Identification of Oxidative Stress-Related Biomarkers for Pain–Depression Comorbidity Based on Bioinformatics
Abstract
:1. Introduction
2. Results
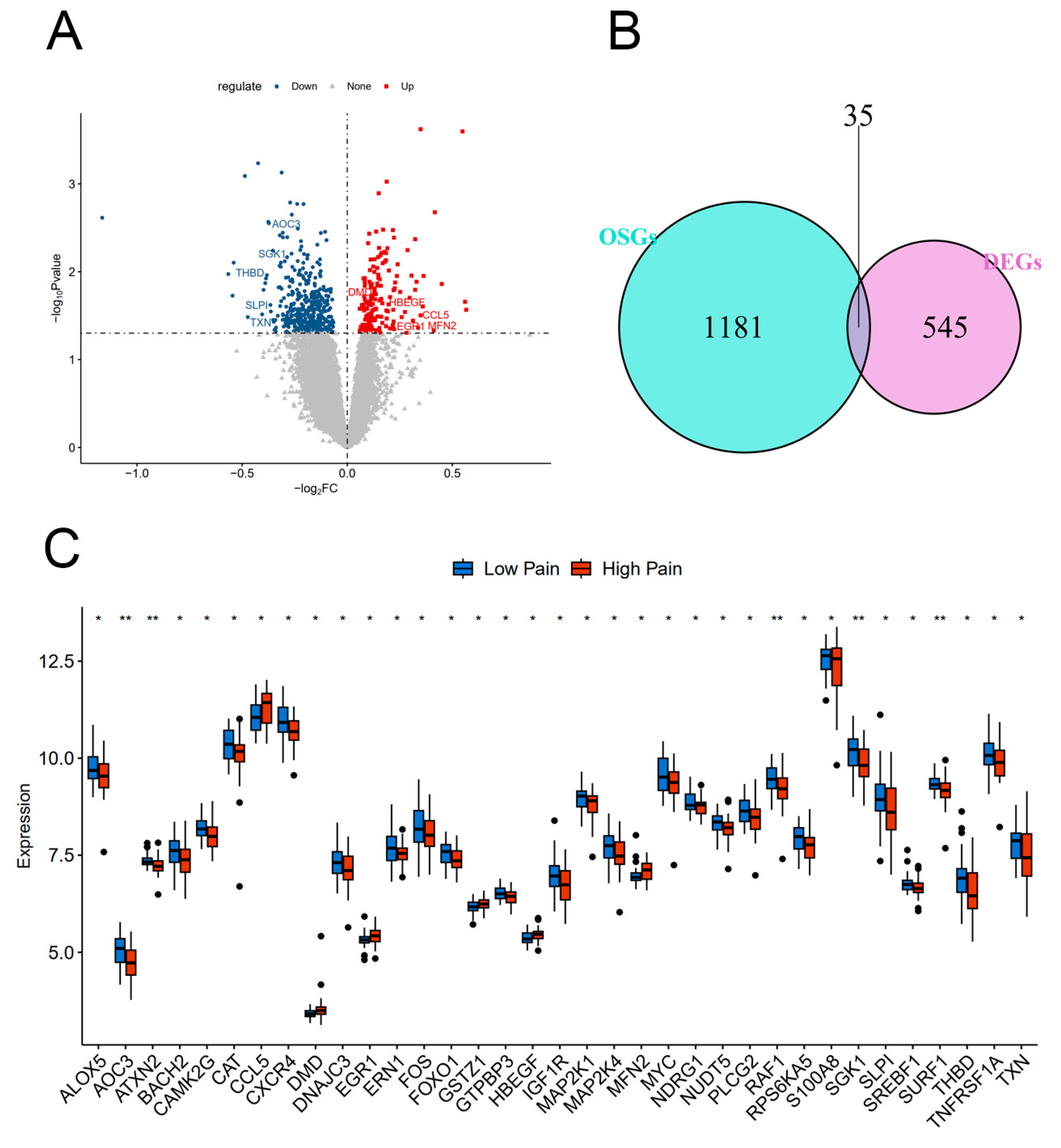
2.1. Identification of DEGs and DEOSGs
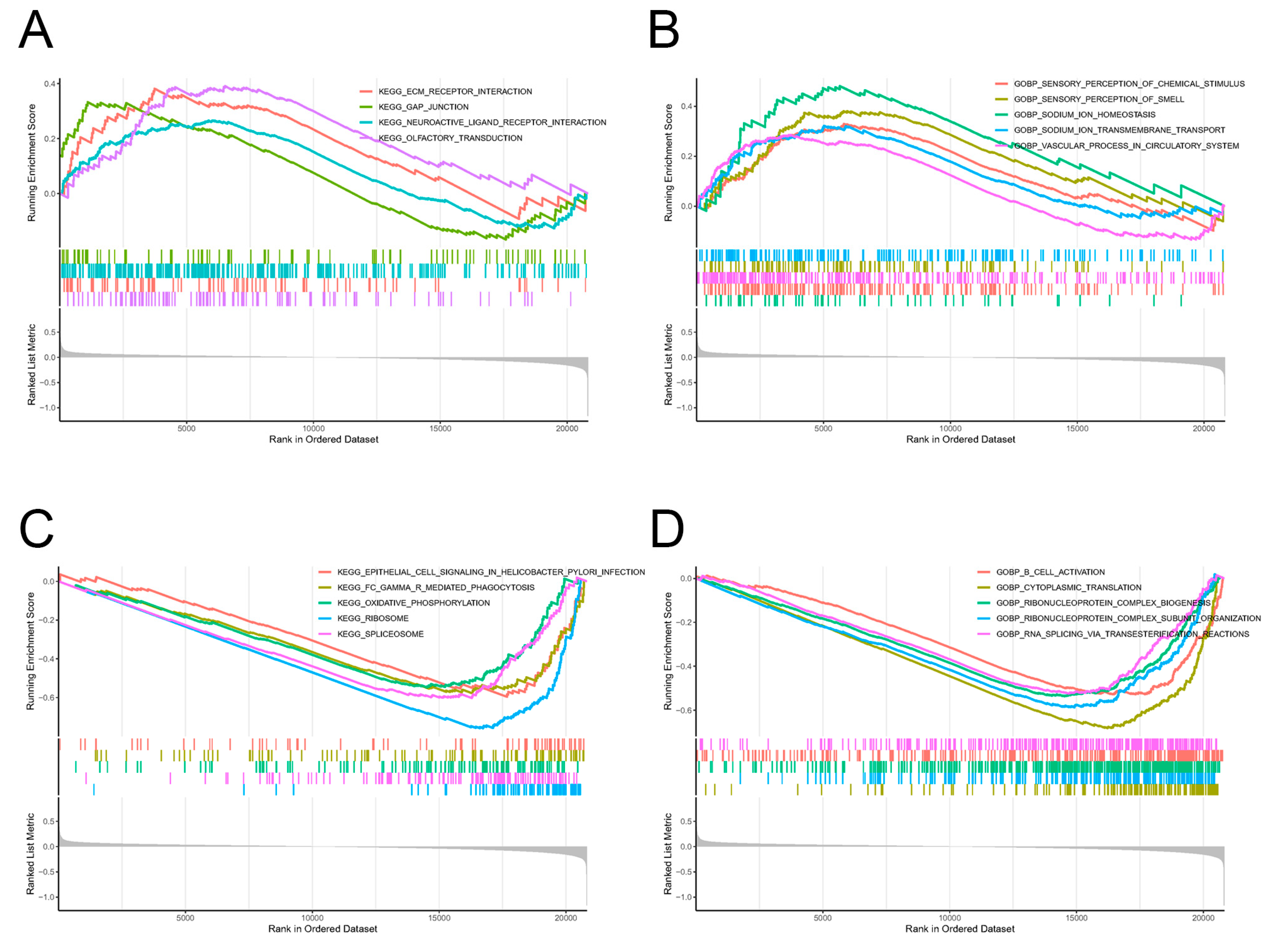
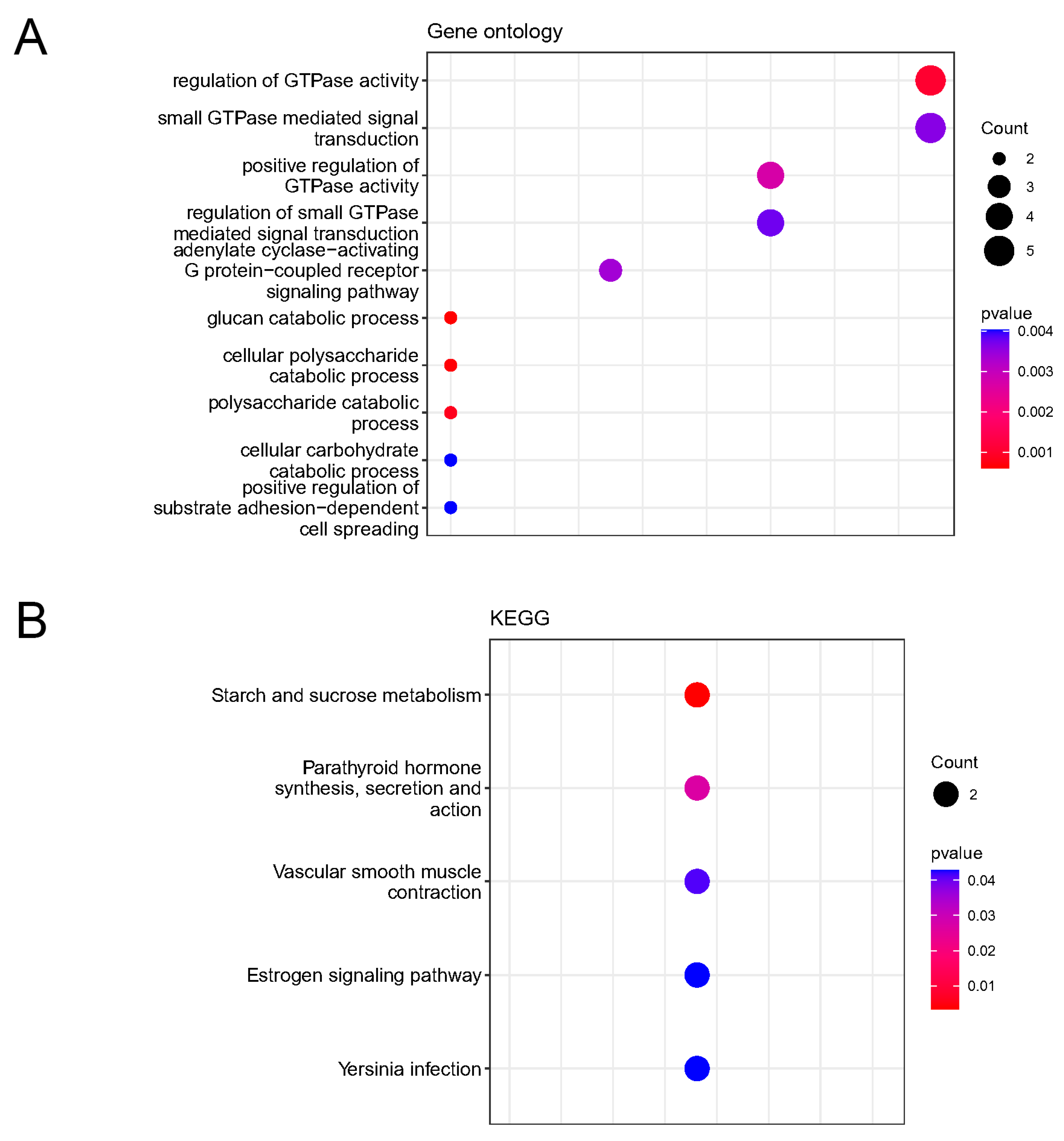
2.2. Enrichment Analysis of KEGG Pathways and GO Functions for DEOSGs
2.3. Screening of Pathway Enrichments by GSEA
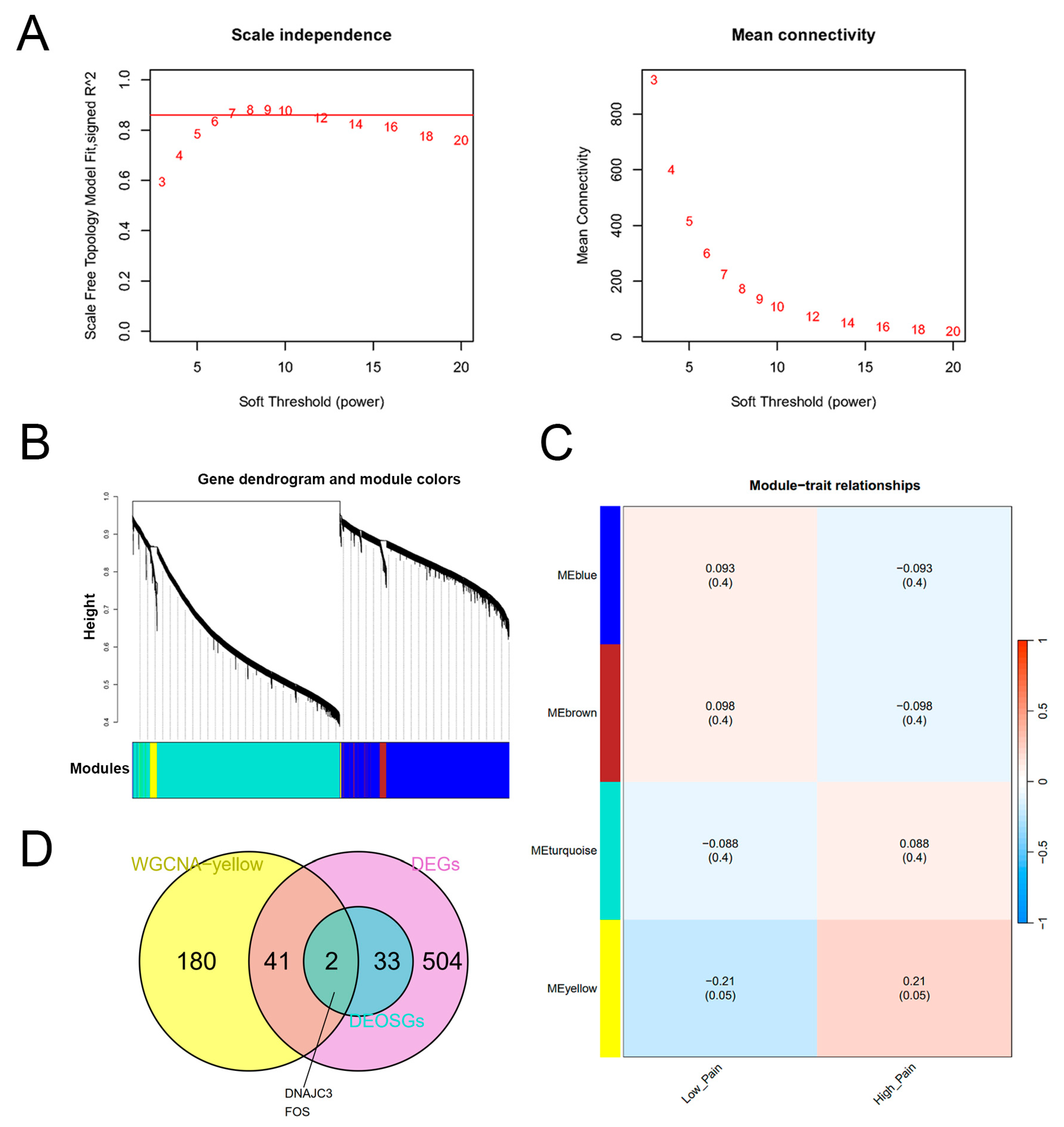
2.4. Construction of the WGCNA Network and Identification of Key Genes
2.5. Enrichment Analysis of Key Genes
2.6. Construction of the PPI Network and Screening of Hub Genes
2.7. Correlation Analysis between Hub Genes and Immune Cells
2.8. Validation of Hub Gene Expression
2.9. Identification of Transcription Factors, microRNAs and Drugs Regulating Key Genes
3. Discussion
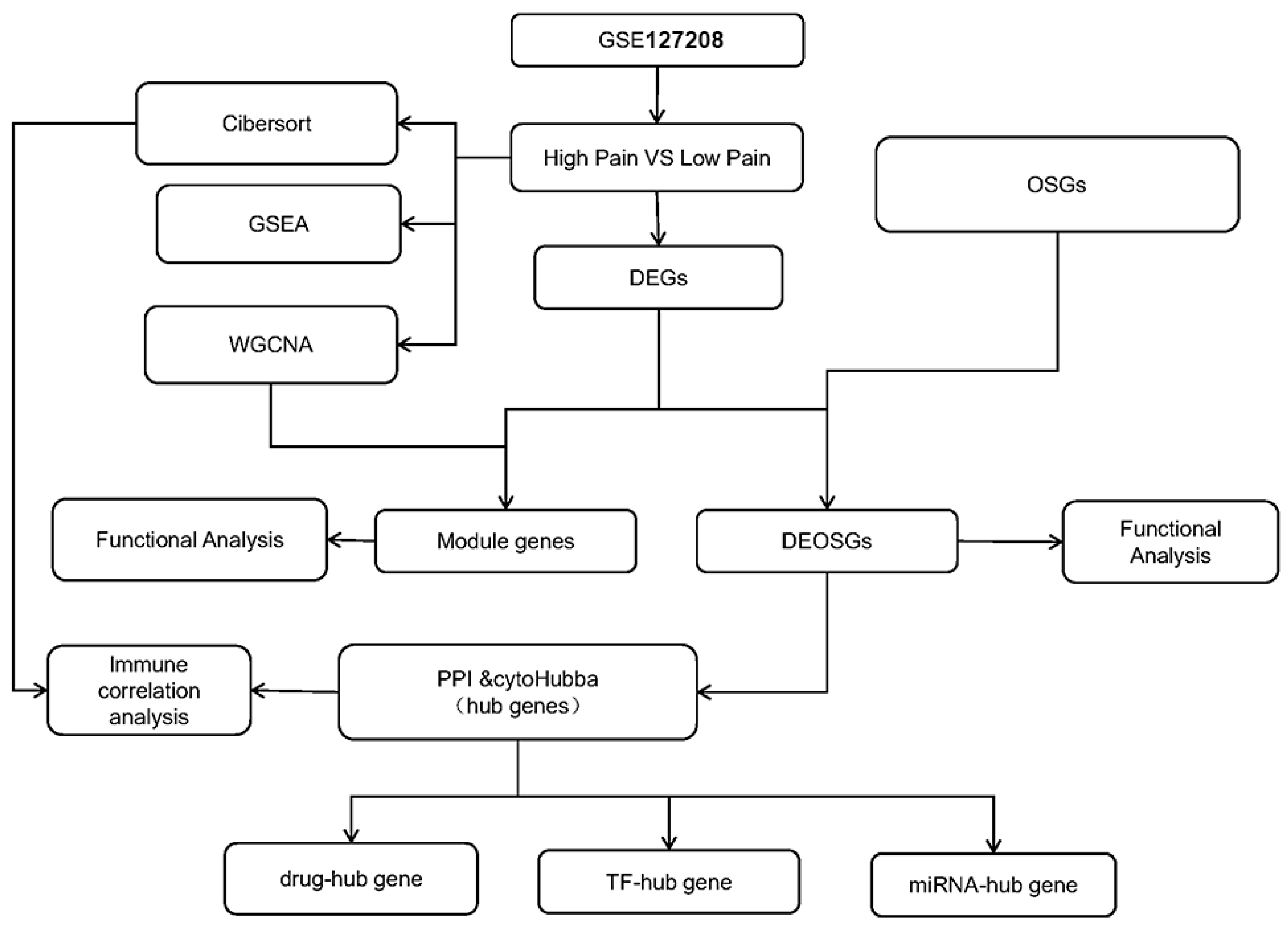
4. Materials and Methods
4.1. Data Collection
4.2. Screening of DEGs
4.3. Functional Enrichment Analysis for DEOSGs
4.4. Gene Set-Enrichment Analysis
4.5. Weighted Gene Co-Expression Network Analysis
4.6. Identification of Key Genes
4.7. Functional Enrichment Analysis for Key Genes
4.8. PPI Network Construction and Topology Network Interaction Analysis
4.9. Immune Cell Infiltration Analysis
4.10. Exploration of miRNAs and TFs Regulating Hub Genes
4.11. Identification of Potentially Effective Drugs Targeting Hub Genes
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, W.; Adams, M.J.; Reel, P.; Rajendrakumar, A.; Huang, Y.; Deary, I.J.; Palmer, C.N.A.; McIntosh, A.M.; Smith, B.H. Genetic correlations between pain phenotypes and depression and neuroticism. Eur. J. Hum. Genet. 2020, 28, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Jaime, H.; Sánchez-Salcedo, J.A.; Estevez-Cabrera, M.M.; Molina-Jiménez, T.; Cortes-Altamirano, J.L.; Alfaro-Rodríguez, A. Depression and Pain: Use of Antidepressants. Curr. Neuropharmacol. 2022, 20, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Petrosky, E.; Harpaz, R.; Fowler, K.A.; Bohm, M.K.; Helmick, C.G.; Yuan, K.; Betz, C.J. Chronic Pain Among Suicide Decedents, 2003 to 2014: Findings from the National Violent Death Reporting System. Ann. Intern. Med. 2018, 169, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Kopala-Sibley, D.C.; Noel, M. The Co-occurrence of Pediatric Chronic Pain and Depression: A Narrative Review and Conceptualization of Mutual Maintenance. Clin. J. Pain 2019, 35, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Satyanarayanan, S.K.; Lin, Y.W.; Su, K.P. Clinical efficacy and immune effects of acupuncture in patients with comorbid chronic pain and major depression disorder: A double-blinded, randomized controlled crossover study. Brain Behav. Immun. 2023, 110, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.R.; Becker, L.J.; Bilbily, J.; Chang, Y.H.; Borges, G.; Dunn, S.S.; Madasu, M.K.; Vazquez, C.R.; Cariello, S.A.; Al-Hasani, R.; et al. Spared nerve injury decreases motivation in long-access homecage-based operant tasks in mice. Pain 2024, 165, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Asaoka, Y.; Kimura, K.; Hara, R.; Arakaki, S.; Sakasai, K.; Suzuki, H.; Yamauchi, N.; Nomura, H.; Amano, T.; et al. Tonic Suppression of the Mesolimbic Dopaminergic System by Enhanced Corticotropin-Releasing Factor Signaling Within the Bed Nucleus of the Stria Terminalis in Chronic Pain Model Rats. J. Neurosci. 2019, 39, 8376–8385. [Google Scholar] [CrossRef]
- Ait Tayeb, A.E.K.; Poinsignon, V.; Chappell, K.; Bouligand, J.; Becquemont, L.; Verstuyft, C. Major Depressive Disorder and Oxidative Stress: A Review of Peripheral and Genetic Biomarkers According to Clinical Characteristics and Disease Stages. Antioxidants 2023, 12, 942. [Google Scholar] [CrossRef]
- Somani, A.; Singh, A.K.; Gupta, B.; Nagarkoti, S.; Dalal, P.K.; Dikshit, M. Oxidative and Nitrosative Stress in Major Depressive Disorder: A Case Control Study. Brain Sci. 2022, 12, 144. [Google Scholar] [CrossRef]
- Lu, Z.; Pu, C.; Zhang, Y.; Sun, Y.; Liao, Y.; Kang, Z.; Feng, X.; Yue, W. Oxidative Stress and Psychiatric Disorders: Evidence from the Bidirectional Mendelian Randomization Study. Antioxidants 2022, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Li, Y.; Jiang, Y.; Huang, J.H.; Wang, F. Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants 2022, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Matsuoka, Y.; Matsuda, M.; Kawai, K.; Amaya, F. Dysregulation of p53 and parkin induce mitochondrial dysfunction and leads to the diabetic neuropathic pain. Neuroscience 2019, 416, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Sun, J.; Zhou, X.R.; Rittner, H.; Mei, W.; Tian, Y.K.; Zhang, H.X.; Chen, F.; et al. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018, 14, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, C.; Baldacci, F.; Cafalli, M.; Chico, L.; Lo Gerfo, A.; Bonuccelli, U.; Siciliano, G.; Gori, S. Evidences of Reduced Antioxidant Activity in Patients with Chronic Migraine and Medication-Overuse Headache. Headache 2015, 55, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, Z.; Han, M.; Chen, K.; Wang, Y.; Qing, J.; Yang, F. Salvianolic acid B alleviates comorbid pain in depression induced by chronic restraint stress through inhibiting GABAergic neuron excitation via an ERK-CREB-BDNF axis-dependent mechanism. J. Psychiatr. Res. 2022, 151, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sarkar, P.; Revanth, M.; Hazarika, D.; Prasanna, A.; Pandol, S.J.; Unnisa, M.; Jakkampudi, A.; Bedarkar, A.P.; Dhagudu, N.; et al. Pain, depression, and poor quality of life in chronic pancreatitis: Relationship with altered brain metabolites. Pancreatology 2022, 22, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.B.; Le-Niculescu, H.; Levey, D.F.; Roseberry, K.; Soe, K.C.; Rogers, J.; Khan, F.; Jones, T.; Judd, S.; McCormick, M.A.; et al. Towards precision medicine for pain: Diagnostic biomarkers and repurposed drugs. Mol. Psychiatry 2019, 24, 501–522. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, J.; Zheng, D.; Luo, Y.; Song, Z.; Chen, F.; Li, A.; Liu, X. Identification of Prognosis-Related Oxidative Stress Model with Immunosuppression in HCC. Biomedicines 2023, 11, 695. [Google Scholar] [CrossRef]
- Kaushik, A.S.; Strath, L.J.; Sorge, R.E. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain. Ther. 2020, 9, 487–498. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, N.; Liu, F.; Lin, J.; Liu, M.; Huang, C.; Liao, D.; Zhou, C.; Wang, H.; Shen, J. Activation of mitogen-activated protein kinases in satellite glial cells of the trigeminal ganglion contributes to substance P-mediated inflammatory pain. Int. J. Oral. Sci. 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Abbah, S.A.; Kilcoyne, M.; Sakai, D.; Dockery, P.; Finn, D.P.; Pandit, A. Implantation of hyaluronic acid hydrogel prevents the pain phenotype in a rat model of intervertebral disc injury. Sci. Adv. 2018, 4, eaaq0597. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Benoit, M.R.; Zhou, J.; Das, B.; Davila-Velderrain, J.; Kellis, M.; Tsai, L.H.; Hu, X.; Yan, R. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci. Adv. 2022, 8, eabo1286. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, W.; Wu, J.; Wu, L.; Xuan, S.; Wang, W.; Shang, A. Neuropeptide Y in the amygdala contributes to neuropathic pain-like behaviors in rats via the neuropeptide Y receptor type 2/mitogen-activated protein kinase axis. Bioengineered 2022, 13, 8101–8114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Gao, S.J.; Sun, J.; Li, D.Y.; Wu, J.Y.; Song, F.H.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. DKK3 ameliorates neuropathic pain via inhibiting ASK-1/JNK/p-38-mediated microglia polarization and neuroinflammation. J. Neuroinflammation 2022, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, W.; Liu, S.; Levitt, R.C.; Candiotti, K.A.; Lubarsky, D.A.; Hao, S. Interleukin 10 mediated by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 in rats. Anesth. Analg. 2014, 119, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.Q.; Chu, Y.X.; Xu, F.; Chen, T.; Gao, L.; Feng, Y.; Hu, X.M.; Yang, W.; Du, L.X.; Zhang, W.W.; et al. Calcium Channel α2δ1 Subunit Mediates Secondary Orofacial Hyperalgesia Through PKC-TRPA1/Gap Junction Signaling. J. Pain. 2020, 21, 238–257. [Google Scholar] [CrossRef]
- Su, Y.; Verkhratsky, A.; Yi, C. Targeting connexins: Possible game changer in managing neuropathic pain? Trends Mol. Med. 2024, 30, 642–659. [Google Scholar] [CrossRef]
- Chen, G.; Park, C.K.; Xie, R.G.; Berta, T.; Nedergaard, M.; Ji, R.R. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 2014, 137(Pt. 8), 2193–2209. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, S.; Tang, S.J. Reactive Oxygen Species (ROS) are Critical for Morphine Exacerbation of HIV-1 gp120-Induced Pain. J. Neuroimmune Pharmacol. 2021, 16, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Hu, D.X.; Zuo, C.; Zhang, W.J. G protein-coupled P2Y12 receptor is involved in the progression of neuropathic pain. Biomed. Pharmacother. 2023, 162, 114713. [Google Scholar] [CrossRef] [PubMed]
- Golan, E.; Haggiag, I.; Os, P.; Bernheim, J. Calcium, parathyroid hormone, and vitamin D: Major determinants of chronic pain in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, E.L.; Kuo, J.; Kohno, K.; Usdin, T.B. Neuropathic and inflammatory pain are modulated by tuberoinfundibular peptide of 39 residues. Proc. Natl. Acad. Sci. USA 2013, 110, 13156–13161. [Google Scholar] [CrossRef]
- Luo, X.; Chen, O.; Wang, Z.; Bang, S.; Ji, J.; Lee, S.H.; Huh, Y.; Furutani, K.; He, Q.; Tao, X.; et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021, 109, 2691–2706.e5. [Google Scholar] [CrossRef] [PubMed]
- Imamov, O.; Yakimchuk, K.; Morani, A.; Schwend, T.; Wada-Hiraike, O.; Razumov, S.; Warner, M.; Gustafsson, J.A. Estrogen receptor beta-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc. Natl. Acad. Sci. USA 2007, 104, 9806–9809. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F. Yersinia infection and acute abdominal pain. Lancet 1987, 1, 1147. [Google Scholar] [CrossRef]
- Lussier, M.P.; Lepage, P.K.; Bousquet, S.M.; Boulay, G. RNF24, a new TRPC interacting protein, causes the intracellular retention of TRPC. Cell Calcium 2008, 43, 432–443. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Monji, A. TRPC Channels and Brain Inflammation. Adv. Exp. Med. Biol. 2017, 976, 111–121. [Google Scholar] [CrossRef]
- Yang, F.; Sivils, A.; Cegielski, V.; Singh, S.; Chu, X.P. Transient Receptor Potential (TRP) Channels in Pain, Neuropsychiatric Disorders, and Epilepsy. Int. J. Mol. Sci. 2023, 24, 4714. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.R.; Wang, Y.; Li, C.L.; Lu, D.; Guan, S.M.; Chen, J. Effects of a non-selective TRPC channel blocker, SKF-96365, on melittin-induced spontaneous persistent nociception and inflammatory pain hypersensitivity. Neurosci. Bull. 2012, 28, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Sagalajev, B.; Yüzer, M.A.; Koivisto, A.; Pertovaara, A. Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neurosci. Lett. 2015, 608, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Harano, N.; Nagahata, S.; Seta, Y.; Tsujisawa, T.; Inenaga, K.; Nakanishi, O. Behavioral characteristics and c-Fos expression in the medullary dorsal horn in a rat model for orofacial cancer pain. Eur. J. Pain. 2009, 13, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sotomayor, M.; Quezada-Calvillo, R.; Avery, S.E.; Chacko, S.K.; Yan, L.K.; Lin, A.H.; Ao, Z.H.; Hamaker, B.R.; Nichols, B.L. Maltase-glucoamylase modulates gluconeogenesis and sucrase-isomaltase dominates starch digestion glucogenesis. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 704–712. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, J.A.; Yezierski, R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial differences in oxidative stress and inflammation: In vitro and in vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.T.; Debs, S.R.; Hayes, J.P.; Duffy, S.S.; Moalem-Taylor, G. Pain-resolving immune mechanisms in neuropathic pain. Nat. Rev. Neurol. 2023, 19, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Krukowski, K.; Laumet, G.O.; Weis, D.; Alexander, J.F.; Heijnen, C.J.; Kavelaars, A. CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight 2022, 7, e154194. [Google Scholar] [CrossRef]
- Durante, M.; Squillace, S.; Lauro, F.; Giancotti, L.A.; Coppi, E.; Cherchi, F.; Di Cesare Mannelli, L.; Ghelardini, C.; Kolar, G.; Wahlman, C.; et al. Adenosine A3 agonists reverse neuropathic pain via T cell-mediated production of IL-10. J. Clin. Investig. 2021, 131, e139299. [Google Scholar] [CrossRef]
- Kim, H.W.; Wang, S.; Davies, A.J.; Oh, S.B. The therapeutic potential of natural killer cells in neuropathic pain. Trends Neurosci. 2023, 46, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Hajhashemi, V.; Abnous, K.; Hosseinzadeh, H. Ceftriaxone, a beta-lactam antibiotic, modulates apoptosis pathways and oxidative stress in a rat model of neuropathic pain. Biomed. Res. Int. 2014, 2014, 937568. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.; Lowes, D.A.; Colvin, L.; Torsney, C.; Galley, H.F. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Luo, Y.; Zhang, L.; Li, C.H.; Khan, A.; Khan, M.I.; Shah, K.; Seo, E.K.; Wang, F.; Khan, S. Daidzein attenuated paclitaxel-induced neuropathic pain via the down-regulation of TRPV1/P2Y and up-regulation of Nrf2/HO-1 signaling. Inflammopharmacology 2023, 31, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Naziroğlu, M.; Uğuz, A.C.; Ismailoğlu, Ö.; Çiğ, B.; Özgül, C.; Borcak, M. Role of TRPM2 cation channels in dorsal root ganglion of rats after experimental spinal cord injury. Muscle Nerve 2013, 48, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Basu, A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Kuhad, A.; Chopra, K. Neuroprotective effect of vitamin E isoforms against chronic alcohol-induced peripheral neurotoxicity: Possible involvement of oxidative-nitrodative stress. Phytother. Res. 2012, 26, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Diakite, D.; Austin, J.; Lee, J.H.; Lantvit, D.D.; Murphy, B.T.; Burdette, J.E. The Flavonoid Baicalein Negatively Regulates Progesterone Target Genes in the Uterus in Vivo. J. Nat. Prod. 2022, 85, 237–247. [Google Scholar] [CrossRef]
- Todd, E.V.; Ortega-Recalde, O.; Liu, H.; Lamm, M.S.; Rutherford, K.M.; Cross, H.; Black, M.A.; Kardailsky, O.; Marshall Graves, J.A.; Hore, T.A.; et al. Stress, novel sex genes, and epigenetic reprogramming orchestrate socially controlled sex change. Sci. Adv. 2019, 5, eaaw7006. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Geng, M.; Li, X.; Gu, Y.; Zhao, W.; Ning, Q.; Zhao, Z.; Wang, L.; Zhang, H.; Zhang, F. Identification of Oxidative Stress-Related Biomarkers for Pain–Depression Comorbidity Based on Bioinformatics. Int. J. Mol. Sci. 2024, 25, 8353. https://doi.org/10.3390/ijms25158353
Zhang T, Geng M, Li X, Gu Y, Zhao W, Ning Q, Zhao Z, Wang L, Zhang H, Zhang F. Identification of Oxidative Stress-Related Biomarkers for Pain–Depression Comorbidity Based on Bioinformatics. International Journal of Molecular Sciences. 2024; 25(15):8353. https://doi.org/10.3390/ijms25158353
Chicago/Turabian StyleZhang, Tianyun, Menglu Geng, Xiaoke Li, Yulin Gu, Wenjing Zhao, Qi Ning, Zijie Zhao, Lei Wang, Huaxing Zhang, and Fan Zhang. 2024. "Identification of Oxidative Stress-Related Biomarkers for Pain–Depression Comorbidity Based on Bioinformatics" International Journal of Molecular Sciences 25, no. 15: 8353. https://doi.org/10.3390/ijms25158353





