Propagermanium as a Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Animal Models
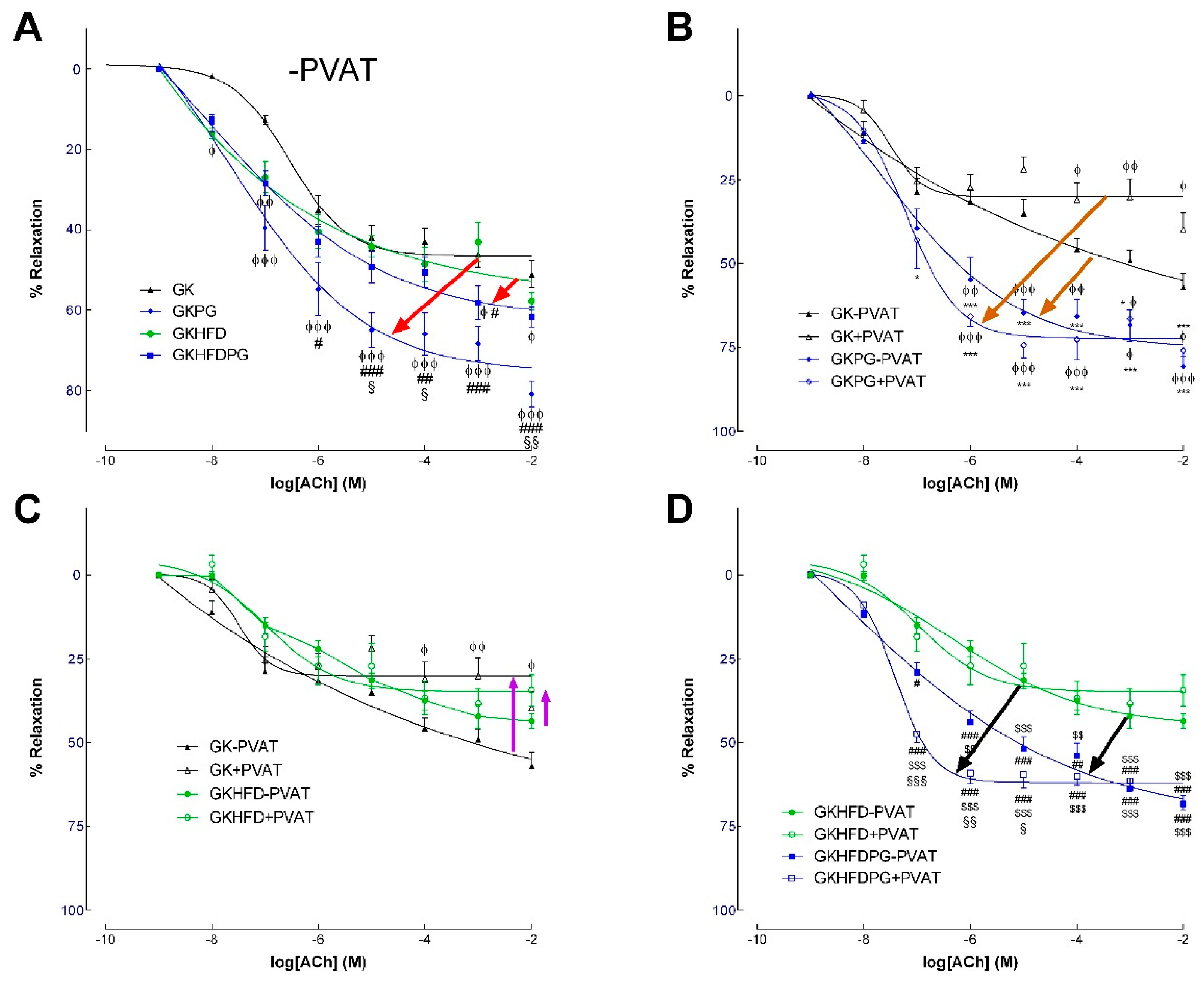
2.2. Vascular Relaxation in the Rat Aorta Dependent on NO
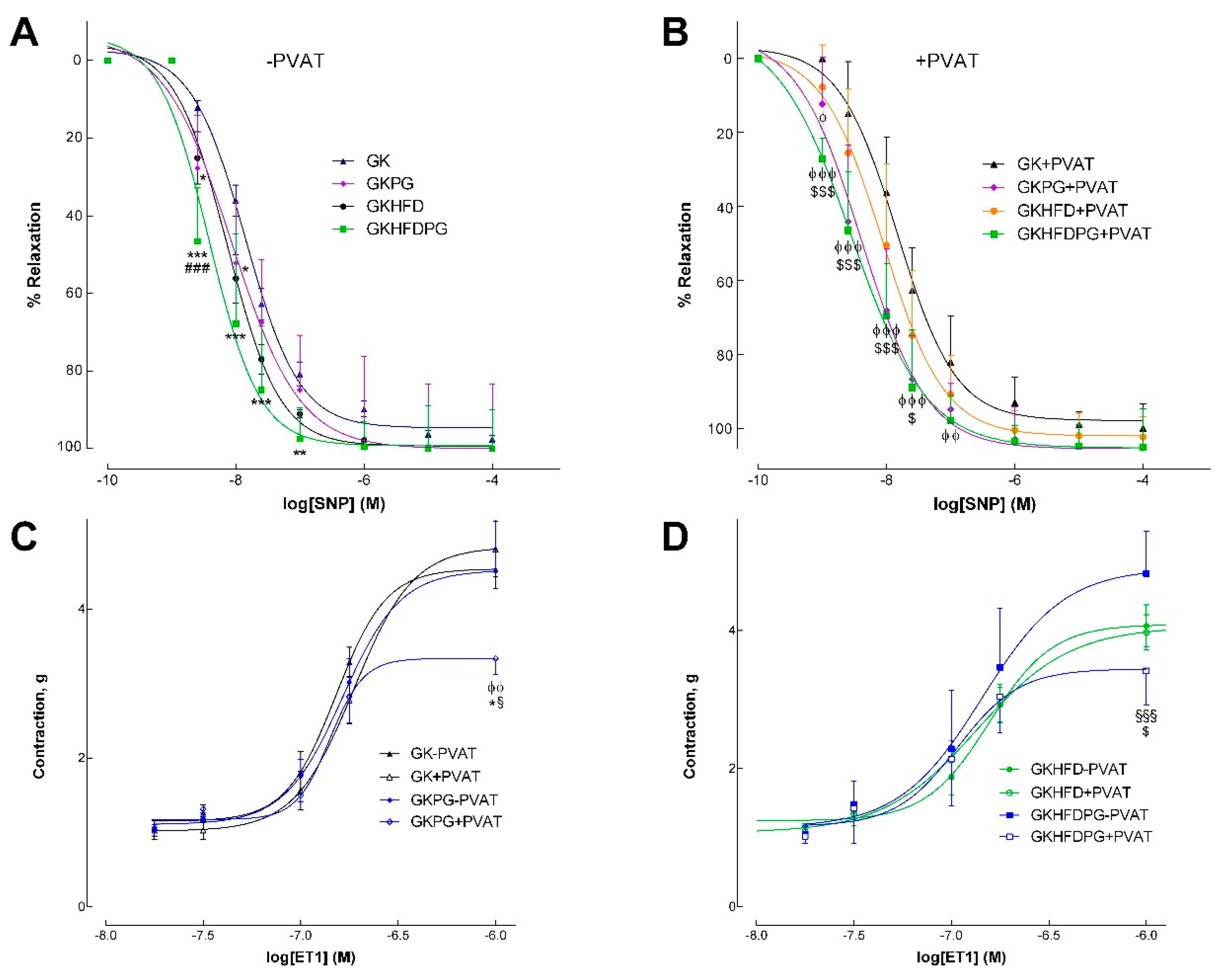
2.3. Response of the Arteries to Endothelin–1
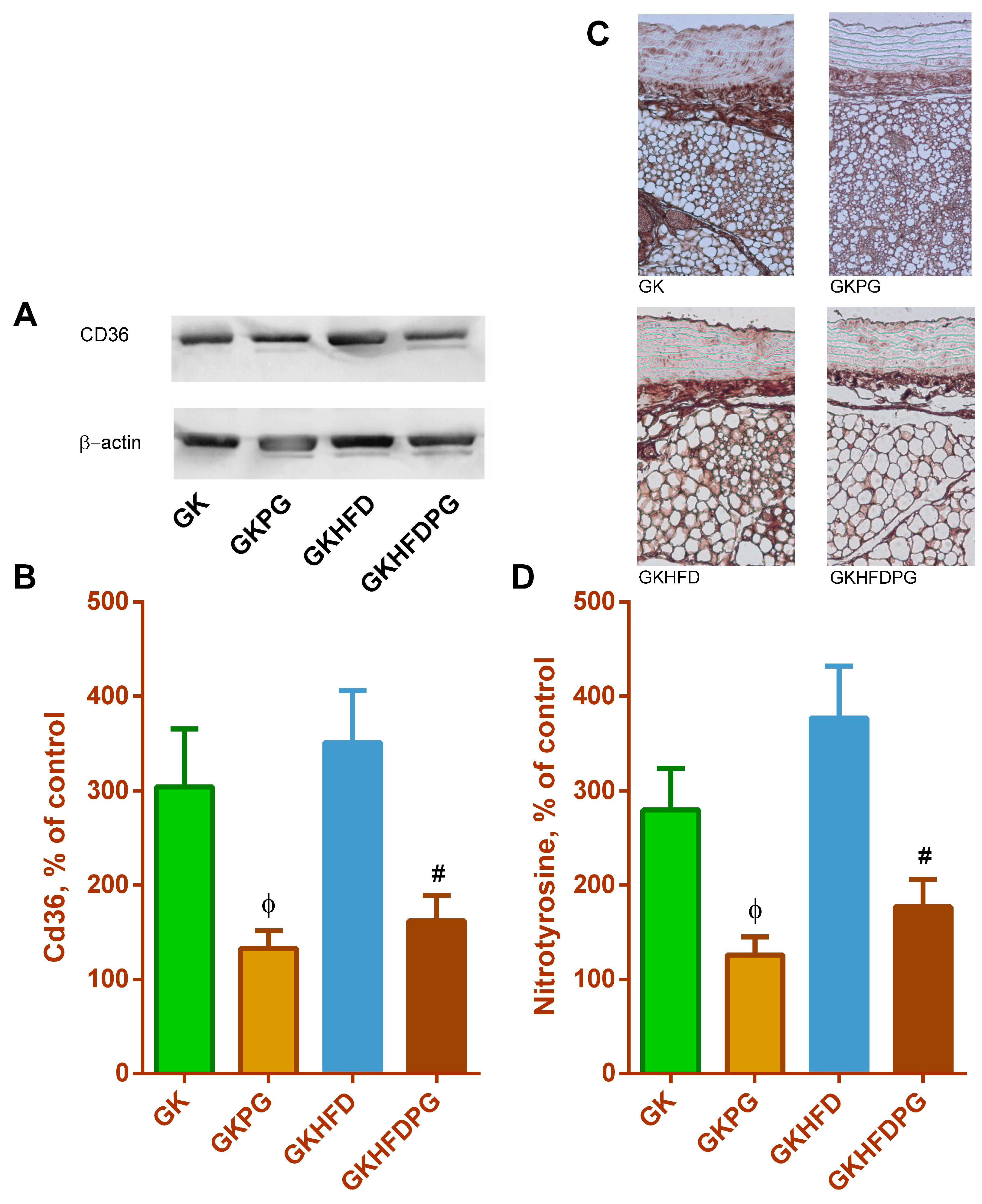
2.4. Vascular Wall Inflammation
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Animals
4.3. Glucose and Insulin Tolerance Tests and Lipid Profile
4.4. Isometric Tension Studies
4.5. Analysis Using Western Blot
4.6. Histology
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stehouwer, C.D.; Henry, R.M.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef]
- Okada, S.; Hiuge, A.; Makino, H.; Nagumo, A.; Takaki, H.; Konishi, H.; Goto, Y.; Yoshimasa, Y.; Miyamoto, Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J. Atheroscl. Thromb. 2010, 17, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Fernandes, R.; Seiça, R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011, 163, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Suganami, T.; Yamauchi, A.; Degawa-Yamauchi, M.; Tanaka, M.; Kouyama, R.; Kobayashi, Y.; Nitta, N.; Yasuda, K.; Hirata, Y.; et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J. Biol. Chem. 2008, 283, 35715–35723. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Morinaga, H.; Talukdar, S.; Bae, E.J.; Olefsky, J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012, 61, 346–354. [Google Scholar] [CrossRef]
- Di Prospero, N.A.; Artis, E.; Andrade-Gordon, P.; Johnson, D.L.; Vaccaro, N.; Xi, L.; Rothenberg, P. CCR2 antagonism in patients with type 2 diabetes mellitus: A randomized, placebo-controlled study. Diabetes Obes. Metab. 2014, 16, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Suzuki, H.; Ito, M.; Okumura, M.; Oda, T. Propagermanium: A nonspecific immune modulator for chronic hepatitis B. J. Gastroenterol. 2003, 38, 525–532. [Google Scholar] [CrossRef]
- Yokochi, S.; Hashimoto, H.; Ishiwata, Y.; Shimokawa, H.; Haino, M.; Terashima, Y.; Matsushima, K. An anti-inflammatory drug, propagermanium, may target GPI-anchored proteins associated with an MCP–1 receptor, CCR2. J. Interferon Cytokine Res. 2001, 21, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kawashima, S.; Ozaki, M.; Namiki, M.; Inoue, N.; Hirata, K.; Yokoyama, M. Propagermanium reduces atherosclerosis in apolipoprotein E knockout mice via inhibition of macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; van den Hoek, A.M.; Kleemann, R. The CCR57 inhibitor propagermanium attenuates diet-induced insulin resistance, adipose tissue inflammation and non-alcoholic steatohepatitis. PLoS ONE 2017, 12, e0169740. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Sugimoto, M.; Murayama, T.; Minami, M.; Nishikaze, Y.; Ariyasu, H.; Akamizu, T.; Kita, T.; Yokode, M.; Arai, H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J. Atheroscler. Thromb. 2010, 17, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Sakai, N.; Furuichi, F.; Sakai, Y.; Takeya, M.; Bucala, R.; Mukaida, N.; Takuwa, Y.; Matsushima, K.; Kaneko, S.; et al. CCL2/CCR2 augments the production of transforming growth factor-beta1, type 1 collagen and CCL2 by human CD45-/collagen 1-positive cells under high glucose concentrations. Clin. Exp. Nephrol. 2013, 17, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.H.; Meehan, D.T.; Delimont, D.; Nakajima, M.; Wada, T.; Gratton, M.A.; Cosgrove, D. Role for macrophage metalloelastase in glomerular basement membrane damage associated with alport syndrome. Am. J. Pathol. 2006, 169, 32–46. [Google Scholar] [CrossRef]
- Tamura, Y.; Sugimoto, M.; Murayama, T.; Ueda, Y.; Kanamori, H.; Ono, K.; Ariyasu, H.; Akamizu, T.; Kita, T.; Yokode, M.; et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Eto, Y.; Miyata, K.; Morishige, K.; Kandabashi, T.; Matsushima, K.; Takeshita, A. Propagermanium suppresses macrophage-mediated formation of coronary arteriosclerotic lesions in pigs in vivo. J. Cardiovasc. Pharmacol. 2003, 41, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Eto, Y.; Shimokawa, H.; Tanaka, E.; Morishige, K.; Fuchigami, M.; Ishiwata, Y.; Matsushima, K.; Takeshita, A. Long-term Treatment with Propagermanium Suppresses Atherosclerosis in WHHL Rabbits. J. Cardiovasc. Pharmacol. 2003, 41, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Wada, T.; Furuichi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Takeya, M.; Kuziel, W.A.; Matsushima, K.; Mukaida, N.; et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 2004, 165, 237–246. [Google Scholar] [CrossRef]
- Bot, I.; Ortiz Zacarías, N.V.; de Witte, W.E.; de Vries, H.; van Santbrink, P.J.; van der Velden, D.; Kröner, M.J.; van der Berg, D.J.; Stamos, D.; de Lange, E.C.; et al. A novel CCR2 antagonist inhibits atherogenesis in apoE deficient mice by achieving high receptor occupancy. Sci. Rep. 2017, 7, 52. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Matsubara, T.; Mima, A.; Sumi, E.; Nagai, K.; Takahashi, T.; Abe, H.; Iehara, N.; Fukatsu, A.; Okamoto, H.; et al. Inhibition of MCP–1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem. Biophys. Res. Commun. 2007, 360, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Wada, T.; Iwata, Y.; Kitagawa, K.; Kobayashi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Wang, H.; Matsushima, K.; et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J. Am. Soc. Nephrol. 2003, 14, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, R.; Li, B.; Huang, L.; Fan, W.; Tembachako, C.R.; Zheng, X.; Xiong, X.; Miyata, M.; Xu, B.; et al. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019, 125, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Silvestre-Roig, C.; Ortega-Gomez, A.; Lemnitzer, P.; Poelman, H.; Schumski, A.; Winter, J.; Drechsler, M.; de Jong, R.; Immler, R.; et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018, 28, 175–182.e5. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge–132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y. Effect of germanium–132 on low-density lipoprotein oxidation and atherosclerosis in Kurosawa and Kusanagi hypercholesterolemic rabbits. Biosci. Biotechnol. Biochem. 2001, 65, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Gato, S.; García-Fernández, V.; Gil-Gómez, A.; Rojas, Á.; Montero-Vallejo, R.; Muñoz-Hernández, R.; Romero-Gómez, M. Navigating the Link Between Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis and Cardiometabolic Syndrome. Eur. Cardiol. 2024, 19, e03. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ghosh, S.; Perrard, X.D.; Feng, L.; Garcia, G.E.; Perrard, J.L.; Sweeney, J.F.; Peterson, L.E.; Chan, L.; Smith, C.W.; et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007, 115, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Toschi, E.; Baldeweg, S.; Ciociaro, D.; Favilla, S.; Saccà, L.; Ferrannini, E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes 2006, 55, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular Adipose Tissue as a Target for Antioxidant Therapy for Cardiovascular Complications. Antioxidants 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Bakos, E.; Thaiss, C.A.; Kramer, M.P.; Cohen, S.; Radomir, L.; Orr, I.; Kaushansky, N.; Ben-Nun, A.; Becker-Herman, S.; Shachar, I. CCR2 Regulates the Immune Response by Modulating the Interconversion and Function of Effector and Regulatory T Cells. J. Immunol. 2017, 198, 4659–4671. [Google Scholar] [CrossRef] [PubMed]
- Boring, L.; Gosling, J.; Chensue, S.W.; Kunkel, S.L.; Farese, R.V.J., Jr.; Broxmeyer, H.E.; Charo, I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 1997, 100, 2552–2561. [Google Scholar] [CrossRef]
- Kurihara, T.; Warr, G.; Loy, J.; Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997, 186, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Fuchigami, M.; Suzuki, T.; Watanabe, N. A novel C-C chemokine receptor 2 antagonist prevents progression of albuminuria and atherosclerosis in mouse models. Biol. Pharm. Bull. 2012, 35, 2069–2074. [Google Scholar] [CrossRef]
- Aiello, R.J.; Perry, B.D.; Bourassa, P.A.; Robertson, A.; Weng, W.; Knight, D.R.; Smith, A.H.; Frederick, K.S.; Kalgutkar, A.; Gladue, R.P. CCR2 receptor blockade alters blood monocyte subpopulations but does not affect atherosclerotic lesions in apoE−/− mice. Atherosclerosis 2010, 208, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Olzinski, A.R.; Turner, G.H.; Bernard, R.E.; Karr, H.; Cornejo, C.A.; Aravindhan, K.; Hoang, B.; Ringenberg, M.A.; Qin, P.; Goodman, K.B.; et al. Pharmacological inhibition of C-C chemokine receptor 2 decreases macrophage infiltration in the aortic root of the human C-C chemokine receptor 2/apolipoprotein E−/− mouse: Magnetic resonance imaging assessment. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Keliher, E.J.; Heidt, T.; Leuschner, F.; Truelove, J.; Sena, B.F.; Gorbatov, R.; Iwamoto, Y.; Dutta, P.; Wojtkiewicz, G.; et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013, 127, 2038–2046. [Google Scholar] [CrossRef] [PubMed]

| GK | GKPG | GKHFD | GKHFDPG | |
|---|---|---|---|---|
| Body weight (g) | 383 ± 4.5 | 386.1 ± 6.5 | 401.9 ± 3.7 ϕ | 417.6 ± 9.4 ϕϕ |
| Fasting glucose (mg/dL) | 96.6 ± 3.3 | 90 ± 3.2 | 99 ± 3.1 | 81.5 ± 3.5 ϕϕ §§§ ## |
| Triglycerides (mg/dL) | 143.1 ± 4.3 | 143.2 ± 5.4 | 168.9 ± 10.2 ϕϕ | 141.0 ± 9.2 # |
| Total cholesterol (mg/dL) | 157.7 ± 1.3 | 158.8 ± 1.4 | 168.7 ± 4.5 ϕ | 203.6 ± 11.2 ϕϕ ## |
| GK−PVAT | GK+PVAT | GKPG−PVAT | GKPG+ PVAT | GKHFD−PVAT | GKHFD+ PVAT | GKHFDPG−PVAT | GKHFDPG+ PVAT | |
|---|---|---|---|---|---|---|---|---|
| ACh | ||||||||
| pEC50 | 6.5 ± 0.14 | 6.2 ± 0.26 | 7.69 ± 0.8 | 7.2 ± 0.17 | 6.35 ± 0.22 | 6.97 ± 0.23 | 8.6 ± 1.5 | 7.41 ± 0.09 |
| Maximal relaxation (%) | 52.6 ± 5.1 | 34.04 ± 6.2 ϕ | 75.6 ± 4.6 ϕ *** | 72.5 ± 6.8 ϕ *** | 46.6 ± 3.1 | 35.04 ± 2.3 ϕ | 73.4 ± 5.1 ϕ ** ## $ | 62.03 ± 1.3 ϕ ** ## $ |
| SNP | ||||||||
| pEC50 | 7.81 ± 0.09 | 7.7 ± 0.1 | 8.07 ± 0.1 | 8.38 ± 0.07 ϕϕ *** | 8.01 ± 0.06 | 8.04 ± 0.1 | 8.7 ± 0.07 ϕϕϕ *** ### $$$ | 8.54 ± 0.09 ϕϕϕ *** ## $$ |
| Maximal relaxation (%) | 94.6 ± 3.09 | 99.9 ± 1.92 | 99.9 ± 3.1 | 94.4 ± 2.18 | 99.3 ± 3.2 | 102.1 ± 1.92 | 96.04 ± 2.4 | 94.7 ± 1.84 |
| ET1 | ||||||||
| pEC50 | 7.02 ± 0.04 | 6.96 ± 0.04 | 6.78 ± 0.07 | 6.85 ± 0.05 | 6.98 ± 0.04 | 6.96 ± 0.04 | 6.84 ± 0.05 | 6.96 ± 0.08 |
| Maximal contraction (g) | 4.57 ± 0.23 | 4.82 ± 0.3 | 4.52 ± 0.2 | 3.34 ± 0.19 ϕϕ *** | 4.57 ± 0.23 | 4.82 ± 0.3 | 4.89 ± 0.25 | 3.44 ± 0.17 ϕϕ # $$ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azul, L.; Leandro, A.; Seiça, R.; Sena, C.M. Propagermanium as a Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 8328. https://doi.org/10.3390/ijms25158328
Azul L, Leandro A, Seiça R, Sena CM. Propagermanium as a Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes. International Journal of Molecular Sciences. 2024; 25(15):8328. https://doi.org/10.3390/ijms25158328
Chicago/Turabian StyleAzul, Lara, Adriana Leandro, Raquel Seiça, and Cristina M. Sena. 2024. "Propagermanium as a Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes" International Journal of Molecular Sciences 25, no. 15: 8328. https://doi.org/10.3390/ijms25158328






