Molecular Modification Enhances Xylose Uptake by the Sugar Transporter KM_SUT5 of Kluyveromyces marxianus
Abstract
:1. Introduction
2. Results
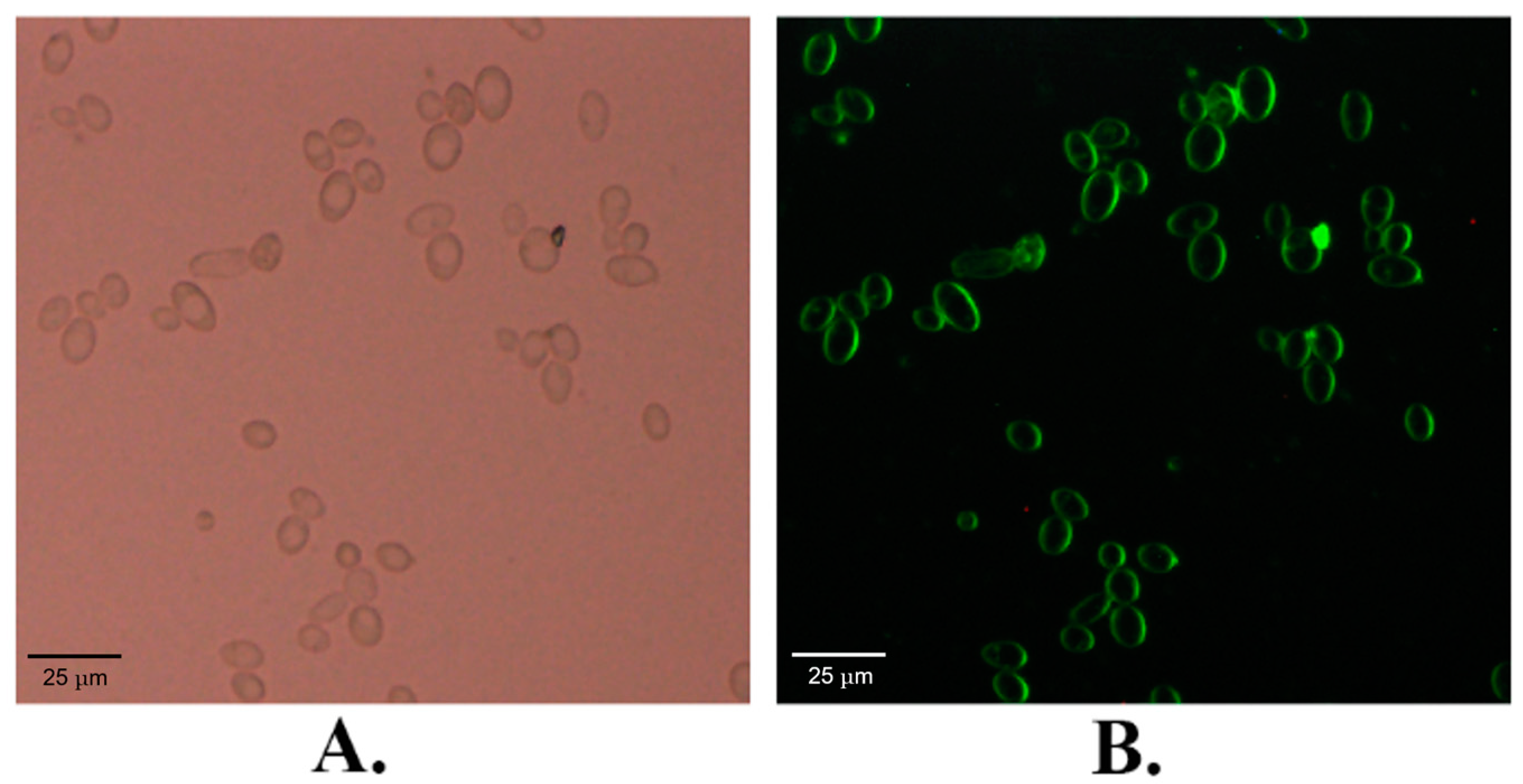
2.1. The Subcellular Localization of KM_SUT5p
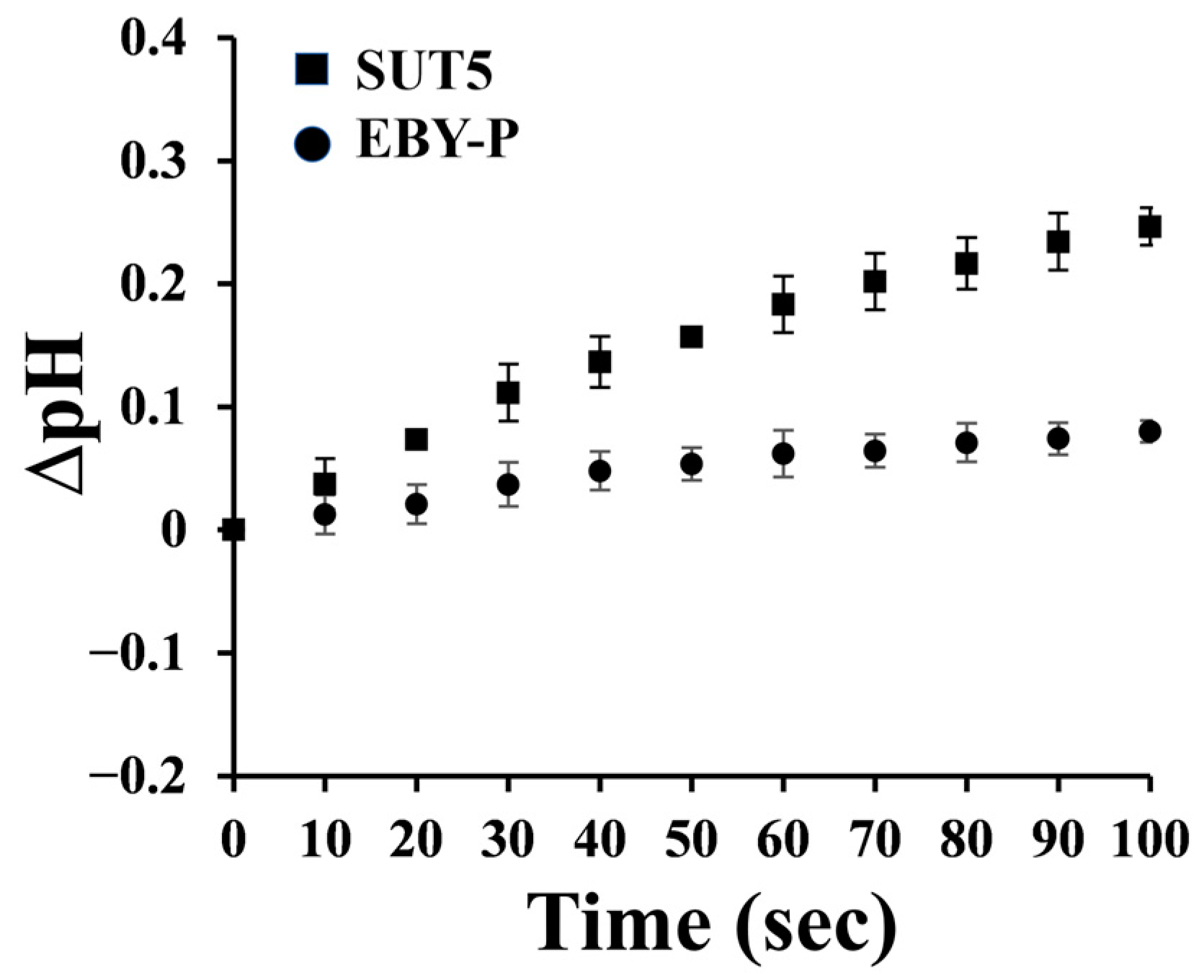
2.2. The Symporter Assay of KM_SUT5p
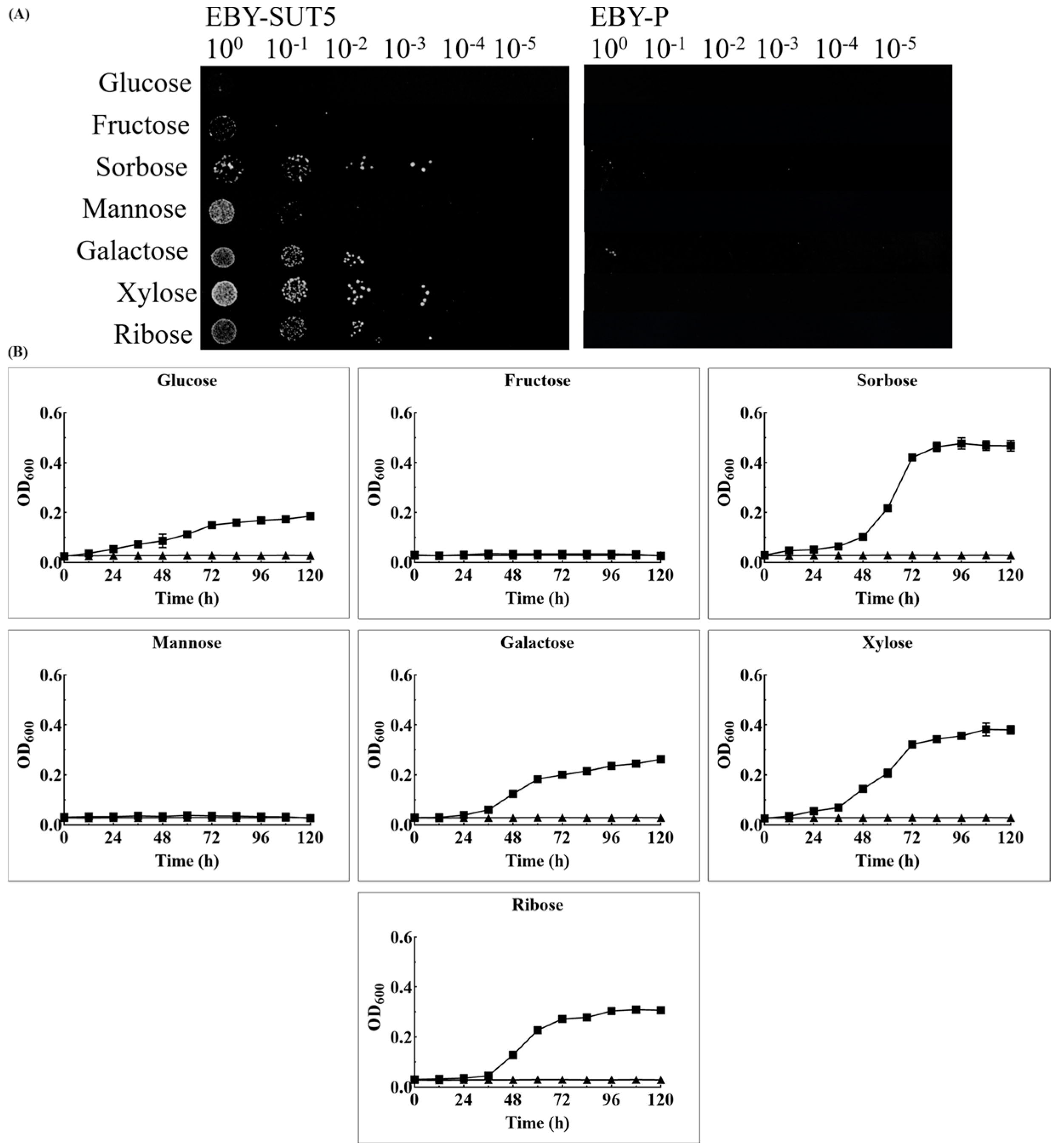
2.3. The Characteristics of KM_SUT5p for Sugar Transportation in EBY-XYL
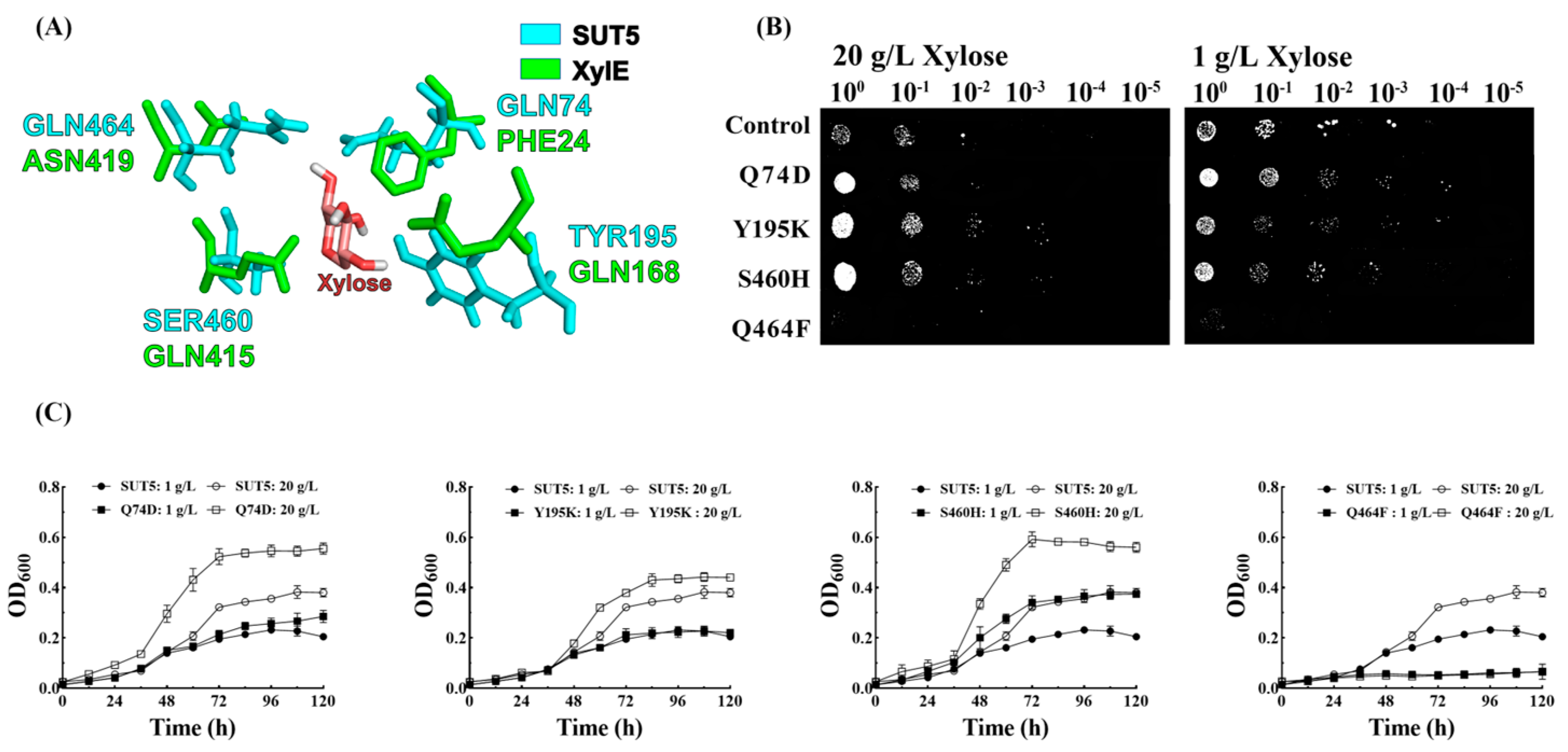
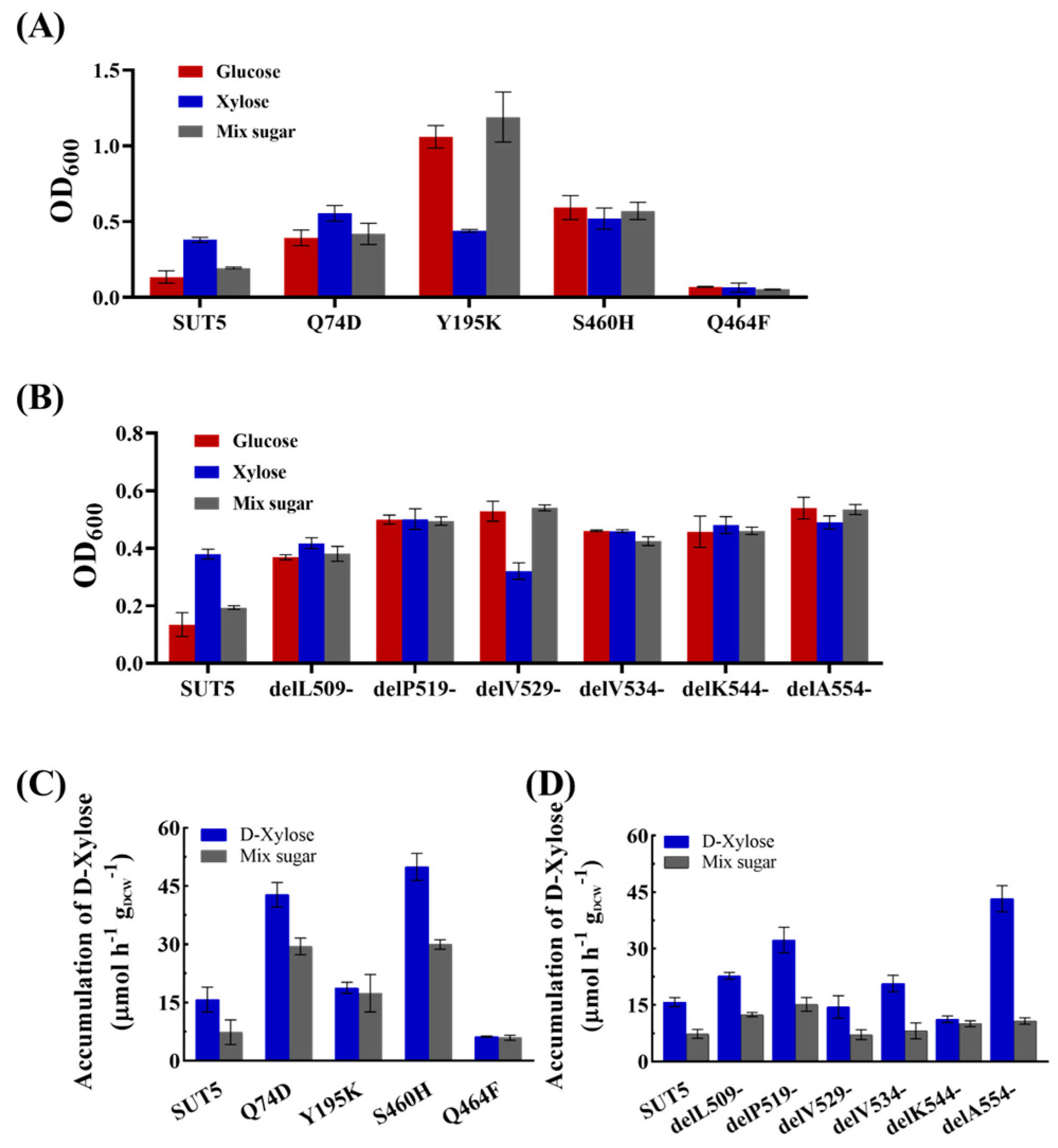
2.4. Effect of the Mutations in the Amino Acid Site of KM_SUT5p on the Affinity for Xylose
2.5. Effect of the C-Terminal Truncation of KM_SUT5p on the Affinity for Xylose
2.6. Effect of Mutations in the Amino Acid Site and C-Terminal Truncation of KM_SUT5p on D-Xylose Transport
3. Discussion
4. Materials and Methods
4.1. Plasmid and Strains
4.2. Cultivation Conditions and Growth Test
4.3. Fluorescence Localization
4.4. Symporter Assay
4.5. Molecular Docking
4.6. Molecular Modification
4.7. Transport Rate of D-Xylose
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, C.S.; Cao, N.J.; Du, J.; Tsao, G.T. Ethanol production from renewable resources. Adv. Biochem. Eng. Biotechnol. 1999, 65, 207–241. [Google Scholar] [CrossRef]
- Gray, K.A.; Zhao, L.; Emptage, M. Bioethanol. Curr. Opin. Chem. Biol. 2006, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, A.; Tamalampudi, S.; Srivastava, A.; Fukuda, H.; Bisaria, V.S.; Kondo, A. Alcoholic fermentation of xylose and mixed sugars using recombinant Saccharomyces cerevisiae engineered for xylose utilization. Appl. Microbiol. Biotechnol. 2009, 82, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, A.; Penttilä, M. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 2000, 11, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.L.; Matsushika, A.; de Sales, B.B.; Goshima, T.; Bon, E.P.; Stambuk, B.U. Xylose and xylose/glucose co-fermentation by recombinant Saccharomyces cerevisiae strains expressing individual hexose transporters. Enzyme Microb. Technol. 2014, 63, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gárdonyi, M.; Osterberg, M.; Rodrigues, C.; Spencer-Martins, I.; Hahn-Hägerdal, B. High capacity xylose transport in Candida intermedia PYCC 4715. FEMS Yeast Res. 2003, 3, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Parachin, N.S.; Bergdahl, B.; van Niel, E.W.; Gorwa-Grauslund, M.F. Kinetic modelling reveals current limitations in the production of ethanol from xylose by recombinant Saccharomyces cerevisiae. Metab. Eng. 2011, 13, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Y.; Hou, J.; Suo, F.; Bao, X. An assay for functional xylose transporters in Saccharomyces cerevisiae. Anal. Biochem. 2013, 442, 241–248. [Google Scholar] [CrossRef]
- Du, J.; Li, S.; Zhao, H. Discovery and characterization of novel d-xylose-specific transporters from Neurospora crassa and Pichia stipitis. Mol. Biosyst. 2010, 6, 2150–2156. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Y.; Gu, L.; Wang, Z.; Su, N.; Niu, K.; Guo, W.; Hou, S.; Bao, X.; Tian, C.; et al. Identification and Characterization of an Efficient d-Xylose Transporter in Saccharomyces cerevisiae. J. Agric. Food Chem. 2020, 68, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Tamayo-Ramos, J.A.; Odoni, D.I.; Laothanachareon, T.; Derntl, C.; Mach-Aigner, A.R.; Martins Dos Santos, V.A.P.; Schaap, P.J. Identification and functional characterization of novel xylose transporters from the cell factories Aspergillus niger and Trichoderma reesei. Biotechnol. Biofuels 2016, 9, 148. [Google Scholar] [CrossRef]
- Leandro, M.J.; Goncalves, P.; Spencer-Martins, I. Two glucose/xylose transporter genes from the yeast Candida intermedia: First molecular characterization of a yeast xylose-H+ symporter. Biochem. J. 2006, 395, 543–549. [Google Scholar] [CrossRef]
- Nijland, J.G.; Shin, H.Y.; de Waal, P.P.; Klaassen, P.; Driessen, A.J.M. Increased xylose affinity of Hxt2 through gene shuffling of hexose transporters in Saccharomyces cerevisiae. J. Appl. Microbiol. 2018, 124, 503–510. [Google Scholar] [CrossRef]
- Reznicek, O.; Facey, S.J.; de Waal, P.P.; Teunissen, A.W.; de Bont, J.A.; Nijland, J.G.; Driessen, A.J.; Hauer, B. Improved xylose uptake in Saccharomyces cerevisiae due to directed evolution of galactose permease Gal2 for sugar co-consumption. J. Appl. Microbiol. 2015, 119, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Nijland, J.G.; Shin, H.Y.; de Jong, R.M.; de Waal, P.P.; Klaassen, P.; Driessen, A.J.M. Engineering of an endogenous hexose transporter into a specific D-xylose transporter facilitates glucose-xylose co-consumption in Saccharomyces cerevisiae. Biotechnol. Biofuels 2014, 7, 168. [Google Scholar] [CrossRef]
- Young, E.M.; Comer, A.D.; Huang, H.; Alper, H.S. A molecular transporter engineering approach to improving xylose catabolism in Saccharomyces cerevisiae. Metab. Eng. 2012, 14, 401–411. [Google Scholar] [CrossRef]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Schmitz, O.; Alper, H.S. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter. Appl. Microbiol. Biotechnol. 2016, 100, 10215–10223. [Google Scholar] [CrossRef]
- Rodrussamee, N.; Lertwattanasakul, N.; Hirata, K.; Suprayogi; Limtong, S.; Kosaka, T.; Yamada, M. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2011, 90, 1573–1586. [Google Scholar] [CrossRef]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Lertwattanasakul, N.; Kosaka, T.; Hosoyama, A.; Suzuki, Y.; Rodrussamee, N.; Matsutani, M.; Murata, M.; Fujimoto, N.; Suprayogi; Tsuchikane, K.; et al. Genetic basis of the highly efficient yeast Kluyveromyces marxianus: Complete genome sequence and transcriptome analyses. Biotechnol. Biofuels 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kim, Y.B.; Cho, K.H.; Kim, J.H. Glucose starvation-induced turnover of the yeast glucose transporter Hxt1. Biochim. Et Biophys. Acta 2014, 1840, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Tamayo Rojas, S.A.; Schmidl, S.; Boles, E.; Oreb, M. Glucose-induced internalization of the S. cerevisiae galactose permease Gal2 is dependent on phosphorylation and ubiquitination of its aminoterminal cytoplasmic tail. FEMS Yeast Res. 2021, 21, foab019. [Google Scholar] [CrossRef]
- Diezemann, A.; Boles, E. Functional characterization of the Frt1 sugar transporter and of fructose uptake in Kluyveromyces lactis. Curr. Genet. 2003, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bao, X.; Li, Y.; Jiao, C.; Hou, J.; Zhang, Q.; Zhang, W.; Liu, W.; Shen, Y. Cloning and characterization of heterologous transporters in Saccharomyces cerevisiae and identification of important amino acids for xylose utilization. Metab. Eng. 2015, 30, 79–88. [Google Scholar] [CrossRef]
- Mueckler, M.; Weng, W.; Kruse, M. Glutamine 161 of Glut1 glucose transporter is critical for transport activity and exofacial ligand binding. J. Biol. Chem. 1994, 269, 20533–20538. [Google Scholar] [CrossRef]
- Díez-Sampedro, A.; Barcelona, S. Sugar binding residue affects apparent Na+ affinity and transport stoichiometry in mouse sodium/glucose cotransporter type 3B. J. Biol. Chem. 2011, 286, 7975–7982. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Bera, A.K. Mutational effects of Pannexin 1 R217H depend on the carboxyl-terminus. Biochem. Biophys. Res. Commun. 2021, 548, 143–147. [Google Scholar] [CrossRef]
- Wang, M.; Yu, C.; Zhao, H. Directed evolution of xylose specific transporters to facilitate glucose-xylose co-utilization. Biotechnol. Bioeng. 2016, 113, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Latimer, L.N.; Lee, M.E.; Medina-Cleghorn, D.; Kohnz, R.A.; Nomura, D.K.; Dueber, J.E. Employing a combinatorial expression approach to characterize xylose utilization in Saccharomyces cerevisiae. Metab. Eng. 2014, 25, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhao, H.; Zhao, H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 2009, 37, e16. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.W.; Liang, J.J.; Qin, X.J.; Wang, J.R.; Feng, J.X.; Huang, R.B. Multiple induced mutagenesis for improvement of ethanol production by Kluyveromyces marxianus. Biotechnol. Lett. 2010, 32, 1847–1851. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.-P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8; Schrodinger, LLC.: New York, NY, USA, 2015. [Google Scholar]
- Wang, W.; Chen, F.; Zheng, F.; Russell, B.T. Optimization of synthesis of carbohydrates and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology (RSM) for improved carbohydrate detection. Food Chem. 2020, 309, 125686. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Tao, X.; Ran, G.; Deng, Y.; Wang, H.; Tan, L.; Pang, Z. Molecular Modification Enhances Xylose Uptake by the Sugar Transporter KM_SUT5 of Kluyveromyces marxianus. Int. J. Mol. Sci. 2024, 25, 8322. https://doi.org/10.3390/ijms25158322
Luo X, Tao X, Ran G, Deng Y, Wang H, Tan L, Pang Z. Molecular Modification Enhances Xylose Uptake by the Sugar Transporter KM_SUT5 of Kluyveromyces marxianus. International Journal of Molecular Sciences. 2024; 25(15):8322. https://doi.org/10.3390/ijms25158322
Chicago/Turabian StyleLuo, Xiuyuan, Xi Tao, Guangyao Ran, Yuanzhen Deng, Huanyuan Wang, Liyan Tan, and Zongwen Pang. 2024. "Molecular Modification Enhances Xylose Uptake by the Sugar Transporter KM_SUT5 of Kluyveromyces marxianus" International Journal of Molecular Sciences 25, no. 15: 8322. https://doi.org/10.3390/ijms25158322





