Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Results
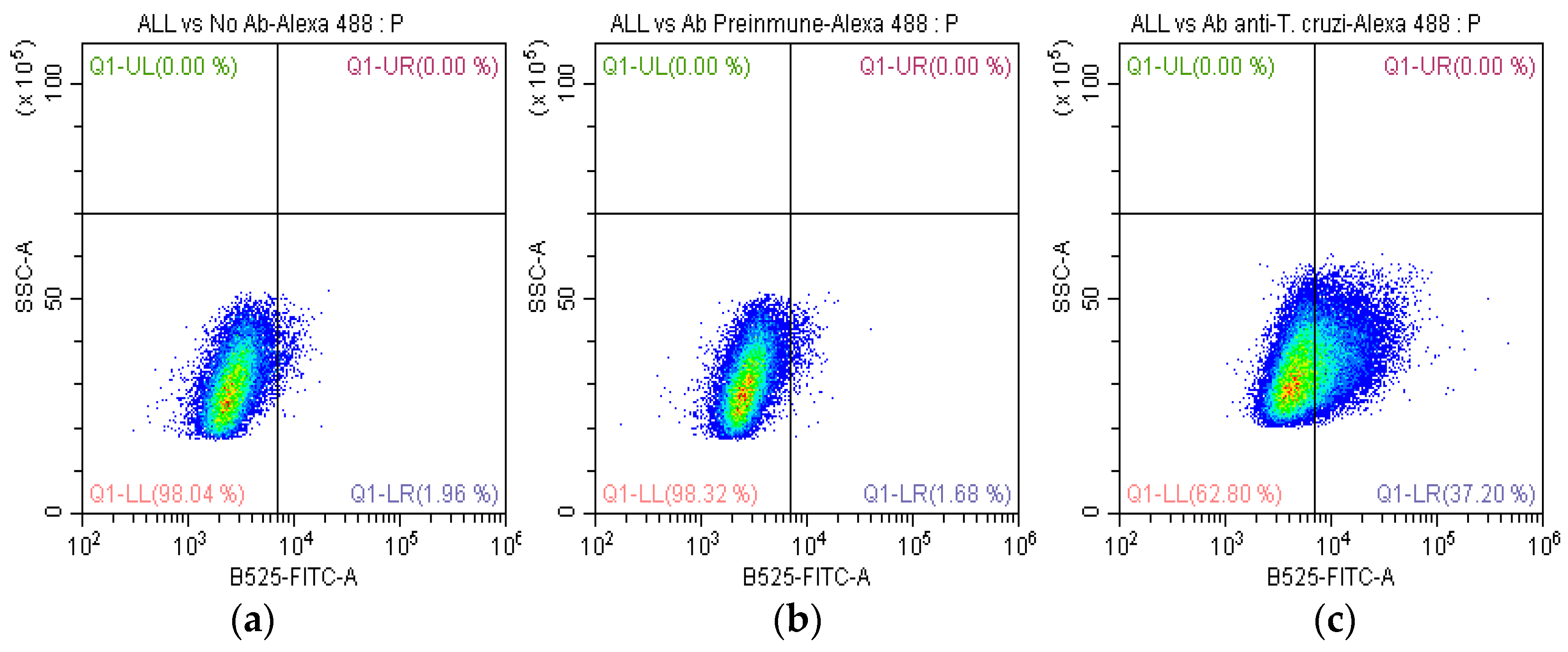
2.1. Recognition of Anti-T. cruzi Antibodies on the Membrane of SUPB15
2.2. Evaluation of Anti-T. cruzi Antibodies with CDC
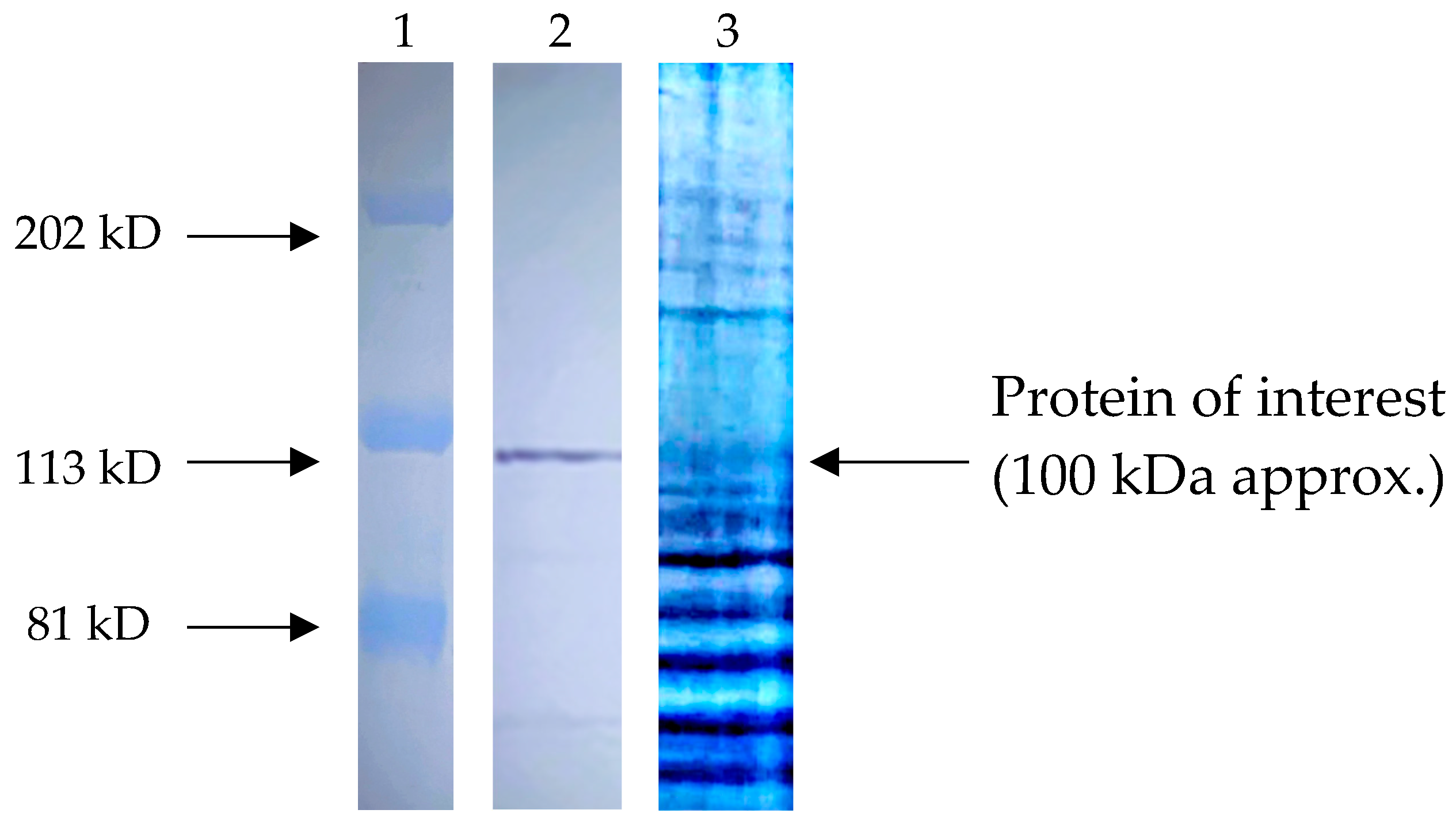
2.3. Recognition of Anti-T. cruzi Antibodies on the Total Proteins of SUPB15
2.4. Identification of the Most Abundant Proteins of a 100 KDa Fragment
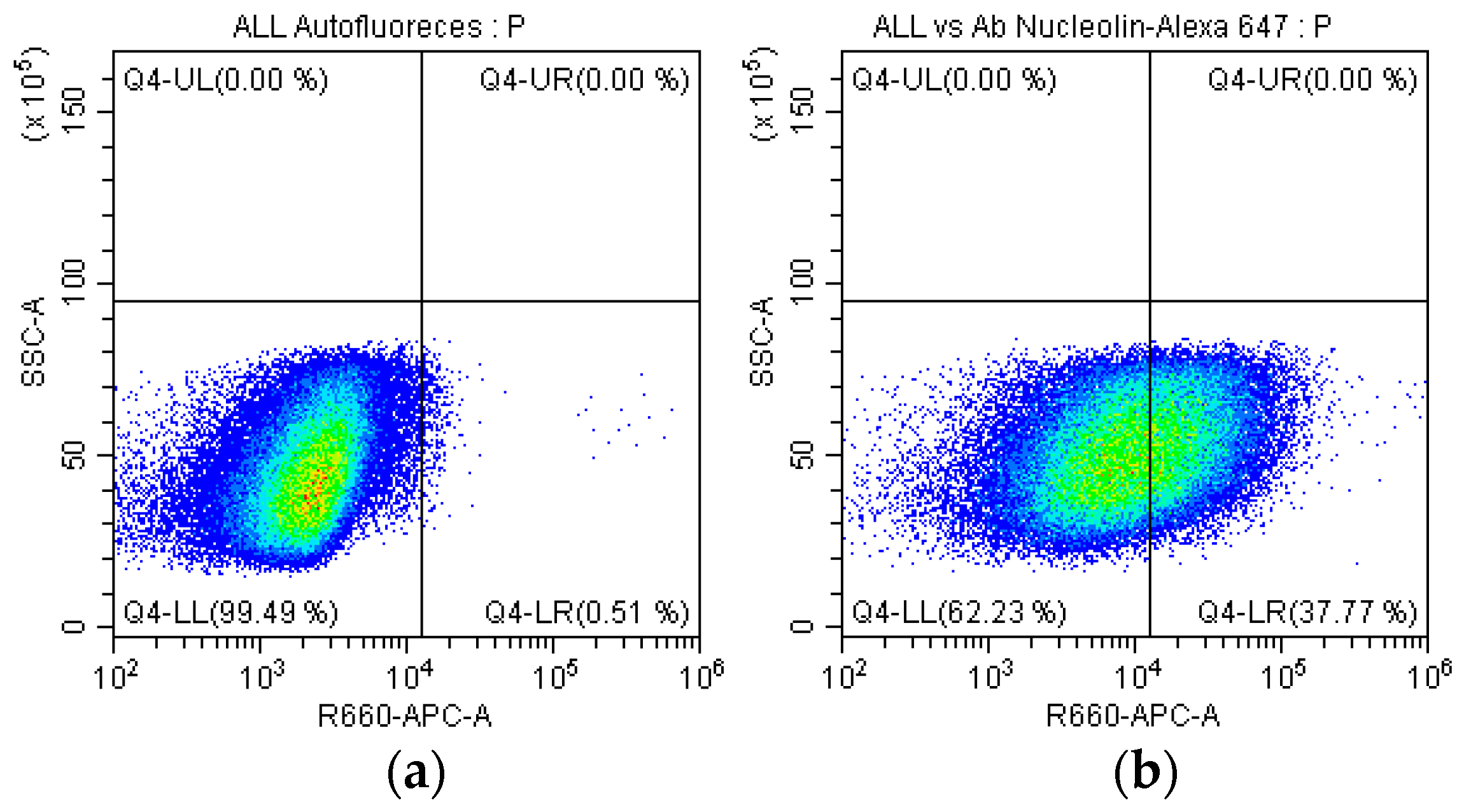
2.5. Identification of the Nucleolin in SUPB15
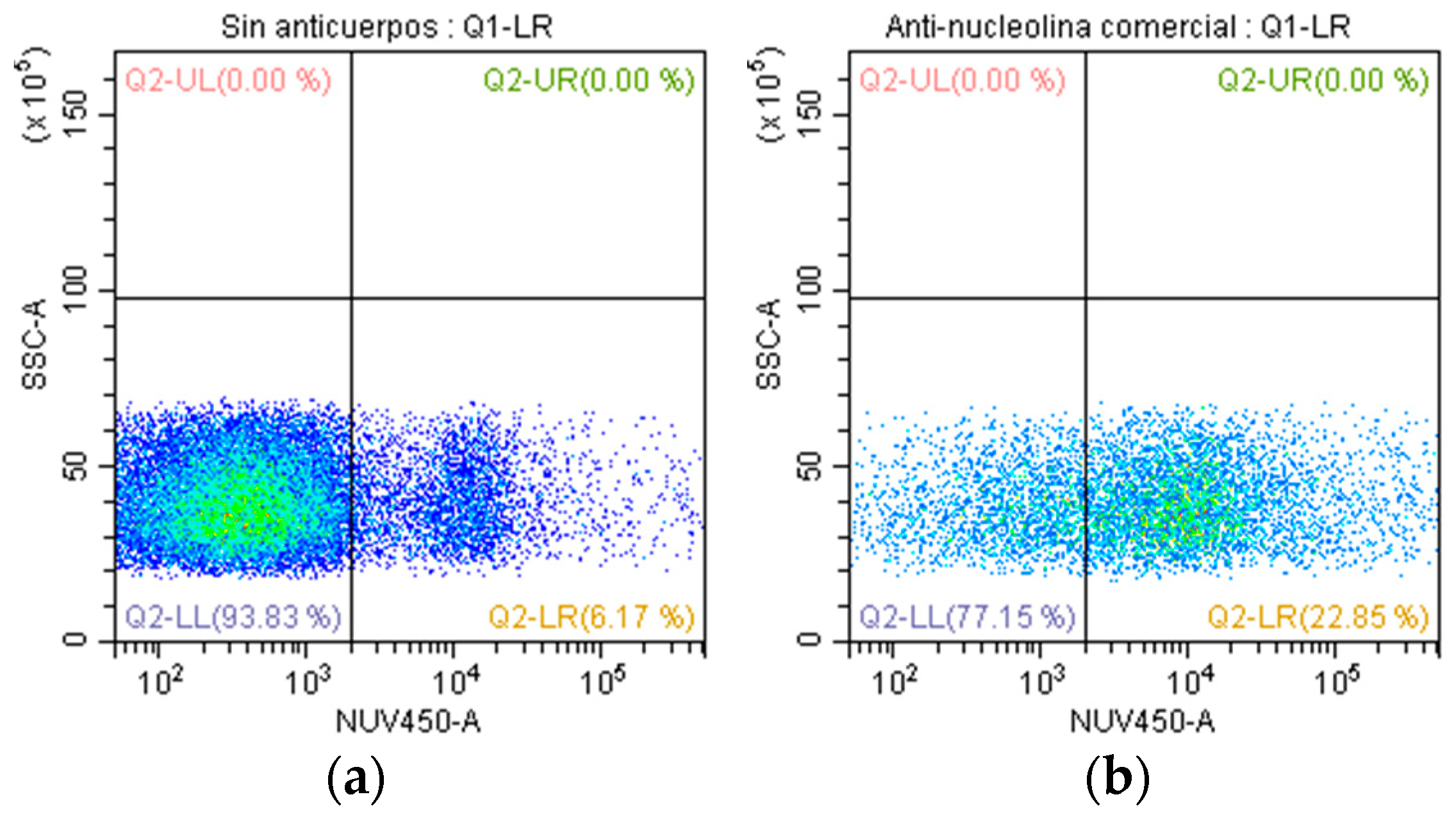
2.6. Evaluation of Anti-Nucleolin Antibodies with CDC
3. Discussion
4. Materials and Methods
4.1. Parasite Culture and Obtaining the Antigen
4.2. Cell Line of SUPB15 and Obtaining the Antigen
4.3. Generation of Anti-T. cruzi Antibodies In Vivo with New Zealand Rabbits
4.4. Purification of Anti-T. cruzi Antibodies by Affinity Chromatography with Protein A/G
4.5. Determination of Anti-T. cruzi Antibodies Recognition with SUPB15 by FC
4.6. CDC on SUP15 by Anti-T. cruzi Antibodies
4.7. Identification of SUP15 Proteins Recognized by Anti-T. cruzi Antibodies by WB
4.8. Mass Spectrometry
4.9. Identification of Nucleolin in SUPB15 by FC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo Clin. Proc. 2016, 91, 1645–1666. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Hernandez, E.; Jaimes-Reyes, E.Z.; Arellano-Galindo, J.; Garcia-Jimenez, X.; Tiznado-Garcia, H.M.; Dueñas-Gonzalez, M.T.; Villegas, O.M.; Sánchez-Jara, B.; Bekker-Méndez, V.C.; Ortíz-Torres, M.G.; et al. Survival of Mexican Children with Acute Lymphoblastic Leukemia under Treatment with the Protocol from the Dana-Farber Cancer Institute 00-01. BioMed Res. Int. 2015, 2015, 576950. [Google Scholar] [CrossRef]
- Flores-Lujano, J.; Duarte-Rodríguez, D.A.; Jiménez-Hernández, E.; Martín-Trejo, J.A.; Allende-López, A.; Peñaloza-González, J.G.; Pérez-Saldivar, M.L.; Medina-Sanson, A.; Torres-Nava, J.R.; Solís-Labastida, K.A.; et al. Persistently high incidence rates of childhood acute leukemias from 2010 to 2017 in Mexico City: A population study from the MIGICCL. Front. Public Health 2022, 10, 918921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giddings, B.M.; Whitehead, T.P.; Metayer, C.; Miller, M.D. Childhood leukemia incidence in California: High and rising in the Hispanic population. Cancer 2016, 122, 2867–2875. [Google Scholar] [CrossRef]
- Valencia-González, M.; Nájera-Castillo, M.F.; Tejocote-Romero, I.; Trujillo-Condes, V.E. Etiological factors of infantile acute lymphoblastic leukemia. Rev. Hematol. Mex. 2021, 22, 155–161. [Google Scholar]
- Vera, A.M.; Pardo, C.; Duarte, M.C.; Suárez, A. Análisis de la mortalidad por leucemia aguda pediátrica en el Instituto Nacional de Cancerología. Biomédica 2012, 32, 355–364. [Google Scholar] [CrossRef]
- Palacio, M.L.; Alvarez, A.M.; Morales, C.E.; Hernández, A.J. Distribución geográfica y temporal de las tasas de mortalidad por Leucemia Linfoblástica Aguda, por municipio y grupo etario, México 2000–2020. In Proceedings of the 1º encuentro PRONAI de Inmunoterapia Multidisciplinaria y Leucemia Infantil, Mexico City, Mexico, 25–26 March 2022. [Google Scholar]
- Aldoss, I.; Stein, A.S. Advances in adult acute lymphoblastic leukemia therapy. Leuk. Lymphoma 2018, 59, 1033–1050. [Google Scholar] [CrossRef]
- Lam, C.G.; Howard, S.C.; Bouffet, E.; Pritchard-Jones, K. Science and health for all children with cancer. Science 2019, 363, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.J.; Barbosa, L.O.; Machado, T.S.; Campos, J.P.; Moura, A.S. Meningoencephalitis Caused by Reactivation of Chagas Disease in Patient Without Known Immunosuppression. Am. J. Trop. Med. Hyg. 2017, 96, 292–294. [Google Scholar] [CrossRef][Green Version]
- Angheben, A.; Boix, L.; Buonfrate, D.; Gobbi, F.; Bisoffi, Z.; Pupella, S.; Gandini, G.; Aprili, G. Chagas Disease and Transfusion Medicine: A Perspective From Non-Endemic Countries. Blood Transfus. 2015, 13, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Maguire, B.J.; Dahal, P.; Rashan, S.; Ngu, R.; Boon, A.; Forsyth, C.; Strub-Wourgaft, N.; Chatelain, E.; Barreira, F.; Sosa-Estani, S.; et al. The Chagas Disease Study Landscape: A Systematic Review of Clinical and Observational Antiparasitic Treatment Studies to Assess the Potential for Establishing an Individual Participant-Level Data Platform. PLoS Negl. Trop. Dis. 2021, 15, e0009697. [Google Scholar] [CrossRef] [PubMed]
- Moretti, N.S.; Mortara, R.A.; Schenkman, S. Trypanosoma cruzi. Trends Parasitol. 2020, 36, 404–405. [Google Scholar] [CrossRef] [PubMed]
- American Trypanosomiasis. Life Cycle. Available online: https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html (accessed on 20 June 2024).
- De Souza, W.; Barrias, E.S. May the epimastigote form of Trypanosoma cruzi be infective? Acta Trop. 2020, 212, 105688. [Google Scholar] [CrossRef] [PubMed]
- Ubillos, L.; Freire, T.; Berriel, E.; Chiribao, M.L.; Chiale, C.; Festari, M.F.; Medeiros, A.; Mazal, D.; Rondán, M.; Bollati-Fogolín, M.; et al. Trypanosoma cruzi extracts elicit protective immune response against chemical-ly induced colon and mammary cancers. Int. J. Cancer 2016, 138, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Sheklakova, L.; Kallinikova, V.; Karpenko, L. Genetic heterogeneity of Trypanosoma cruzi and its direct anticancer effect in cultured human tumor cells. Bull. Exp. Biol. Med. 2003, 135, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Zenina, A.V.; Kravtsov, E.G.; Tsetsegsaikhan, B.; Yashina, N.V.; Dalin, M.V.; Karpenko, L.P.; Sheklakova, L.A.; Kallinikova, V. The study of immunological component in antitumor effect of Trypanosoma cruzi. Bull. Exp. Biol. Med. 2008, 145, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.J.; Valdéz, G.C.; Campos, A.C.; Sanchez, R.d.l.L.; Mendoza, C.R.; Hernández, A.P.; Ramírez, M.H.; Habana, J.R.; González, E.B.; Matzumura, P.D.; et al. Maternal fetal transmission of Trypanosoma cruzi: A problem of public health little studied in Mexico. Exp. Parasitol. 2012, 131, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Roskin, G.; Exempliarskaia, E. Protozoeninfektion und Experimenteller Krebs. I. Mitteilung. Z. Krebsforsch. 1931, 34, 628–645. [Google Scholar] [CrossRef]
- Darani, H.Y.; Yousefi, M. Parasites and cancers: Parasite antigens as possible targets for cancer immunotherapy. Future Oncol. 2012, 8, 1529–1535. [Google Scholar] [CrossRef]
- Morillo, D.A.; Gonzalez, B.E.; Sulbarán, A.J.; Ibarra, A.; Alvarez, C.; Colmenares, M.V.; Bonfante-Cabarcas, R.A. La infección por Trypanosoma cruzi disminuye el desarrollo del melanoma maligno e incrementa la supervivencia en ratones C57BL/6. Investig. Clin. 2014, 55, 227–237. [Google Scholar]
- Campo, V.L.; Riul, T.B.; Carvalho, I.; Baruffi, M.D. Antibodies against mucin-based glycopeptides affect Trypanosoma cruzi cell invasion and tumor cell viability. ChemBioChem 2014, 15, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- López, N.C.; Valck, C.; Ramirez, G.; Rodriguez, M.; Ribeiro, C.; Orellana, J.; Maldonado, I.; Albini, A.; Anacona, D.; Lemus, D.; et al. Antiangiogenic and antitumor effects of Trypanosoma cruzi Calreticulin. PLoS Negl. Trop. Dis. 2010, 4, e730. [Google Scholar] [CrossRef] [PubMed]
- Abello-Cáceres, P.; Pizarro-Bauerle, J.; Rosas, C.; Maldonado, I.; Aguilar-Guzmán, L.; González, C.; Ramírez, G.; Ferreira, J.; Ferreira, A. Does native Trypanosoma cruzi calreticulin mediate growth inhibition of a mammary tumor during infection? BMC Cancer 2016, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Eligio-Garcia, L.; Crisostomo-Vazquez, M.D.P.; Maravelez-Acosta, V.A.; Soria-Guerrero, M.; Cortes-Campos, A.; Jimenez-Cardoso, E. Trypanosoma cruzi Antigenic Proteins Shared with Acute Lymphoblastic Leukemia and Neuroblastoma. Pharmaceuticals 2022, 15, 1421. [Google Scholar] [CrossRef] [PubMed]
- Sadr, S.; Ghiassi, S.; Lotfalizadeh, N.; Simab, P.A.; Hajjafari, A.; Borji, H. Antitumor Mechanisms of Molecules Secreted by Trypanosoma cruzi in Colon and Breast Cancer: A Review. Anticancer Agents Med. Chem. 2023, 23, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Sosoniuk-Roche, E.; Cruz, P.; Maldonado, I.; Duaso, L.; Pesce, B.; Michalak, M.; Valck, C.; Ferreira, A. In vitro Treatment of a Murine Mammary Adenocarcinoma Cell Line with Recombinant Trypanosoma cruzi Calreticulin Promotes Immunogenicity and Phagocytosis. Mol. Immunol. 2020, 124, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Vicco, M.H.; Ferini, F.; Rodeles, L.; Cardona, P.; Bontempi, I.; Lioi, S.; Beloscar, J.; Marcipar, I.; Bottasso, O.A. Valoración de anticuerpos con reactividad cruzada patogeno-huesped en pacientes con diferentes estadios de cardiopatia Chagasica cronica. Rev. Española Cardiol. 2013, 66, 791–796. [Google Scholar] [CrossRef]
- Joo, E.J.; Wasik, B.R.; Parrish, C.; Paz, H.; Mϋhlenhoff, M.; Abdel-Azim, H.; Heisterkamp, N. Pre-B acute lymphoblastic leukemia expresses cell surface nucleolin as a 9-O-acetylated sialoglycoprotein. Sci. Rep. 2018, 8, 17174. [Google Scholar] [CrossRef]
- De Lederkremer, R.; Lima, C.; Ramirez, M.; Ferguson, M.; Homans, S.; Thomas-Oates, J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi Epimastigotes. J. Biol. Chem. 1991, 266, 23670–23675. [Google Scholar] [CrossRef]
- Acosta, S.A.; Almeida, I.C.; Freitas, L.H.; Yoshida, N.; Schenkman, S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: Structure and biological roles. Mol. Biochem. Parasitol. 2001, 114, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Chioccola, V.L.; Acosta-Serrano, A.; de Almeida, I.C.; Ferguson, M.A.; Souto-Padron, T.; Rodrigues, M.M.; Travassos, L.R.; Schenkman, S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 2000, 113, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.E.; De Lederkremer, R.M. Trans-sialidase and mucins of Trypanosoma cruzi: An important interplay for the parasite. Carbohydr. Res. 2011, 346, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.E.; Lederkremer, R.M. The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose. Molecules 2020, 25, 3913. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, P.L.; Penha, L.; Garcez, T.C.; Jones, C.; Previato, J.O. Addition of alpha-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi. Glycoconj. J. 2013, 30, 659–766. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef]
- Jacobs, T.; Erdmann, H.; Fleischer, B. Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi. Eur. J. Cell Biol. 2010, 89, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.P.; Fortes, B.; Silva-Filho, J.L.; Terra-Granado, E.; Santos, L.; Conde, L.; de Araújo Oliveira, I.; Freire-de-Lima, L.; Martins, M.V.; Pinheiro, A.A.S.; et al. Inhibitory effects of Trypanosoma cruzi sialoglycoproteins on CD4+ T cells are associated with increased susceptibility to infection. PLoS ONE 2013, 8, e77568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, W.; Yao, Z.; Zhao, J.; Guan, Q.; Gao, L. New perspectives of physiological and pathological functions of nucleolin (NCL). Life Sci. 2017, 186, 1–10. [Google Scholar] [CrossRef]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef]
- Fogal, V.; Sugahara, K.N.; Ruoslahti, E.; Christian, S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis 2009, 12, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, H.; Zhou, H.; Song, X.; Yuan, S.; Luo, Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 2006, 107, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Arnal, A.; Waleckx, E.; Rico-Chávez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.M.; Peterson, A.T.; Carmona-Castro, O.; Moo-Llanes, D.A.; Nakazawa, Y.; Butrick, M.; Tun-Ku, E.; De La Cruz-Félix, K.; Ibarra-Cerdeña, C.N. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 2015, 110, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Castro, O.; Moo-Llanes, D.A.; Ramsey, J.M. Impact of climate change on vector transmission of Trypanosoma cruzi (Chagas, 1909) in North America. Med. Vet. Entomol. 2018, 32, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, G.; Figueroa-Lara, A.; Elizondo-Cano, M.; Wilson, L.; Novelo-Garza, B.; Valiente-Banuet, L.; Ramsey, J.M. Cost-effectiveness of blood donation screening for Trypanosoma cruzi in Mexico. PLoS Negl. Trop. Dis. 2016, 10, e0004528. [Google Scholar] [CrossRef]
- Velasco-Castrejón, O.; Valdespino, J.L.; Tapia-Conyer, R.; Salvatierra, B.; Guzman-Bracho, C.; Magos, C.; Llausas, A.; Gutierrez, G.; Sepulveda, J. Seroepidemiología de la enfermedad de Chagas en México. Salud Publica Mex. 1992, 34, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Park, H.I.; Yoon, H.W.; Jung, S.T. The highly evolvable antibody Fc domain. Trends Biotechnol. 2016, 34, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Kegyes, D.; Ghiaur, G.; Bancos, A.; Tomuleasa, C.; Gale, R.P. Immune therapies of B-cell acute lymphoblastic leukaemia in children and adults. Crit. Rev. Oncol. Hematol. 2024, 196, 104317. [Google Scholar] [CrossRef] [PubMed]
- Dho, S.H.; Lim, J.C.; Kim, L.K. Beyond the role of CD55 as a complement component. Immune Netw. 2018, 18, e11. [Google Scholar] [CrossRef]
- Loeff, F.C.; van Egmond, H.M.E.; Nijmeijer, B.A.; Falkenburg, J.H.F.; Halkes, C.J.; Jedema, I. Complement-dependent cytotoxicity induced by therapeutic antibodies in B-cell acute lymphoblastic leukemia is dictated by target antigen expression levels and augmented by loss of membrane-bound complement inhibitors. Leuk. Lymphoma 2017, 58, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Hara, T.; Kojima, A.; Fukuda, H.; Masaoka, T.; Fukumori, Y.; Matsumoto, M.; Seya, T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br. J. Haematol. 1992, 82, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Min, S.-W.; Kwon, H.S.; Bae, G.-D.; Jung, J.H.; Park, H.I.; Lee, S.H.; Lim, C.S.; Ko, B.J.; Lee, J.C.; et al. Effective clearance of rituximab-resistant tumor cells by breaking the mirror-symmetry of immunoglobulin G and simultaneous binding to CD55 and CD20. Sci. Rep. 2023, 13, 18275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romano, S.; Moura, V.; Simoes, S.; Moreira, J.N.; Goncalves, J. Anticancer activity and antibody-dependent cell-mediated cytotoxicity of novel anti-nucleolin antibodies. Sci. Rep. 2018, 8, 7450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Klinman, D.M. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev. Vaccines 2014, 13, 299–312. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Matsuda, T.; Washizaki, A.; Murakami, H.; Yamamoto, T.; Yoshioka, Y. Elucidation of the role of nucleolin as a cell surface receptor for nucleic acid-based adjuvants. npj Vaccines 2022, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.; Panebra, A.; Santa-Cruz, C. Relevance of hemin for in vitro differentiation of Trypanosoma cruzi. J. Protozool. 1985, 32, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The Protein Protocols Handbook, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Johnson, C.A.; Kleshchenko, Y.Y.; Ikejiani, A.O.; Udoko, A.N.; Cardenas, T.C.; Pratap, S.; Duquette, M.A.; Lima, M.F.; Lawler, J.; Villalta, F.; et al. Thrombospondin-1 interacts with Trypanosoma cruzi surface calreticulin to enhance cellular infection. PLoS ONE 2012, 7, e40614. [Google Scholar] [CrossRef] [PubMed]
- Piwocka, K.; Podszywalow-Bartnicka, P.; Swatler, J.; Kolba, M.D.; Kominek, A.; Kozlowska, E. Insight into the Leukemia Microenvironment and Cell-cell Interactions Using Flow Cytometry. In Multidimensional Flow Cytometry Techniques for Novel Highly Informative Assays; Gemei, M., Ed.; Intech: London, UK, 2018. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maravelez Acosta, V.A.; Crisóstomo Vázquez, M.d.P.; Eligio García, L.; Franco Sandoval, L.O.; Castro Pérez, D.; Patiño López, G.; Medina Contreras, O.; Jiménez Cardoso, E. Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 8307. https://doi.org/10.3390/ijms25158307
Maravelez Acosta VA, Crisóstomo Vázquez MdP, Eligio García L, Franco Sandoval LO, Castro Pérez D, Patiño López G, Medina Contreras O, Jiménez Cardoso E. Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2024; 25(15):8307. https://doi.org/10.3390/ijms25158307
Chicago/Turabian StyleMaravelez Acosta, Víctor Alberto, María del Pilar Crisóstomo Vázquez, Leticia Eligio García, Luz Ofelia Franco Sandoval, Denia Castro Pérez, Genaro Patiño López, Oscar Medina Contreras, and Enedina Jiménez Cardoso. 2024. "Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 25, no. 15: 8307. https://doi.org/10.3390/ijms25158307
APA StyleMaravelez Acosta, V. A., Crisóstomo Vázquez, M. d. P., Eligio García, L., Franco Sandoval, L. O., Castro Pérez, D., Patiño López, G., Medina Contreras, O., & Jiménez Cardoso, E. (2024). Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences, 25(15), 8307. https://doi.org/10.3390/ijms25158307





