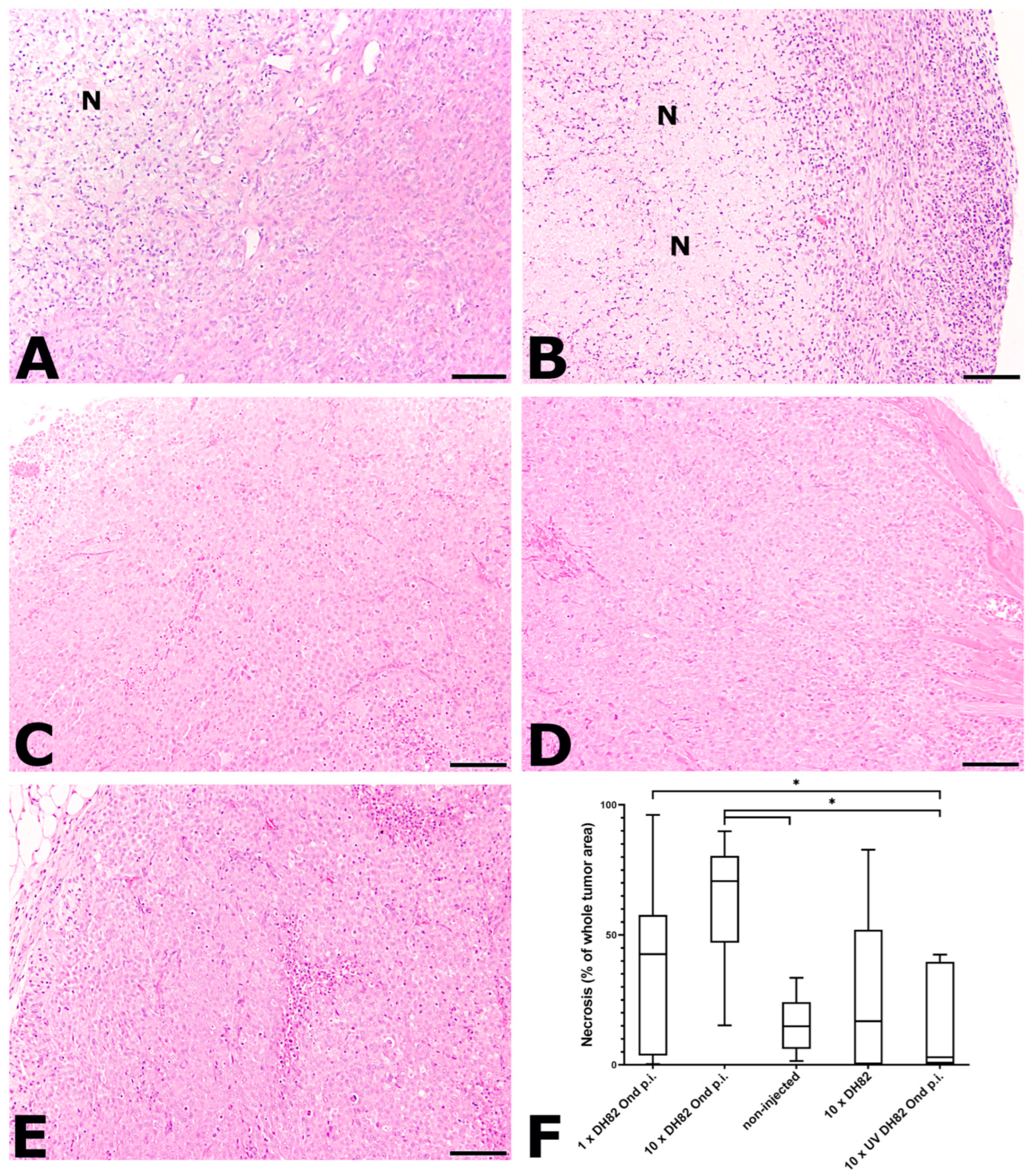
2.2.1. Necrosis
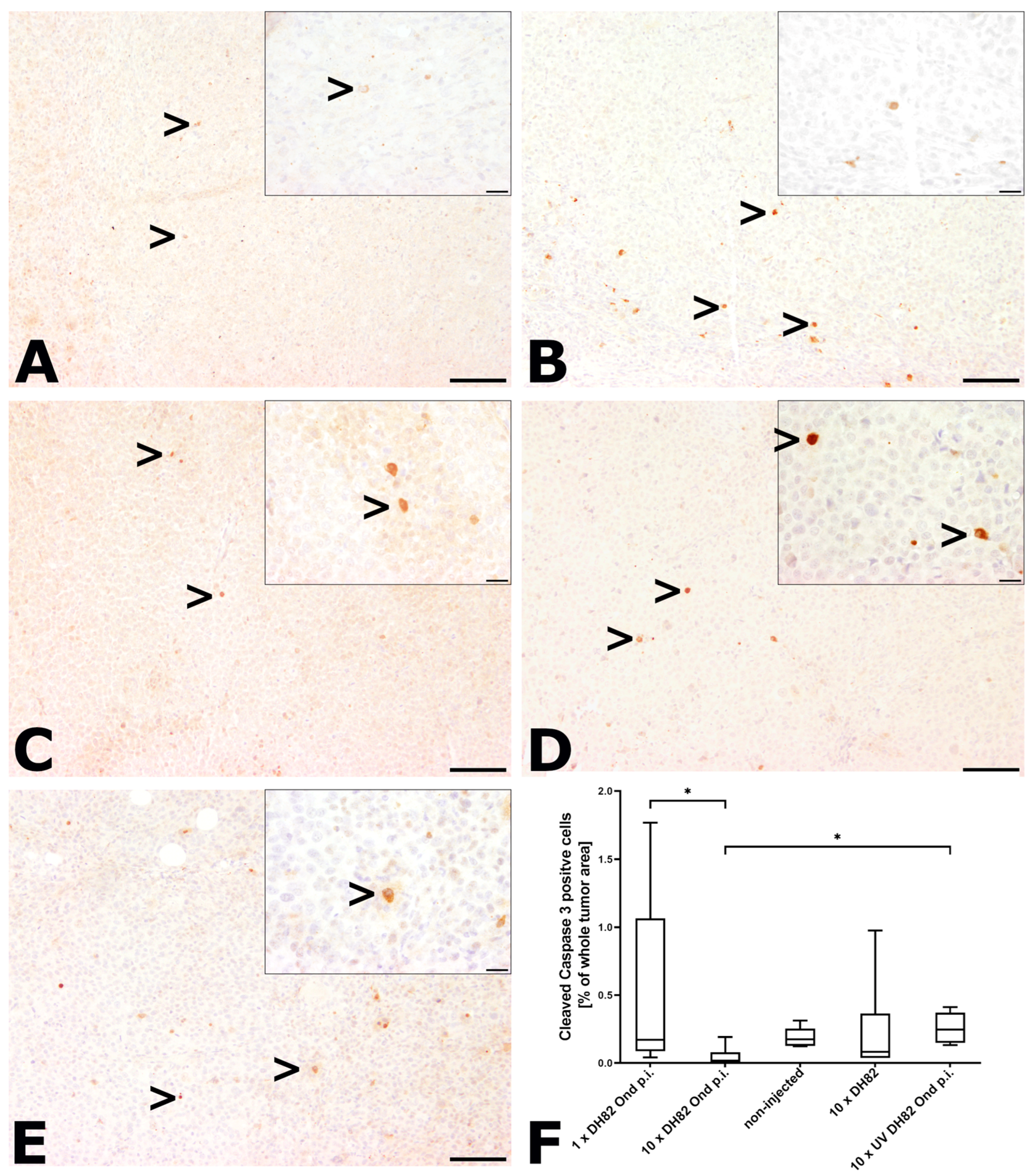
2.2.2. Intratumoral Apoptotic Rate
2.2.3. Mitotic Rate
2.2.4. Intratumoral Vessel Density
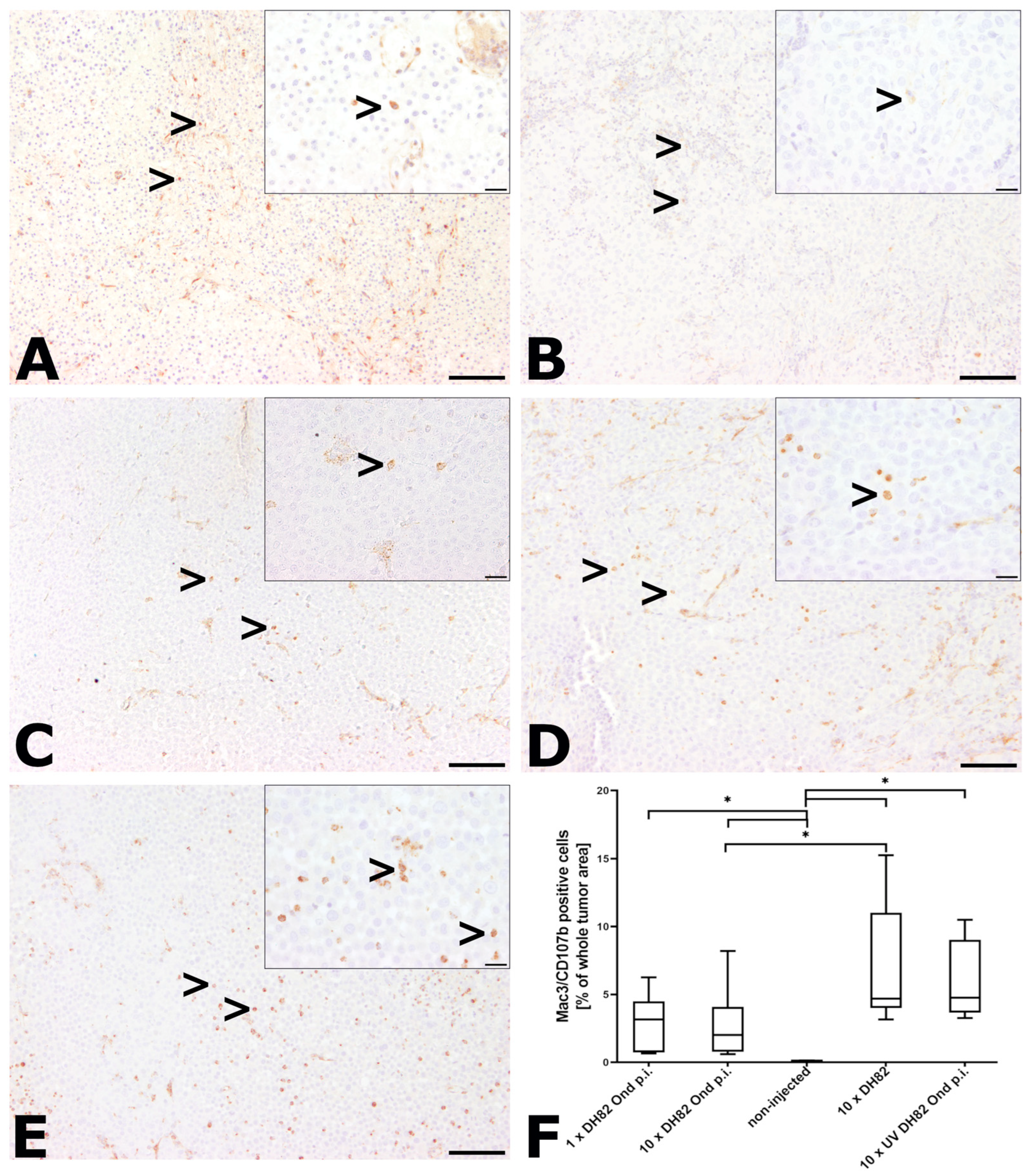
2.2.5. Intratumoral Infiltration with Murine Macrophages
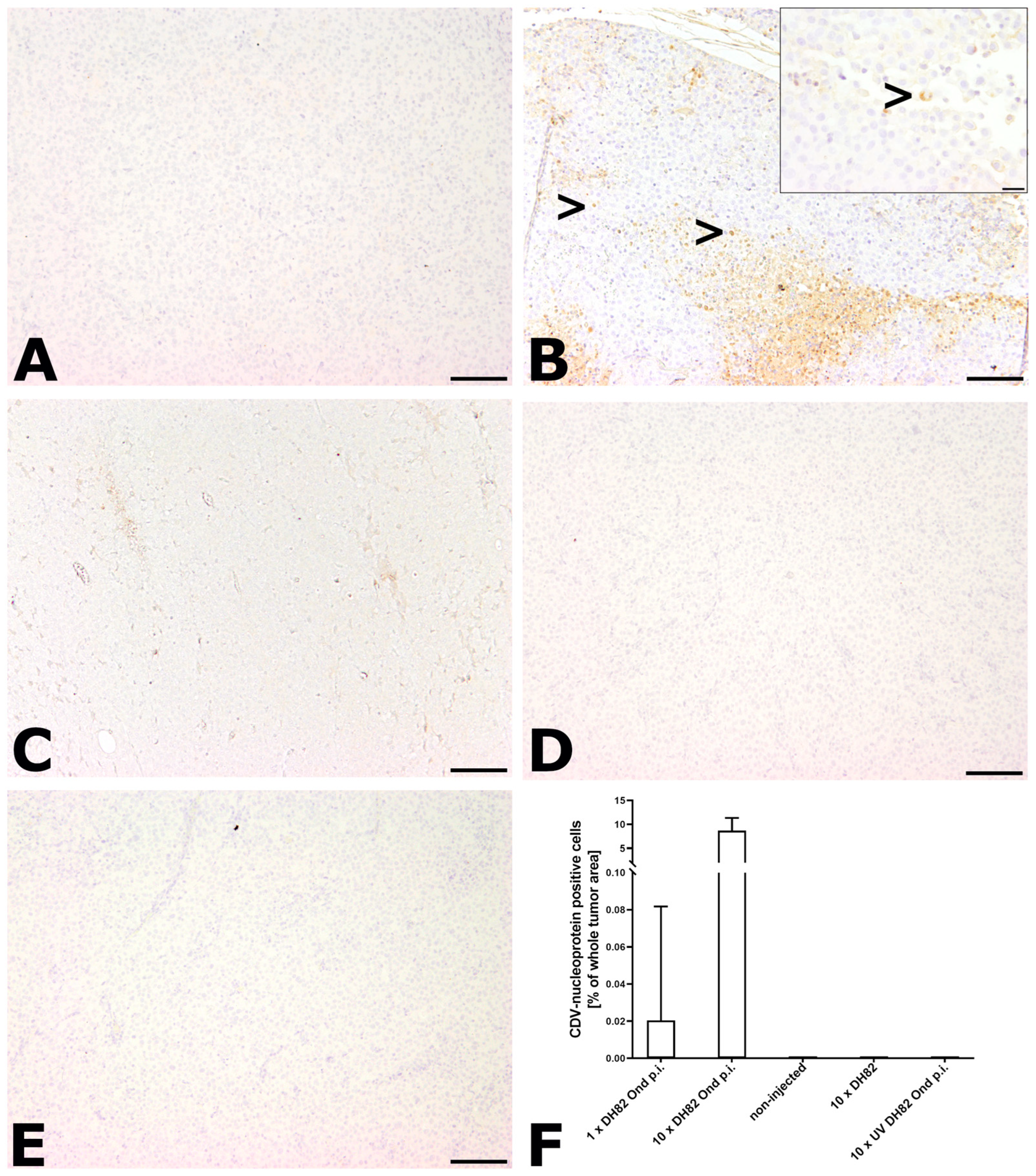
2.2.6. Immunohistological Detection of CDV Nucleoprotein
2.3. RT-qPCR for Detection of CDV mRNA Transcripts
2.4. Virus Titration
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Xenotransplantational Mouse Model
4.3. Tumor Treatment and Measurement
4.4. Necropsy and Sample Collection
4.5. Histological and Immunochistological Staining
4.6. Histological and Immunohistological Evaluation
4.7. RNA Isolation, Reverse Transcription (RT) and RT-qPCR
4.8. Virus Titration
4.9. Immunofluorescence
4.10. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cline, M.J. Histiocytes and histiocytosis. Blood 1994, 84, 2840–2853. [Google Scholar] [CrossRef] [PubMed]
- Favara, B.E.; Feller, A.C.; Pauli, M.; Jaffe, E.S.; Weiss, L.M.; Arico, M.; Bucsky, P.; Egeler, R.M.; Elinder, G.; Gadner, H.; et al. Contemporary classification of histiocytic disorders. The WHO committee on histiocytic/reticulum cell proliferations. Reclassification working group of the histiocyte society. Med. Pediatr. Oncol. 1997, 29, 157–166. [Google Scholar] [CrossRef]
- Moore, P.F. A review of histiocytic diseases of dogs and cats. Vet. Pathol. 2014, 51, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F.; Affolter, V.K.; Vernau, W. Canine hemophagocytic histiocytic sarcoma: A proliferative disorder of CD11d+ macrophages. Vet. Pathol. 2006, 43, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Schlick, K.; Aigelsreiter, A.; Pichler, M.; Reitter, S.; Neumeister, P.; Hoefler, G.; Beham-Schmid, C.; Linkesch, W. Histiocytic sarcoma—Targeted therapy: Novel therapeutic options? A series of 4 cases. Onkologie 2012, 35, 447–450. [Google Scholar] [CrossRef]
- Fidel, J.; Schiller, I.; Hauser, B.; Jausi, Y.; Rohrer-Bley, C.; Roos, M.; Kaser-Hotz, B. Histiocytic sarcomas in flat-coated retrievers: A summary of 37 cases (November 1998-March 2005). Vet. Comp. Oncol. 2006, 4, 63–74. [Google Scholar] [CrossRef]
- Mahoney, D.J.; Stojdl, D.F.; Laird, G. Can viruses treat cancer? Sci. Am. 2014, 311, 54–59. [Google Scholar] [CrossRef]
- Sinkovics, J.G.; Horvarth, J.C. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancer. Arch. Immunol. Ther. Exp. 2008, 56, 3–59. [Google Scholar] [CrossRef]
- Zygiert, Z. Hodgkin’s disease: Remission after measles. Lancet 1971, 297, 593. [Google Scholar] [CrossRef]
- Angarita, F.A.; Acuna, S.A.; Ottolino-Perry, K.; Zerhouni, S.; McCart, J.A. Mounting a strategic offense: Fighting tumor vasculature with oncolytic viruses. Trends Mol. Med. 2013, 19, 378–392. [Google Scholar] [CrossRef]
- De Silva, N.; Atkins, H.; Kirn, D.H.; Bell, J.C.; Breitbach, C.J. Double trouble for tumours: Exploiting the tumour microenvironment to enhance anticancer effect of oncolytic viruses. Cytokine Growth Factor Rev. 2010, 21, 135–141. [Google Scholar] [CrossRef]
- Singh, P.K.; Doley, J.; Kumar, G.R.; Sahoo, A.P.; Tiwari, A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J. Med. Res. 2012, 139, 571–584. [Google Scholar]
- Pfankuche, V.M.; Spitzbarth, I.; Lapp, S.; Ulrich, R.; Deschel, U.; Kalkuhl, A.; Baumgärtner, W.; Puff, C. Reduced angiogenic gene expression in morbillivirus-triggered oncolysis in a translational model for histiocytic sarcoma. J. Cell. Mol. Med. 2017, 21, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Bourke, M.G.; Salwa, S.; Harrington, K.J.; Kucharczyk, M.J.; Forde, P.F.; de Kruijf, M.; Soden, D.; Tangney, M.; Collins, J.K.; O’Sullivan, G.C. The emerging role of viruses in the treatment of solid tumours. Cancer Treat. Rev. 2011, 37, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Kirn, D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): Results of phase I and phase II trials. Expert Opin. Biol. Ther. 2001, 1, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Milhem, M.M.; Minor, D.; Hamid, O.; Li, A.; Chen, L.; Chastain, M.; Gorski, K.S.; Anderson, A.; Chou, J.; et al. Talimogene Laherparepvec in combination with Ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2006, 34, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. China appoves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006, 98, 298–300. [Google Scholar] [CrossRef]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Caner Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Alldinger, S.; Baumgärtner, W.; Kremmer, E.; Fonfara, S. Characterization of a canine CD44 specific monoclonal antibody. Zentralbl. Veterinarmed. 1999, 46, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Empl, M.T.; Macke, S.; Winterhalter, P.; Puff, C.; Lapp, S.; Stoica, G.; Baumgärtner, W.; Steinberg, P. The growth of the canine glioblastoma cell line D-GBM and the canine histiocytic sarcoma cell line DH82 is inhibited by the resveratrol oligomers hopeaphenol and r2-viniferin. Vet. Comp. Oncol. 2014, 12, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Waner, T.; Friedmann-Morvinski, D.; Fishman, Z.; Bark, H.; Harmelin, A. Down-regulation of MHC class II receptors of DH82 cells, following infection with Ehrlichia canis. Vet. Immunol. Immunopathol. 2003, 96, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Puff, C.; Krudewig, C.; Imbschweiler, I.; Baumgärtner, W.; Alldinger, S. Influence of persistent canine distemper virus infection on expression of RECK, matrix-metalloproteinases and their inhibitors in a canine macrophage/monocytic tumour cell line (DH82). Vet. J. 2009, 182, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Armando, F.; Fayyad, A.; Arms, S.; Barthel, Y.; Schaudien, D.; Rohn, K.; Gambini, M.; Lombardo, M.S.; Beineke, A.; Baumgärtner, W.; et al. Intratumoral canine distemper virus infection inhibits tumor growth by modulation of the tumor microenvironment in a murine xenograft model of canine histiocytic sarcoma. Int. J. Mol. Sci. 2021, 22, 3578. [Google Scholar] [CrossRef] [PubMed]
- Lapp, S. In Vivo Untersuchungen über die Onkolytische Wirkung des Staupevirus bei dem Disseminierten Histiozytären Sarkom des Hundes in Einem Mausmodell; University of Veterinary Medicine Hannover: Hannover, Germany, 11 November 2013. [Google Scholar]
- Kirn, D.H.; Wang, Y.; Le Boeuf, F.; Bell, J.; Thorne, S.H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007, 4, e353. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, S.; Ahonen, M.; Diaconu, I.; Kipar, A.; Siurala, M.; Vaha-Koskela, M.; Cerullo, V.; Hemminki, A. GMCSF-armed vaccinia virus induces an antitumor immune response. Int. J. Cancer 2015, 136, 1065–1072. [Google Scholar] [CrossRef]
- Currier, M.A.; Adams, L.C.; Mahller, Y.Y.; Cripe, T.P. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005, 12, 407–416. [Google Scholar] [CrossRef]
- Boisgerault, N.; Guillerme, J.B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fonteneau, J.F.; Tangy, F.; Gregoire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 387362. [Google Scholar] [CrossRef]
- Armando, F.; Gambini, M.; Corradi, A.; Giudice, C.; Pfankuche, V.M.; Brogden, G.; Attig, F.; von Köckritz-Blickwede, M.; Baumgärtner, W.; Puff, C. Oxidative stress in canine histiocytic sarcoma cells induced by an infection with canine distemper virus led to a dysregulation of HIF-1alpha downstream pathway resulting in a reduced expression of VEGF-B in vitro. Viruses 2020, 12, 200. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Chen, G.; Zhang, X.; Lin, D.; Hou, Y.; Yu, Y.; Liu, W.; Zhang, D. Oncolytic activity of canine distemper virus in canine mammary tubular adenocarcinoma cells. Vet. Comp. Oncol. 2019, 17, 174–183. [Google Scholar] [CrossRef]
- Igase, M.; Shousu, K.; Fujiki, N.; Sakurai, M.; Bonkobara, M.; Hwang, C.C.; Coffey, M.; Noguchi, S.; Nemoto, Y.; Mizuno, T. Anti-tumour activity of oncolytic reovirus against canine histiocytic sarcoma cells. Vet. Comp. Oncol. 2019, 17, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 1992, 13, 265–270. [Google Scholar] [CrossRef]
- Fayyad, A.; Lapp, S.; Risha, E.; Pfankuche, V.M.; Rohn, K.; Barthel, Y.; Schaudien, D.; Baumgärtner, W.; Puff, C. Matrix metalloproteinases expression in spontaneous canine histiocytic sarcomas and its xenograft model. Vet. Immunol. Immunopathol. 2018, 198, 54–64. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Marek, K.; Armando, F.; Asawapattanakul, T.; Nippold, V.M.; Plattet, P.; Gerold, G.; Baumgärtner, W.; Puff, C. Functional granulocyte–macrophage colony-stimulating factor (GM-CSF) delivered by canine histiocytic sarcoma cells persistently infected with engineered attenuated canine distemper virus. Pathogens 2023, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Cattaneo, R.; Fielding, A.K. Neutrophils contribute to the measles virus-induced antitumor effect: Enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003, 63, 6463–6468. [Google Scholar]
- Mullen, J.T.; Donahue, J.M.; Chandrasekhar, S.; Yoon, S.S.; Liu, W.; Ellis, L.M.; Nakamura, H.; Kasuya, H.; Pawlik, T.M.; Tanabe, K.K. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer 2004, 101, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Armando, F.; Nippold, V.M.; Rohn, K.; Plattet, P.; Brogden, G.; Gerold, G.; Baumgärtner, W.; Puff, C. Persistent infection of a canine histiocytic sarcoma cell line with attenuated canine distemper virus expressing vasostatin or granulocyte-macrophage colony-stimulating factor. Int. J. Mol. Sci. 2022, 23, 6156. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Techangamsuwan, S.; Haas, L.; Rohn, K.; Baumgärtner, W.; Wewetzer, K. Distinct cell tropism of canine distemper virus strains to adult olfactory ensheathing cells and Schwann cells in vitro. Virus Res. 2009, 144, 195–201. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.S.; Armando, F.; Marek, K.; Rohn, K.; Baumgärtner, W.; Puff, C. Persistence of Infectious Canine Distemper Virus in Murine Xenotransplants of Canine Histiocytic Sarcoma Cells after Intratumoral Application. Int. J. Mol. Sci. 2024, 25, 8297. https://doi.org/10.3390/ijms25158297
Lombardo MS, Armando F, Marek K, Rohn K, Baumgärtner W, Puff C. Persistence of Infectious Canine Distemper Virus in Murine Xenotransplants of Canine Histiocytic Sarcoma Cells after Intratumoral Application. International Journal of Molecular Sciences. 2024; 25(15):8297. https://doi.org/10.3390/ijms25158297
Chicago/Turabian StyleLombardo, Mara Sophie, Federico Armando, Katarzyna Marek, Karl Rohn, Wolfgang Baumgärtner, and Christina Puff. 2024. "Persistence of Infectious Canine Distemper Virus in Murine Xenotransplants of Canine Histiocytic Sarcoma Cells after Intratumoral Application" International Journal of Molecular Sciences 25, no. 15: 8297. https://doi.org/10.3390/ijms25158297
APA StyleLombardo, M. S., Armando, F., Marek, K., Rohn, K., Baumgärtner, W., & Puff, C. (2024). Persistence of Infectious Canine Distemper Virus in Murine Xenotransplants of Canine Histiocytic Sarcoma Cells after Intratumoral Application. International Journal of Molecular Sciences, 25(15), 8297. https://doi.org/10.3390/ijms25158297






