Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus
Abstract
:1. Introduction
2. Background and Mechanisms of Base Editing Technology
3. Types and Optimization of DNA Base Editors
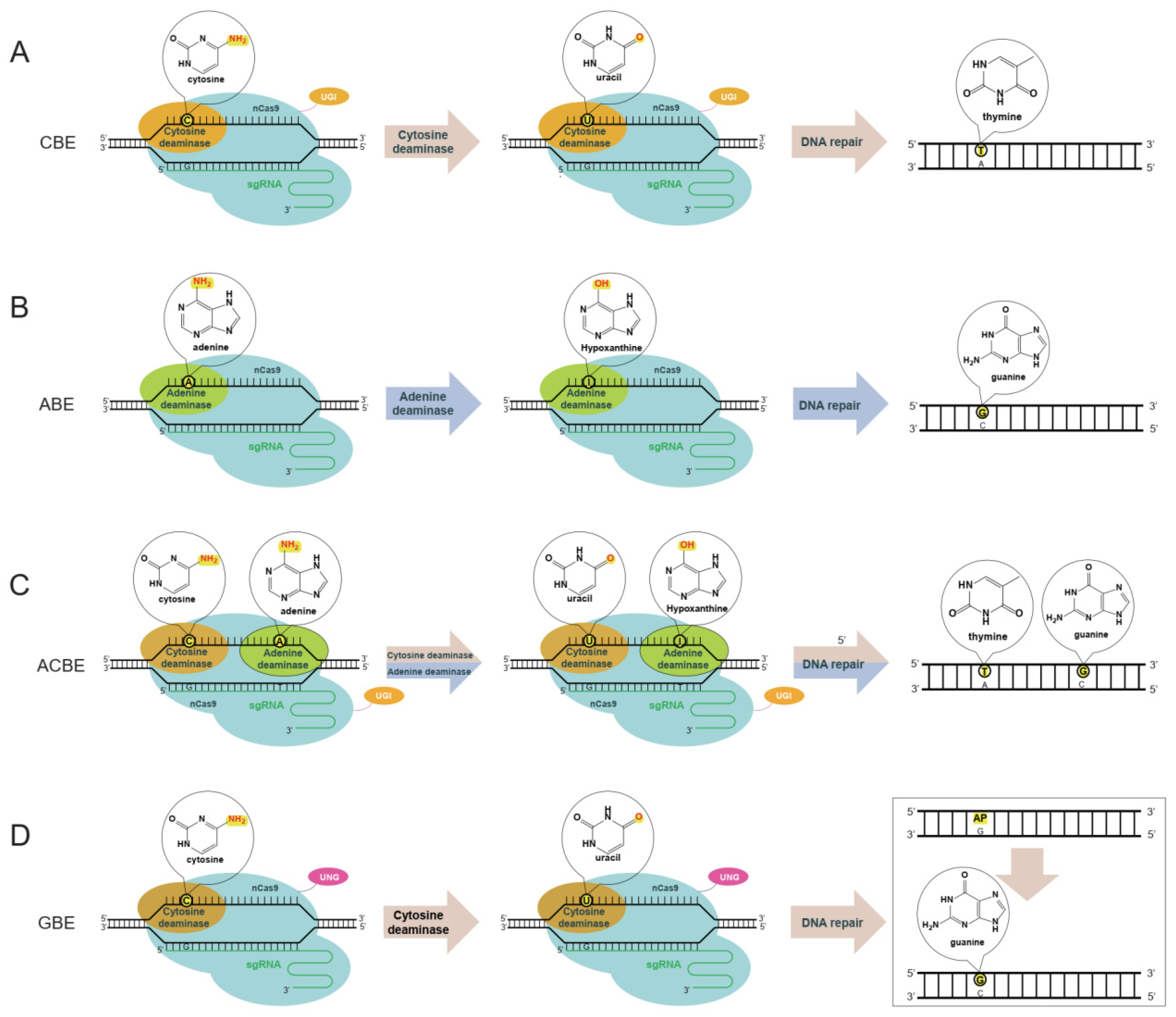
3.1. Cytidine Base Editor (CBE)
3.2. Adenine Base Editor (ABE)
3.3. Dual Base Editor
3.4. Glycosylase Base Editor
4. Current Status and Methods of Improving Base Editors
4.1. Strategies for Enhancing Editing Sites and Windows
4.2. Approaches to Optimize Editing Efficiency
5. Application of Base Editors in Crop Genetic Improvement
6. Potential Application of Base Editors in Populus Breeding
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Doudna, J.A. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Su, F.; Huang, S.; Mei, F.; Niu, X.; Ma, C.; Zhang, H.; Zhu, X.; Zhu, J.K.; Zhang, J. Novel Wx alleles generated by base editing for improvement of rice grain quality. J. Integr. Plant Biol. 2021, 63, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-Editing-Mediated Artificial Evolution of OsALS1 In Planta to Develop Novel Herbicide-Tolerant Rice Germplasms. Mol. Plant 2020, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- McGinn, J.; Marraffini, L.A. Molecular mechanisms of CRISPR-Cas spacer acquisition. Nat. Rev. Microbiol. 2019, 17, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Embden, J.D.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151 Pt 8, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Koo, T. Recent advances in CRISPR technologies for genome editing. Arch. Pharm. Res. 2021, 44, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Taylor, M.R.G.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef]
- Tuladhar, R.; Yeu, Y.; Tyler Piazza, J.; Tan, Z.; Rene Clemenceau, J.; Wu, X.; Barrett, Q.; Herbert, J.; Mathews, D.H.; Kim, J.; et al. CRISPR-Cas9-based mutagenesis frequently provokes on-target mRNA misregulation. Nat. Commun. 2019, 10, 4056. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, R.; Meng, X.; Chen, S.; Zong, Y.; Lu, C.; Qiu, J.L.; Chen, Y.H.; Li, J.; Gao, C. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 2020, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grunewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Ahmad, M.J.; Asif, A.R.; Adnan, M.; Iqbal, M.K.; Mehmood, K.; Muhammad, S.A.; Bhuiyan, A.A.; Elokil, A.; Du, X.; et al. A Review of CRISPR-Based Genome Editing: Survival, Evolution and Challenges. Curr. Issues Mol. Biol. 2018, 28, 47–68. [Google Scholar] [CrossRef]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef] [PubMed]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Song, L.T.; Yuan, B.; Zhang, C.; Cao, J.X.; Chen, J.L.; Qiu, J.Y.; Tai, Y.L.; Chen, J.Q.; Qiu, Z.L.; et al. TadA reprogramming to generate potent miniature base editors with high precision. Nat. Commun. 2023, 14, 413. [Google Scholar] [CrossRef]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zeng, D.; Zhao, Y.; Wang, Y.; Liu, T.; Li, S.; Xue, Y.; Luo, Y.; Xie, X.; Chen, L.; et al. PhieABEs: A PAM-less/free high-efficiency adenine base editor toolbox with wide target scope in plants. Plant Biotechnol. J. 2022, 20, 934–943. [Google Scholar] [CrossRef]
- Sakata, R.C.; Ishiguro, S.; Mori, H.; Tanaka, M.; Tatsuno, K.; Ueda, H.; Yamamoto, S.; Seki, M.; Masuyama, N.; Nishida, K.; et al. Base editors for simultaneous introduction of C-to-T and A-to-G mutations. Nat. Biotechnol. 2020, 38, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Zhou, R.; Lareau, C.A.; Garcia, S.P.; Iyer, S.; Miller, B.R.; Langner, L.M.; Hsu, J.Y.; Aryee, M.J.; Joung, J.K. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat. Biotechnol. 2020, 38, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kong, F.; Qin, R.; Li, J.; Liu, X.; Wei, P. Development of an efficient plant dual cytosine and adenine editor. J. Integr. Plant Biol. 2021, 63, 1600–1605. [Google Scholar] [CrossRef]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nat. Commun. 2021, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, X.; Liu, Y.; Liu, N.; Li, Y.; Luo, J.; Ma, Q.; Wu, D.; Li, J.; Xu, C.; et al. Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase. Nat. Biotechnol. 2023, 41, 1080–1084. [Google Scholar] [CrossRef]
- Neugebauer, M.E.; Hsu, A.; Arbab, M.; Krasnow, N.A.; McElroy, A.N.; Pandey, S.; Doman, J.L.; Huang, T.P.; Raguram, A.; Banskota, S.; et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity. Nat. Biotechnol. 2023, 41, 673–685. [Google Scholar] [CrossRef]
- Yang, L.; Huo, Y.; Wang, M.; Zhang, D.; Zhang, T.; Wu, H.; Rao, X.; Meng, H.; Yin, S.; Mei, J.; et al. Engineering APOBEC3A deaminase for highly accurate and efficient base editing. Nat. Chem. Biol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Sola-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.J.; Liquori, A.J.; et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Jakimo, N.; Jacobson, J.M. Minimal PAM specificity of a highly similar SpCas9 ortholog. Sci. Adv. 2018, 4, eaau0766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Dong, C.; Zheng, Z.; Shen, R.; Cao, X.; Chen, X.; Wang, M.; Zhu, J.K.; Tian, Y. Efficient and heritable A-to-K base editing in rice and tomato. Hortic. Res. 2024, 11, uhad250. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, J.; Harrison, P.T. Prime editing—An update on the field. Gene Ther. 2021, 28, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, J.; Zhang, Q.; Wang, X.; Gou, S.; Lin, L.; Chen, T.; Ge, W.; Zhuang, Z.; Lian, M.; et al. AGBE: A dual deaminase-mediated base editor by fusing CGBE with ABE for creating a saturated mutant population with multiple editing patterns. Nucleic Acids Res. 2022, 50, 5384–5399. [Google Scholar] [CrossRef]
- Yu, M.; Kuang, Y.; Wang, C.; Wu, X.; Li, S.; Zhang, D.; Sun, W.; Zhou, X.; Ren, B.; Zhou, H. Diverse nucleotide substitutions in rice base editing mediated by novel TadA variants. Plant Commun. 2024, 100926. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Komor, A.C.; Levy, J.M.; Packer, M.S.; Zhao, K.T.; Liu, D.R. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 2017, 35, 371–376. [Google Scholar] [CrossRef]
- Lei, L.; Chen, H.; Xue, W.; Yang, B.; Hu, B.; Wei, J.; Wang, L.; Cui, Y.; Li, W.; Wang, J.; et al. APOBEC3 induces mutations during repair of CRISPR-Cas9-generated DNA breaks. Nat. Struct. Mol. Biol. 2018, 25, 45–52. [Google Scholar] [CrossRef]
- Chatterjee, P.; Lee, J.; Nip, L.; Koseki, S.R.T.; Tysinger, E.; Sontheimer, E.J.; Jacobson, J.M.; Jakimo, N. A Cas9 with PAM recognition for adenine dinucleotides. Nat. Commun. 2020, 11, 2474. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, H.; Chen, S.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Efficient base editing with expanded targeting scope using an engineered Spy-mac Cas9 variant. Cell Discov. 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Oakes, B.L.; Fellmann, C.; Rishi, H.; Taylor, K.L.; Ren, S.M.; Nadler, D.C.; Yokoo, R.; Arkin, A.P.; Doudna, J.A.; Savage, D.F. CRISPR-Cas9 Circular Permutants as Programmable Scaffolds for Genome Modification. Cell 2019, 176, 254–267.e16. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.P.; Zhao, K.T.; Miller, S.M.; Gaudelli, N.M.; Oakes, B.L.; Fellmann, C.; Savage, D.F.; Liu, D.R. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat. Biotechnol. 2019, 37, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Yarra, R.; Krysan, P.J. An SpG-Cas9-based cytosine base editor expands the scope of genome editing in carrot plants. Plant Cell Rep. 2024, 43, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, F.; Xu, Z.; Wang, Y.; Zhang, C.; Zhou, Y.; Hui, F.; Yang, X.; Nie, X.; Zhang, X.; et al. Precise fine-turning of GhTFL1 by base editing tools defines ideal cotton plant architecture. Genome Biol. 2024, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, S.; Huang, S.; Yu, W.; Li, G.; Yang, G.; Liu, Y.; Zhang, Y.; Zhang, L.; Hou, Y.; et al. BE-PLUS: A new base editing tool with broadened editing window and enhanced fidelity. Cell Res. 2018, 28, 855–861. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Liu, N.; Yao, S. BE-PIGS: A base-editing tool with deaminases inlaid into Cas9 PI domain significantly expanded the editing scope. Signal Transduct. Target. Ther. 2019, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, B.; Cao, J.; Chen, J.; Chen, J.; Qiu, J.; Zhao, X.M.; Wang, X.; Qiu, Z.; Cheng, T.L. Docking sites inside Cas9 for adenine base editing diversification and RNA off-target elimination. Nat. Commun. 2020, 11, 5827. [Google Scholar] [CrossRef]
- Ryu, S.M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.T.; Kim, H.S.; Kim, D.E.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef]
- Villiger, L.; Schmidheini, L.; Mathis, N.; Rothgangl, T.; Marquart, K.; Schwank, G. Replacing the SpCas9 HNH domain by deaminases generates compact base editors with an alternative targeting scope. Mol. Ther. Nucleic Acids 2021, 26, 502–510. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, Y.; Ma, Y.; Liu, S.; Fan, T.; Liang, Y.; Tang, X.; Zheng, X.; Wu, Y.; Zhang, T.; et al. Expanding plant genome editing scope and profiles with CRISPR-FrCas9 systems targeting palindromic TA sites. Plant Biotechnol. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Lee, S.; Hwang, G.H.; Hong, S.A.; Park, S.E.; Kim, J.S.; Woo, J.S.; Bae, S. Adenine base editor engineering reduces editing of bystander cytosines. Nat. Biotechnol. 2021, 39, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, J.M.; Cervantes, O.; Clement, M.K.; Wu, Y.; Zeng, J.; Bauer, D.E.; Pinello, L.; Joung, J.K. An APOBEC3A-Cas9 base editor with minimized bystander and off-target activities. Nat. Biotechnol. 2018, 36, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ding, N.; Sun, Y.; Yuan, T.; Li, J.; Yuan, Q.; Liu, L.; Yang, J.; Wang, Q.; Kolomeisky, A.B.; et al. Single C-to-T substitution using engineered APOBEC3G-nCas9 base editors with minimum genome- and transcriptome-wide off-target effects. Sci. Adv. 2020, 6, eaba1773. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Expanding the genome-targeting scope and the site selectivity of high-precision base editors. Nat. Commun. 2020, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, G.; Li, J.; Chen, X.; Li, S.; Wang, J.; Zhou, Z.; Pu, S.; Dai, Z.; Ma, Y.; et al. Imperfect guide-RNA (igRNA) enables CRISPR single-base editing with ABE and CBE. Nucleic Acids Res. 2022, 50, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bae, S.; Park, J.; Kim, E.; Kim, S.; Yu, H.R.; Hwang, J.; Kim, J.I.; Kim, J.S. Digenome-seq: Genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 2015, 12, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, D.E.; Lee, G.; Cho, S.I.; Kim, J.S. Genome-wide target specificity of CRISPR RNA-guided adenine base editors. Nat. Biotechnol. 2019, 37, 430–435. [Google Scholar] [CrossRef]
- Liang, P.; Xie, X.; Zhi, S.; Sun, H.; Zhang, X.; Chen, Y.; Chen, Y.; Xiong, Y.; Ma, W.; Liu, D.; et al. Genome-wide profiling of adenine base editor specificity by EndoV-seq. Nat. Commun. 2019, 10, 67. [Google Scholar] [CrossRef]
- Lei, Z.; Meng, H.; Lv, Z.; Liu, M.; Zhao, H.; Wu, H.; Zhang, X.; Liu, L.; Zhuang, Y.; Yin, K.; et al. Detect-seq reveals out-of-protospacer editing and target-strand editing by cytosine base editors. Nat. Methods 2021, 18, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, J.; Zhong, Z.; Vanegas, J.A.; Gao, X.; Kolomeisky, A.B. A general theoretical framework to design base editors with reduced bystander effects. Nat. Commun. 2021, 12, 6529. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, B.; Ru, G.; Meng, H.; Yan, Y.; Hong, M.; Zhang, D.; Luan, C.; Zhang, S.; Wu, H.; et al. Re-engineering the adenine deaminase TadA-8e for efficient and specific CRISPR-based cytosine base editing. Nat. Biotechnol. 2023, 41, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bharat, S.S.; Li, S.; Li, J.; Yan, L.; Xia, L. Base editing in plants: Current status and challenges. Crop J. 2020, 8, 384–395. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Chai, Z.; Chen, S.; Bai, Y.; Zong, Y.; Chen, K.; Li, J.; Jiang, L.; Gao, C. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat. Plants 2019, 5, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Pan, H.; Li, K.; Zhou, Y.; Zhao, F.; Ye, L.; Ruan, S.; Deng, Q.; Xu, J.; Lu, Y. Targeted A-to-T and A-to-C base replacement in maize using an optimized adenine base editor. Plant Biotechnol. J. 2024, 22, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, A.; Shimizu, T.; Kaku, K.; Kawai, K.; Toriyama, K. A novel mutated acetolactate synthase gene conferring specific resistance to pyrimidinyl carboxy herbicides in rice. Plant Mol. Biol. 2007, 64, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y.; Li, W.; Chen, Z.; Wang, J.; Fan, F.; Tao, Y.; Jiang, Y.; Zhu, Q.-H.; Yang, J. Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. Crop J. 2021, 9, 305–312. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Ni, H.; Xu, Y.; Chen, Q.; Jiang, L. CRISPR/Cas9-mediated base-editing system efficiently generates gain-of-function mutations in Arabidopsis. Sci. China Life Sci. 2017, 60, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Xie, H.; Cao, X.; Song, M.; Wang, X.; Li, S.; Pang, K.; Zhang, Y.; Zhu, J.K.; Zhu, J. Engineering soybean with high levels of herbicide resistance with a Cas12-SF01-based cytosine base editor. Plant Biotechnol. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Jiang, Y.; Tao, X.; Zhu, J.K. Precision genome engineering in rice using prime editing system. Plant Biotechnol. J. 2020, 18, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J.K. Precise Editing of a Target Base in the Rice Genome Using a Modified CRISPR/Cas9 System. Mol. Plant 2017, 10, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Q.; Li, X.; Wang, F.; Chen, Z.; Wang, J.; Li, W.; Fan, F.; Tao, Y.; Jiang, Y.; et al. Fine-tuning the amylose content of rice by precise base editing of the Wx gene. Plant Biotechnol. J. 2021, 19, 11–13. [Google Scholar] [CrossRef]
- Zeng, D.; Li, X.; Huang, J.; Li, Y.; Cai, S.; Yu, W.; Li, Y.; Huang, Y.; Xie, X.; Gong, Q.; et al. Engineered Cas9 variant tools expand targeting scope of genome and base editing in rice. Plant Biotechnol. J. 2020, 18, 1348–1350. [Google Scholar] [CrossRef]
- Li, H.; Qin, R.; Liu, X.; Liao, S.; Xu, R.; Yang, J.; Wei, P. CRISPR/Cas9-Mediated Adenine Base Editing in Rice Genome. Rice Sci. 2019, 26, 125–128. [Google Scholar] [CrossRef]
- Zhang, D.; Pries, V.; Boch, J. Targeted C•G-to-T•A base editing with TALE-cytosine deaminases in plants. BMC Biol. 2024, 22, 99. [Google Scholar] [CrossRef]
- Li, J.Y.; Jiao, G.A.; Sun, Y.W.; Chen, J.; Zhong, Y.X.; Yan, L.; Jiang, D.; Ma, Y.Z.; Xia, L.Q. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2021, 19, 937–951. [Google Scholar] [CrossRef]
- Li, G.; Sretenovic, S.; Eisenstein, E.; Coleman, G.; Qi, Y. Highly efficient C-to-T and A-to-G base editing in a Populus hybrid. Plant Biotechnol. J. 2021, 19, 1086–1088. [Google Scholar] [CrossRef]
- Yao, T.; Yuan, G.L.; Lu, H.W.; Liu, Y.; Zhang, J.; Tuskan, G.A.; Muchero, W.; Chen, J.G.; Yang, X.H. CRISPR/Cas9-based gene activation and base editing in Populus. Hortic. Res. 2023, 10, uhad085. [Google Scholar] [CrossRef] [PubMed]
| Base Editor | Deaminase | Cas Protein | Editor Features | References | |
|---|---|---|---|---|---|
| Cytosine base editor | BE1 | APOBEC1 | dCas9 | C–G to T–A | [11] |
| BE3 | APOBEC1 | nCas9 | [11] | ||
| BE4 | APOBEC1 | nCas9 | An additional UGI is connected next to the UGI of the BE3 to improve efficiency | [18,19] | |
| nCas9-PBE | APOBEC1 | nCas9 | Editing efficiency up to 43.5% | [20] | |
| Target-AID | PmCDA1 | nCas9 | [21] | ||
| miniCBE | TadA8e | nCas9 | High accuracy and minimal off-target effects | [22] | |
| Adenine base editor | A–T to G–C | ||||
| ABE7.10 | ecTadA | nCas9 | [13] | ||
| ABEmax | ecTadA | nCas9 | [19] | ||
| ABE8e | TadA8e | nCas9 | [23] | ||
| PhieABEs | TadA8e | nCas9-NG | The editing window is extensive | [24] | |
| Dual base editor | Edit K–G–-T to T–A and A–T to George-K at the same time | ||||
| ACBE | PmCDA1 ecTadA:ecTadA | nCas9 | [25] | ||
| SPACE | PmCDA1 ecTadA* | nCas9 | [26] | ||
| STEMEs | hAPOBEC3A ecTadA:ecTadA* | nCas9 | The efficiency of simultaneous editing of C–G to T–A and A–T to G–C is up to more than 15%. | [14] | |
| pDuBE1 | TadA8e | nCas9 | In rice, there is a co-editing efficiency of up to 49.7%. | [27] | |
| Glycosylase base editor | Conversion between purines and pyrimidines | ||||
| GBE (AID-nCas9-Ung) | AID | nCas9 | Convert C to A | [15] | |
| GBE (APOBEC-nCas9-Ung) | APOBEC1 | nCas9 | Convert C to G | [15] | |
| CGBE1 | APOBEC1 (R33A) | nCas9 | Low off-target rate | [28] | |
| AYBE | ABE8e | nCas9 | A to T and A to C conversions | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhu, P.; Gui, J. Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus. Int. J. Mol. Sci. 2024, 25, 8314. https://doi.org/10.3390/ijms25158314
Yang X, Zhu P, Gui J. Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus. International Journal of Molecular Sciences. 2024; 25(15):8314. https://doi.org/10.3390/ijms25158314
Chicago/Turabian StyleYang, Xuefei, Ping Zhu, and Jinshan Gui. 2024. "Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus" International Journal of Molecular Sciences 25, no. 15: 8314. https://doi.org/10.3390/ijms25158314
APA StyleYang, X., Zhu, P., & Gui, J. (2024). Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus. International Journal of Molecular Sciences, 25(15), 8314. https://doi.org/10.3390/ijms25158314





