Low-Penetrance Susceptibility Variants in Colorectal Cancer—Current Outlook in the Field
Abstract
:1. Introduction
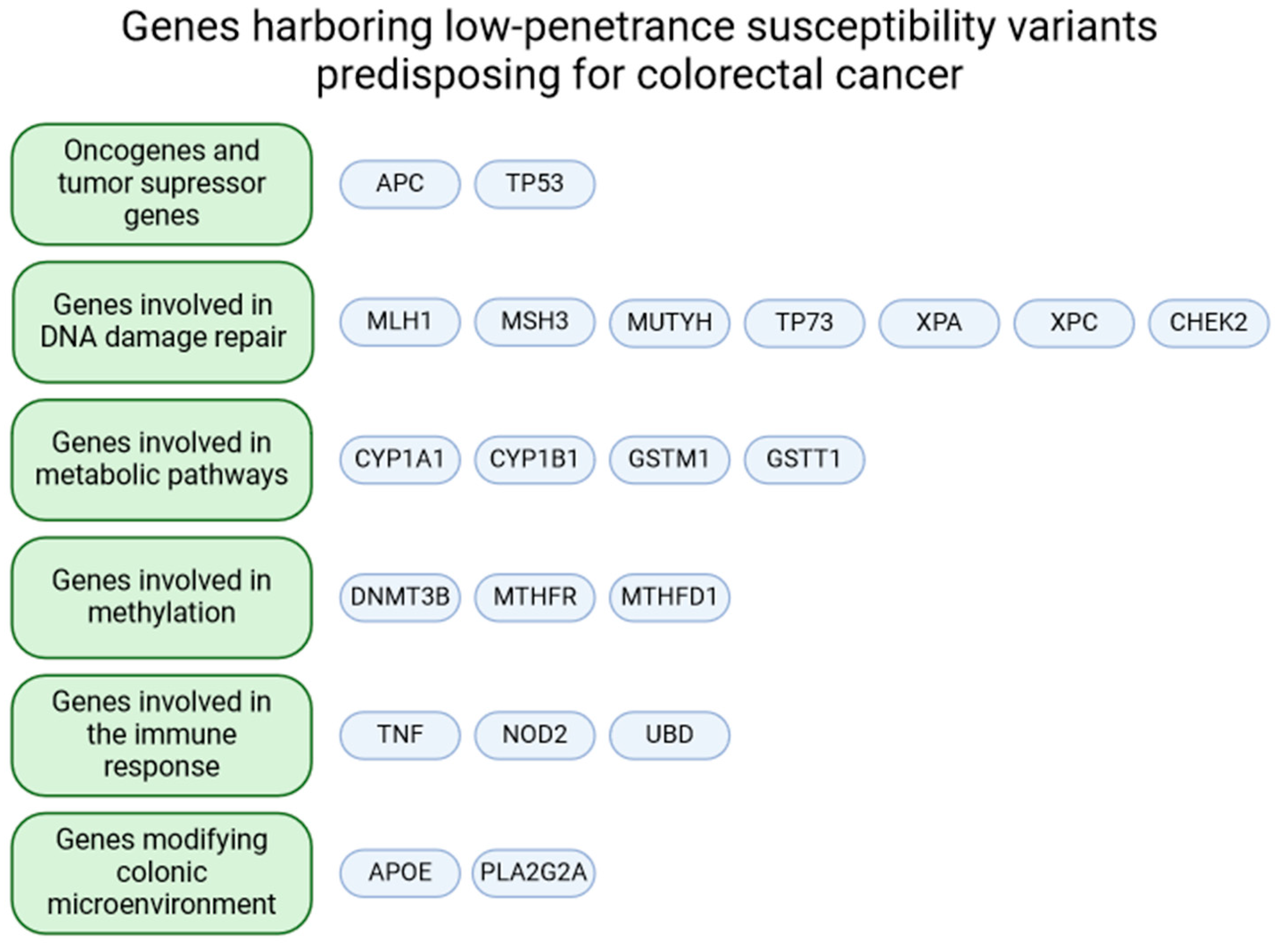
2. Genes of Interest
2.1. Oncogenes and Tumor Suppressor Genes
2.1.1. APC
2.1.2. TP53
2.2. Genes Involved in DNA Damage Repair
2.2.1. MLH1
2.2.2. MSH3
2.2.3. MUTYH
2.2.4. TP73
2.2.5. XPA
2.2.6. XPC
2.2.7. CHEK2
2.3. Genes Involved in Metabolic Pathways
2.3.1. CYP1A1
2.3.2. CYP1B1
2.3.3. GSTM1 and GSTT1
2.4. Genes Involved in Methylation
2.4.1. DNMT3B
2.4.2. MTHFR
2.4.3. MTHFD1
2.5. Genes Involved in the Immune Response
2.5.1. TNF
2.5.2. NOD2
2.5.3. UBD
2.6. Genes Modifying Colonic Microenvironment
2.6.1. APOE
2.6.2. PLA2G2A
3. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Montazeri, Z.; Li, X.; Nyiraneza, C.; Ma, X.; Timofeeva, M.; Svinti, V.; Meng, X.; He, Y.; Bo, Y.; Morgan, S.; et al. Systematic meta-analyses, field synopsis and global assessment of the evidence of genetic association studies in colorectal cancer. Gut 2020, 69, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Montazeri, Z.; Hawken, S.; Allum, G.C.; Gong, J.; Tait, V.; Kirac, I.; Tazari, M.; Farrington, S.M.; Demarsh, A.; et al. Systematic meta-analyses and field synopsis of genetic association studies in colorectal cancer. J. Natl. Cancer Inst. 2012, 104, 1433–1457. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Zheng, W. Genetic variants associated with colorectal cancer risk: Comprehensive research synopsis, meta-analysis, and epidemiological evidence. Gut 2014, 63, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Bisgaard, M.L.; Fenger, K.; Bülow, S.; Niebuhr, E.; Mohr, J. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum. Mutat. 1994, 3, 121–125. [Google Scholar] [CrossRef]
- Friedl, W.; Aretz, S. Familial Adenomatous Polyposis: Experience from a Study of 1164 Unrelated German Polyposis Patients. Hered. Cancer Clin. Pract. 2005, 3, 95–114. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Woodage, T.; King, S.M.; Wacholder, S.; Hartge, P.; Struewing, J.P.; McAdams, M.; Laken, S.J.; Tucker, M.A.; Brody, L.C. The APC I1307K allele and cancer risk in a community-based study of Ashkenazi Jews. Nat. Genet. 1998, 20, 62–65. [Google Scholar] [CrossRef]
- Rozen, P.; Naiman, T.; Strul, H.; Taussky, P.; Karminsky, N.; Shomrat, R.; Samuel, Z.; Yaron, Y.; Orr-Urtreger, A. Clinical and screening implications of the I1307K adenomatous polyposis coli gene variant in Israeli Ashkenazi Jews with familial colorectal neoplasia. Cancer 2002, 94, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Sella, T.; Liberman, E.; Shapira, S.; David, M.; Kazanov, D.; Arber, N.; Kraus, S. The APC p.I1307K polymorphism is a significant risk factor for CRC in average risk Ashkenazi Jews. Eur. J. Cancer 2013, 49, 3680–3685. [Google Scholar] [CrossRef] [PubMed]
- Surget, S.; Khoury, M.P.; Bourdon, J.-C. Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. OncoTargets Ther. 2013, 7, 57–68. [Google Scholar] [CrossRef]
- Khan, M.H.; Khalil, A.; Rashid, H. Evaluation of the p53 Arg72Pro polymorphism and its association with cancer risk: A HuGE review and meta-analysis. Genet. Res. 2015, 97, e7. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Shanaehbandi, D.; Zarintan, A.; Pedram, N.; Baradaran, B.; Zafari, V.; Shirmohamadi, M.; Hashemzadeh, S. TP53 Gene Pro72Arg (rs1042522) Single Nucleotide Polymorphism as Not a Risk Factor for Colorectal Cancer in the Iranian Azari Population. Asian Pac. J. Cancer Prev. 2017, 18, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; Kandil, E.; Fawzy, M.S. Genetic polymorphisms of TP53 (rs1042522) and MDM2 (rs2279744) and colorectal cancer risk: An updated meta-analysis based on 59 case-control studies. Gene 2020, 734, 144391. [Google Scholar] [CrossRef]
- Raptis, S.; Mrkonjic, M.; Green, R.C.; Pethe, V.V.; Monga, N.; Chan, Y.M.; Daftary, D.; Dicks, E.; Younghusband, B.H.; Parfrey, P.S.; et al. MLH1 –93 G > A Promoter Polymorphism and the Risk of Microsatellite-Unstable Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Curtin, K.; Wolff, R.K.; Albertsen, H.; Sweeney, C.; Caan, B.J.; Ulrich, C.M.; Potter, J.D.; Slattery, M.L. The MLH1 −93 G > A promoter polymorphism and genetic and epigenetic alterations in colon cancer. Genes. Chromosomes Cancer 2008, 47, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Nejda, N.; Iglesias, D.; Moreno Azcoita, M.; Medina Arana, V.; González-Aguilera, J.J.; Fernández-Peralta, A.M. A MLH1 polymorphism that increases cancer risk is associated with better outcome in sporadic colorectal cancer. Cancer Genet. Cytogenet. 2009, 193, 71–77. [Google Scholar] [CrossRef]
- Studamire, B.; Price, G.; Sugawara, N.; Haber, J.E.; Alani, E. Separation-of-function mutations in Saccharomyces cerevisiae MSH2 that confer mismatch repair defects but do not affect nonhomologous-tail removal during recombination. Mol. Cell. Biol. 1999, 19, 7558–7567. [Google Scholar] [CrossRef]
- Nicholson, A.; Hendrix, M.; Jinks-Robertson, S.; Crouse, G.F. Regulation of mitotic homeologous recombination in yeast: Functions of mismatch repair and nucleotide excision repair genes. Genetics 2000, 154, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Wilson, T.; Gradia, S.; Kane, M.F.; Guerrette, S.; Marsischky, G.T.; Kolodner, R.; Fishel, R. hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc. Natl. Acad. Sci. USA 1996, 93, 13629–13634. [Google Scholar] [CrossRef] [PubMed]
- Genschel, J.; Littman, S.J.; Drummond, J.T.; Modrich, P. Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J. Biol. Chem. 1998, 273, 19895–19901. [Google Scholar] [CrossRef] [PubMed]
- Berndt, S.I.; Platz, E.A.; Fallin, M.D.; Thuita, L.W.; Hoffman, S.C.; Helzlsouer, K.J. Mismatch repair polymorphisms and the risk of colorectal cancer. Int. J. Cancer 2007, 120, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, J.P.; Sampson, J.R. Exposing the MYtH about base excision repair and human inherited disease. Hum. Mol. Genet. 2003, 12, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, S.J.; di Bernardo, M.C.; Chandler, I.P.; Houlston, R.S. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J. Clin. Oncol. 2009, 27, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fry, E.A. Haploinsufficient tumor suppressor genes. Adv. Med. Biol. 2017, 118, 83–122. [Google Scholar] [PubMed]
- Barreiro, R.A.S.; Sabbaga, J.; Rossi, B.M.; Achatz, M.I.W.; Bettoni, F.; Camargo, A.A.; Asprino, P.F.; Galante, P.A.F. Heterozygous deleterious MUTYH variants as a driver for tumorigenesis. J. Pathol. 2022, 2, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Levrero, M.; de Laurenzi, V.; Costanzo, A.; Gong, J.; Wang, J.Y.; Melino, G. The p53/p63/p73 family of transcription factors: Overlapping and distinct functions. J. Cell Sci. 2000, 113, 1661–1670. [Google Scholar] [CrossRef]
- Meng, J.; Wang, S.; Zhang, M.; Fan, S.; Zhang, L.; Liang, C. TP73 G4C14-A4T14 polymorphism and cancer susceptibility: Evidence from 36 case-control studies. Biosci. Rep. 2018, 38, BSR20181452. [Google Scholar] [CrossRef]
- Hamajima, N.; Matsuo, K.; Suzuki, T.; Nakamura, T.; Matsuura, A.; Hatooka, S.; Shinoda, M.; Kodera, Y.; Yamamura, Y.; Hirai, T.; et al. No associations of p73 G4C14-to-A4T14 at exon 2 and p53 Arg72Pro polymorphisms with the risk of digestive tract cancers in Japanese. Cancer Lett. 2002, 181, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Arfaoui, A.T.; Ben Mahmoud, L.K.; Hmida, A.B.; Khiari, M.; Mohamed, A.L.; Chaar, I.; Khalfallah, T.; Regaya, S.M.; Bouraoui, S. Relationship Between p73 Polymorphism and the Immunohistochemical Profile of the Full-length (TAp73) and NH2-truncated (ΔNp73) Isoforms in Tunisian Patients. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 546. [Google Scholar] [CrossRef]
- Lee, K.-E.; Hong, Y.-S.; Kim, B.-G.; Kim, N.-Y.; Lee, K.-M.; Kwak, J.-Y.; Roh, M.-S. p73 G4C14 to A4T14 polymorphism is associated with colorectal cancer risk and survival. World J. Gastroenterol. 2010, 16, 4448–4454. [Google Scholar] [CrossRef]
- Sugitani, N.; Sivley, R.M.; Perry, K.E.; Capra, J.A.; Chazin, W.J. XPA: A key scaffold for human nucleotide excision repair. DNA Repair 2016, 44, 123–135. [Google Scholar] [CrossRef]
- Yuan, M.; Yu, C.; Yu, K. Association of human XPA rs1800975 polymorphism and cancer susceptibility: An integrative analysis of 71 case-control studies. Cancer Cell Int. 2020, 20, 164. [Google Scholar] [CrossRef]
- Rajalakshmi, T.R.; AravindhaBabu, N.; Shanmugam, K.T.; Masthan, K.M.K. DNA adducts-chemical addons. J. Pharm. Bioallied Sci. 2015, 7, S197–S199. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Lao, X.; Tang, W.; Chen, Z.; Li, R.; Qin, X.; Li, S. XPC Lys939Gln polymorphism contributes to colorectal cancer susceptibility: Evidence from a meta-analysis. Diagn. Pathol. 2014, 9, 120. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Horejsí, Z.; Koed, K.; Krämer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- Cragun, D.; Radford, C.; Dolinsky, J.; Caldwell, M.; Chao, E.; Pal, T. Panel-based testing for inherited colorectal cancer: A descriptive study of clinical testing performed by a US laboratory. Clin. Genet. 2014, 86, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Meijers-Heijboer, H.; Wijnen, J.; Vasen, H.; Wasielewski, M.; Wagner, A.; Hollestelle, A.; Elstrodt, F.; van den Bos, R.; de Snoo, A.; Fat, G.T.A.; et al. The CHEK2 1100delC mutation identifies families with a hereditary breast and colorectal cancer phenotype. Am. J. Hum. Genet. 2003, 72, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, C.; Wokołorczyk, D.; Kładny, J.; Kurzawski, G.; Suchy, J.; Grabowska, E.; Gronwald, J.; Huzarski, T.; Byrski, T.; Górski, B.; et al. Germline CHEK2 mutations and colorectal cancer risk: Different effects of a missense and truncating mutations? Eur. J. Hum. Genet. 2007, 15, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Djureinovic, T.; Lindblom, A.; Dalén, J.; Dedorson, S.; Edler, D.; Hjern, F.; Holm, J.; Lenander, C.; Lindforss, U.; Lundqvist, N.; et al. The CHEK2 1100delC variant in Swedish colorectal cancer. Anticancer Res. 2006, 26, 4885–4888. [Google Scholar] [PubMed]
- Weischer, M.; Bojesen, S.E.; Tybjaerg-Hansen, A.; Axelsson, C.K.; Nordestgaard, B.G. Increased risk of breast cancer associated with CHEK2*1100delC. J. Clin. Oncol. 2007, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Näslund-Koch, C.; Nordestgaard, B.G.; Bojesen, S.E. Increased Risk for Other Cancers in Addition to Breast Cancer for CHEK2*1100delC Heterozygotes Estimated from the Copenhagen General Population Study. J. Clin. Oncol. 2016, 34, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Kilpivaara, O.; Laiho, P.; Aaltonen, L.A.; Nevanlinna, H. CHEK2 1100delC and colorectal cancer. J. Med. Genet. 2003, 40, e110. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.M.; Nolte, I.M.; Meerman, G.J.T.; van der Graaf, W.T.A.; Oosterom, E.; Bruinenberg, M.; van der Steege, G.; Oosterwijk, J.C.; van der Hout, A.H.; Boezen, H.M.; et al. No increased susceptibility to breast cancer from combined CHEK2 1100delC genotype and the HLA class III region risk factors. Eur. J. Cancer 2005, 41, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Geng, X.; Ge, W.; Li, H. Meta-analysis of CHEK2 1100delC variant and colorectal cancer susceptibility. Eur. J. Cancer 2011, 47, 2546–2551. [Google Scholar] [CrossRef]
- Li, J.; Williams, B.L.; Haire, L.F.; Goldberg, M.; Wilker, E.; Durocher, D.; Yaffe, M.B.; Jackson, S.P.; Smerdon, S.J. Structural and functional versatility of the FHA domain in DNA-damage signaling by the tumor suppressor kinase Chk2. Mol. Cell 2002, 9, 1045–1054. [Google Scholar] [CrossRef]
- Cybulski, C.; Górski, B.; Huzarski, T.; Masojć, B.; Mierzejewski, M.; Debniak, T.; Teodorczyk, U.; Byrski, T.; Gronwald, J.; Matyjasik, J.; et al. CHEK2 is a multiorgan cancer susceptibility gene. Am. J. Hum. Genet. 2004, 75, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Irmejs, A.; Miklasevics, E.; Boroschenko, V.; Gardovskis, A.; Vanags, A.; Melbarde-Gorkusa, I.; Bitina, M.; Suchy, J.; Gardovskis, J. Pilot study on low penetrance breast and colorectal cancer predisposition markers in latvia. Hered. Cancer Clin. Pract. 2006, 4, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Suchy, J.; Cybulski, C.; Wokołorczyk, D.; Oszurek, O.; Górski, B.; Debniak, T.; Jakubowska, A.; Gronwald, J.; Huzarski, T.; Byrski, T.; et al. CHEK2 mutations and HNPCC-related colorectal cancer. Int. J. Cancer 2010, 126, 3005–3009. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Q.-S.; Wang, Y.-J. The CHEK2 I157T variant and colorectal cancer susceptibility: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2012, 13, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Guo, C.; Liu, L. The effect of CHEK2 variant I157T on cancer susceptibility: Evidence from a meta-analysis. DNA Cell Biol. 2013, 32, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Thirthagiri, E.; Cheong, L.S.; Yip, C.H.; Teo, S.H. CHEK2*1100delC Does Not Contribute to Risk to Breast Cancer Among Malay, Chinese and Indians in Malaysia. Fam. Cancer 2009, 8, 355–358. [Google Scholar] [CrossRef]
- Zhang, S.; Phelan, C.M.; Zhang, P.; Rousseau, F.; Ghadirian, P.; Robidoux, A.; Foulkes, W.; Hamel, N.; McCready, D.; Trudeau, M.; et al. Frequency of the CHEK2 1100delC Mutation among Women with Breast Cancer: An International Study. Cancer Res. 2008, 68, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S.; Topaktaş, M.; Akkız, H.; Bekar, A.; Akgöllü, E. CHEK2 1100delC, IVS2 + 1 G > A and I157T mutations are not present in colorectal cancer cases from Turkish population. Cancer Epidemiol. 2012, 36, 453–457. [Google Scholar] [CrossRef]
- Fan, Z.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xu, Y.; Xie, Y. Identification and analysis of CHEK2 germline mutations in Chinese BRCA1/2-negative breast cancer patients. Breast Cancer Res. Treat. 2018, 169, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.-J.; Sun, L.; Li, H.-L. Association between CYP1A1 polymorphism and colorectal cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-Q.; Hu, Y.-Y.; Niu, Y.-M.; Yang, G.-L.; Wu, Y.-Y.; Leng, W.-D.; Xia, L.-Y. CYP1A1 Ile462Val polymorphism contributes to colorectal cancer risk: A meta-analysis. World J. Gastroenterol. 2011, 17, 260. [Google Scholar] [CrossRef] [PubMed]
- Carrera, A.N.; Grant, M.K.O.; Zordoky, B.N. CYP1B1 as a therapeutic target in cardio-oncology. Clin. Sci. 2020, 134, 2897–2927. [Google Scholar] [CrossRef] [PubMed]
- Shiizaki, K.; Kawanishi, M.; Yagi, T. Modulation of benzo [a] pyrene—DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ. 2017, 39, 14. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.; Gill, J.H.; Khan, P.A.; Seargent, J.M.; Martin, S.W.; Batman, P.A.; Griffith, J.; Bradley, C.; Double, J.A.; Bibby, M.C.; et al. Cytochrome P450 1B1 (CYP1B1) is overexpressed in human colon adenocarcinomas relative to normal colon: Implications for drug development. Mol. Cancer Ther. 2003, 2, 527–534. [Google Scholar] [PubMed]
- Hlavata, I.; Vrana, D.; Smerhovsky, Z.; Pardini, B.; Naccarati, A.; Vodicka, P.; Novotny, J.; Mohelnikova-Duchonova, B.; Soucek, P. Association between exposure-relevant polymorphisms in CYP1B1, EPHX1, NQO1, GSTM1, GSTP1 and GSTT1 and risk of colorectal cancer in a Czech population. Oncol. Rep. 2010, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Trubicka, J.; Grabowska-Kłujszo, E.; Suchy, J.; Masojć, B.; Serrano-Fernandez, P.; Kurzawski, G.; Cybulski, C.; Górski, B.; Huzarski, T.; Byrski, T.; et al. Variant alleles of the CYP1B1 gene are associated with colorectal cancer susceptibility. BMC Cancer 2010, 10, 420. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, G.-Q.; Miao, X.-Y.; Liu, Y.; Zhou, W.; Zhong, D.-W. CYP1B1 Leu432Val polymorphism and colorectal cancer risk among Caucasians: A meta-analysis. Tumour Biol. 2012, 33, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Cotton, S.C.; Sharp, L.; Little, J.; Brockton, N. Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. Am. J. Epidemiol. 2000, 151, 7–32. [Google Scholar] [CrossRef]
- Raimondi, S.; Botteri, E.; Iodice, S.; Lowenfels, A.B.; Maisonneuve, P. Gene-smoking interaction on colorectal adenoma and cancer risk: Review and meta-analysis. Mutat. Res. 2009, 670, 6–14. [Google Scholar] [CrossRef]
- Vlaykova, T.; Gulubova, M.; Vlaykova, D.; Cirovski, G.; Yovchev, Y.; Dimov, D.; Chilingirov, P. Possible Influence of GSTM1 and GSTT1 Null Genotype on the Risk for Development of Sporadic Colorectal Cancer. Biotechnol. Biotechnol. Equip. 2009, 23, 1084–1089. [Google Scholar] [CrossRef]
- Bao, Q.; He, B.; Pan, Y.; Tang, Z.; Zhang, Y.; Qu, L.; Xu, Y.; Zhu, C.; Tian, F.; Wang, S. Genetic variation in the promoter of DNMT3B is associated with the risk of colorectal cancer. Int. J. Color. Dis. 2011, 26, 1107–1112. [Google Scholar] [CrossRef]
- Jung, A.Y.; Poole, E.M.; Bigler, J.; Whitton, J.; Potter, J.D.; Ulrich, C.M. DNA Methyltransferase and Alcohol Dehydrogenase: Gene-Nutrient Interactions in Relation to Risk of Colorectal Polyps. Cancer Epidemiol. Biomark. Prev. 2008, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Daraei, A.; Salehi, R.; MohamadHashem, F. DNA-methyltransferase 3B 39179 G > T polymorphism and risk of sporadic colorectal cancer in a subset of Iranian population. J. Res. Med. Sci. 2011, 16, 807–813. [Google Scholar]
- Trimmer, E.E. Methylenetetrahydrofolate reductase: Biochemical characterization and medical significance. Curr. Pharm. Des. 2013, 19, 2574–2593. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.P.; Yu, C.H. Meta-Prediction of MTHFR Gene Polymorphism Mutations and Associated Risk for Colo-rectal Cancer. Biol. Res. Nurs. 2016, 18, 357–369. [Google Scholar] [CrossRef]
- Taioli, E.; Garza, M.A.; Ahn, Y.O.; Bishop, D.T.; Bost, J.; Budai, B.; Chen, K.; Gemignani, F.; Keku, T.; Lima, C.S.P.; et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: A HuGE-GSEC review. Am. J. Epidemiol. 2009, 170, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Moruzzi, S.; Guarini, P.; Udali, S.; Ruzzenente, A.; Guglielmi, A.; Conci, S.; Pattini, P.; Martinelli, N.; Olivieri, O.; Tammen, S.A.; et al. One-carbon genetic variants and the role of MTHFD1 1958 G > A in liver and colon cancer risk according to global DNA methylation. PLoS ONE 2017, 12, e0185792. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, K.; Wang, X.; Zhang, C.; Xu, Y. TNF-α-308G/A polymorphism and the risk of colorectal cancer: A systematic review and an updated meta-analysis. J. BUON 2018, 23, 1616–1624. [Google Scholar]
- Li, Z.; Li, S.; Sun, Y.; Liu, Y.; Li, W.; Yang, L.; Duan, Y.; Li, J.; Guo, H.; Zou, T.; et al. TNF-α-308 A allele is associated with an increased risk of distant metastasis in rectal cancer patients from Southwestern China. PLoS ONE 2017, 12, e0178218. [Google Scholar] [CrossRef]
- Banday, M.Z.; Balkhi, H.M.; Hamid, Z.; Sameer, A.S.; Chowdri, N.A.; Haq, E. Tumor necrosis factor-α (TNF-α)-308G/A promoter polymorphism in colorectal cancer in ethnic Kashmiri population—A case control study in a detailed perspective. Meta Gene 2016, 9, 128–136. [Google Scholar] [CrossRef]
- Mahla, R.S.; Reddy, M.C.; Prasad, D.V.R.; Kumar, H. Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology. Front. Immunol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Udden, S.M.N.; Peng, L.; Gan, J.-L.; Shelton, J.M.; Malter, J.S.; Hooper, L.V.; Zaki, M.d.H. NOD2 suppresses colorectal tumorigenesis via downregulation of the TLR pathways. Cell Rep. 2017, 19, 2756–2770. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, C.; Xu, Q.; Xing, C.; Yuan, Y. NOD2 Polymorphisms Associated with Cancer Risk: A Meta-Analysis. PLoS ONE 2014, 9, e89340. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Hu, Z.; Wang, D.; Sun, X.; Ren, C. Differential effects of NOD2 polymorphisms on colorectal cancer risk: A meta-analysis. Int. J. Color. Dis. 2010, 25, 161–168. [Google Scholar] [CrossRef]
- Papaconstantinou, I.; Theodoropoulos, G.; Gazouli, M.; Panoussopoulos, D.; Mantzaris, G.J.; Felekouras, E.; Bramis, J. Association between mutations in the CARD15/NOD2 gene and colorectal cancer in a Greek population. Int. J. Cancer 2005, 114, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Hitre, E.; Szalay, F.; Zinober, K.; Fuszek, P.; Lakatos, L.; Fischer, S.; Osztovits, J.; Gemela, O.; Veres, G.; et al. Common NOD2/CARD15 variants are not associated with susceptibility or the clinicopathologic characteristics of sporadic colorectal cancer in Hungarian patients. BMC Cancer 2007, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Möckelmann, N.; von Schönfels, W.; Buch, S.; von Kampen, O.; Sipos, B.; Egberts, J.H.; Rosenstiel, P.; Franke, A.; Brosch, M.; Hinz, S.; et al. Investigation of innate immunity genes CARD4, CARD8 and CARD15 as germline susceptibility factors for colorectal cancer. BMC Gastroenterol. 2009, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.; Portela, F.; Donato, M.M.; Figueiredo, P.; Ferreira, M.; Amaro, P.; Sá, A.; Andrade, P.; Gouveia, H.; Sofia, C. CARD15 mutations and colorectal cancer in a South European country. Int. J. Color. Dis. 2010, 25, 1211–1219. [Google Scholar] [CrossRef]
- Bates, E.E.; Ravel, O.; Dieu, M.C.; Ho, S.; Guret, C.; Bridon, J.M.; Ait-Yahia, S.; Brière, F.; Caux, C.; Banchereau, J.; et al. Identification and analysis of a novel member of the ubiquitin family expressed in dendritic cells and mature B cells. Eur. J. Immunol. 1997, 27, 2471–2477. [Google Scholar] [CrossRef]
- Frank, B.; Hoffmeister, M.; Klopp, N.; Illig, T.; Chang-Claude, J.; Brenner, H. Polymorphisms in inflammatory pathway genes and their association with colorectal cancer risk. Int. J. Cancer 2010, 127, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Tanzi, R.E.; Kovacs, D.M. Alzheimer’s disease: The cholesterol connection. Nat. Neurosci. 2003, 6, 345–351. [Google Scholar] [CrossRef]
- Kervinen, K.; Södervik, H.; Mäkelä, J.; Lehtola, J.; Niemi, M.; Kairaluoma, M.I.; Kesäniemi, Y.A. Is the development of adenoma and carcinoma in proximal colon related to apolipoprotein E phenotype? Gastroenterology 1996, 110, 1785–1790. [Google Scholar] [CrossRef]
- Watson, M.A.; Gay, L.; Stebbings, W.S.; Speakman, C.T.; Bingham, S.A.; Loktionov, A. Apolipoprotein E gene polymorphism and colorectal cancer: Gender-specific modulation of risk and prognosis. Clin. Sci. 2003, 104, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.R.S.; Nakazone, M.A.; Pinhel, M.a.S.; Alvares, R.M.; Monaco, A.C.; Pinheiro, A.; Barros, C.F.D.C.; Cury, P.M.; Cunrath, G.S.; Netinho, J.G. Association between Apolipoprotein E Genotype, Serum Lipids, and Colorectal Cancer in Brazilian Individuals. Braz. J. Med. Biol. Res. 2009, 42, 397–403. [Google Scholar] [CrossRef]
- Nicolas, J.P.; Lin, Y.; Lambeau, G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 1997, 272, 7173–7181. [Google Scholar] [CrossRef]
- Küry, S.; Buecher, B.; Robiou-du-Pont, S.; Scoul, C.; Colman, H.; Le Neel, T.; Le Houérou, C.; Faroux, R.; Ollivry, J.; Lafraise, B.; et al. Low-penetrance alleles predisposing to sporadic colorectal cancers: A French case-controlled genetic association study. BMC Cancer 2008, 8, 326. [Google Scholar] [CrossRef]
- Corduck, L. Genetic Testing Can Reduce Suffering and Save Lives. Am. J. Manag. Care 2019, 25, SP270–SP271. [Google Scholar]

| Gene | Variant | dbSNP rs Number | Mutated Allele Zygosity | Colorectal Cancer Risk * | ClinVar Accession Number ** | Clinical Significance *** |
|---|---|---|---|---|---|---|
| Oncogenes and tumor suppressor genes | ||||||
| APC | c.3920T>A | rs1801155 | - | 1.96 | VCV000000822.89 | Pathogenic/Likely pathogenic/Established risk allele; risk factor (7) Risk factor (6) Conflicting interpretations of pathogenicity; risk factor (15) Conflicting interpretations of pathogenicity; association; risk factor (13) Uncertain significance (6) Not provided (2) |
| TP53 | c.215C>G | rs1042522 | homozygous | 2.73 | VCV000012351.66 | Pathogenic (1) Benign (26) |
| heterozygous | 1.65/1.13 | |||||
| Genes involved in DNA damage repair | ||||||
| MLH1 | c.-93G>A | rs1800734 | homozygous | 3.23/8.88 | VCV000089600.24 | Benign (11) |
| heterozygous | 1.84/2.56 | |||||
| c.655A>G | rs1799977 | - | 2.53 | VCV000036557.40 | Benign (27) | |
| MSH3 | c.3133G>A | rs26279 | - | 1.1/RR = 1.34 | VCV000822964.23 | Benign (5) |
| c.2846A>G | rs184967 | - | 1.11/RR = 1.29 | VCV000821892.22 | Benign (5) | |
| MUTYH | c.22G>A | rs3219484 | - | 0.95 | VCV000041760.50 | Benign (22) |
| c.930G>C | rs3219489 | - | 1.09 | VCV000041767.45 | Benign (20) | |
| TP73 | c.-30G>T | rs2273953 | - | - | Not reported | Not reported |
| c.-20C>T | rs1801173 | - | - | Not reported | Not reported | |
| G4C14-A4T14 | - | - | 1.204 | - | - | |
| XPA | c.-4A>G | rs1800975 | - | 0.87 | VCV000190206.14 | Benign (7) |
| XPC | c.2815C>A | rs2228001 | - | 1.26/1.08 | VCV000190215.17 | Benign (8) |
| CHEK2 | c.470T>C | rs17879961 | - | 1.5 | VCV000005591.86 | Pathogenic (1) Likely pathogenic (8) Established risk allele (1) Risk factor (8) Conflicting interpretation of pathogenicity (15) Uncertain significance (6) |
| c.252A>G | rs1805129 | - | 1.0 (inconclusive) | VCV000142139.40 | Benign (18) | |
| c.1100del | rs555607708 | - | 1.0 (inconclusive) | VCV000128042.109 | Pathogenic (67) Uncertain significance (1) | |
| Genes involved in metabolic pathways | ||||||
| CYP1A1 | c.1384A>G | rs1048943 | - | 1.45/1.47 | Not reported | Not reported |
| CYP1B1 | c.1358A>G | rs1800440 | heterozygous | 0.69 | VCV000166969.34 | Benign/Likely benign (5) |
| homozygous | 0.52 | |||||
| homozygous + heterozygous | 0.68 | |||||
| c.355G>T | rs1056827 | heterozygous | 0.84 | VCV000092437.19 | Benign/Likely benign (3) Benign (1) | |
| homozygous | 1.04/1.3 | |||||
| homozygous + heterozygous | 0.88 | |||||
| c.1358A>G and c.355G>T diplotype | - | - | 0.53 | - | - | |
| c.142C>G | rs10012 | homozygous | 1.0 | VCV000092436.18 | Benign/Likely benign (3) Benign (1) | |
| c.1294C>G | rs1056836 | homozygous | 0.8 | VCV000456637.10 | Benign (1) | |
| homozygous-wild c.142C>G and c.355G>T | - | heterozygous | 2.4 | - | - | |
| homozygous-wild c.142C>G and c.355G>T | - | homozygous | 7.1 | - | - | |
| homozygous-mutated c.355G>T and c.1294C>G | - | heterozygous | 6.1 | - | - | |
| GSTM1 | null genotype | - | homozygous | 1.30/2.32 | - | - |
| GSTT1 | null genotype | - | homozygous | 1.07/5.69 | - | - |
| GSTM1 + GSTT1 | combined null genotype | - | homozygous | 1.58/21.53 | - | - |
| Genes involved in methylation | ||||||
| DNMT3B | c.-579G>T | rs1569686 | - | 0.50/0.848 (for wild-type allele) | Not reported | Not reported |
| MTHFR | c.665C>T | rs1801133 | - | 0.83/RR = 0.92 | VCV000003520.112 | Conflicting interpretations of pathogenicity (3) Risk factor (1) Uncertain significance (2) Benign/Likely benign (4) Benign (8) |
| c.1286A>C | rs1801131 | - | RR = 0.95 | VCV000003521.92 | Benign/Likely benign (4) Benign (5) Uncertain significance (1) | |
| MTHFD1 | c.401A>G | rs1950902 | - | 0.90 | VCV000403114.12 | Benign (3) |
| c.1958G>A | rs2236225 | - | - | VCV000013633.17 | Benign/Likely benign (2) Benign (2) | |
| Genes involved in the immune response | ||||||
| TNF | c.308G/A | rs1800629 | - | 0.96 | VCV000225964.9 | not reported |
| NOD2 | c.3019dup | rs2066847 | - | 1.23–1.35 | VCV000004691.38 | Likely benign (1) Benign (1) Established risk allele (1) Risk factor (1) Likely risk allele; risk factor (1) Association (1) Uncertain significance (1) Conflicting interpretations of pathogenicity (4) |
| c.2023C>T | rs2066844 | - | 1.32–1.35 | VCV000004693.32 | Likely benign (2) Benign (1) Association (1) Uncertain significance (2) Conflicting interpretations of pathogenicity (2) Not provided (1) | |
| c.2641G>C | rs2066845 | - | 1.32–1.39 | VCV000004692.41 | Risk factor (1) Association (1) Uncertain significance (2) Conflicting interpretations of pathogenicity (5) | |
| UBD | c.3527T>C | rs2076485 | homozygous | 1.14/1.43 | Not reported | Not reported |
| heterozygous | 1.02/1.19 | |||||
| Genes modifying the colonic microenvironment | ||||||
| APOE | APOEε2 | rs7412 | heterozygous | 1.91 | Not reported | Not reported |
| APOEε4 | rs429358 | - | 0.35/0.36 | Not reported | Not reported | |
| PLA2G2A | c.132C>T | rs4744 | homozygous | 1.16 | Not reported | Not reported |
| heterozygous | 1.20 | |||||
| c.435+230C>T | rs11677 | homozygous | 1.13 | Not reported | Not reported | |
| heterozygous | 1.17 | |||||
| c.185+88G>A | rs2236772 | homozygous | - | Not reported | Not reported | |
| heterozygous | 1.30 | |||||
| c.-859C>G | rs11573156 | homozygous | 0.50 | Not reported | Not reported | |
| heterozygous | 0.82 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szuman, M.; Kaczmarek-Ryś, M.; Hryhorowicz, S.; Kryszczyńska, A.; Grot, N.; Pławski, A. Low-Penetrance Susceptibility Variants in Colorectal Cancer—Current Outlook in the Field. Int. J. Mol. Sci. 2024, 25, 8338. https://doi.org/10.3390/ijms25158338
Szuman M, Kaczmarek-Ryś M, Hryhorowicz S, Kryszczyńska A, Grot N, Pławski A. Low-Penetrance Susceptibility Variants in Colorectal Cancer—Current Outlook in the Field. International Journal of Molecular Sciences. 2024; 25(15):8338. https://doi.org/10.3390/ijms25158338
Chicago/Turabian StyleSzuman, Marcin, Marta Kaczmarek-Ryś, Szymon Hryhorowicz, Alicja Kryszczyńska, Natalia Grot, and Andrzej Pławski. 2024. "Low-Penetrance Susceptibility Variants in Colorectal Cancer—Current Outlook in the Field" International Journal of Molecular Sciences 25, no. 15: 8338. https://doi.org/10.3390/ijms25158338





