Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress
Abstract
1. Introduction
2. Results
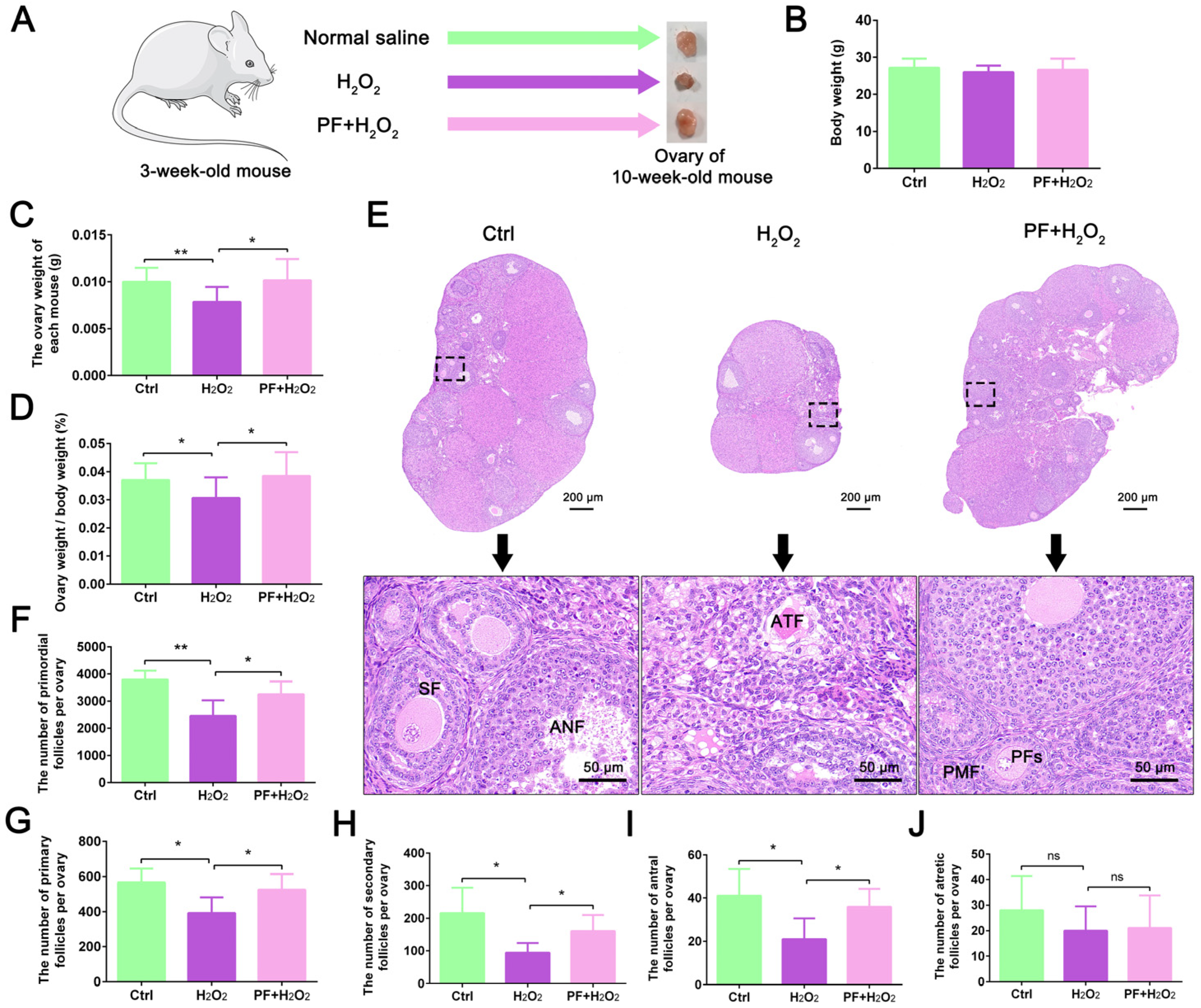
2.1. H2O2-Induced Ovarian Developmental Delay in Mice Is Attenuated by PF Administration
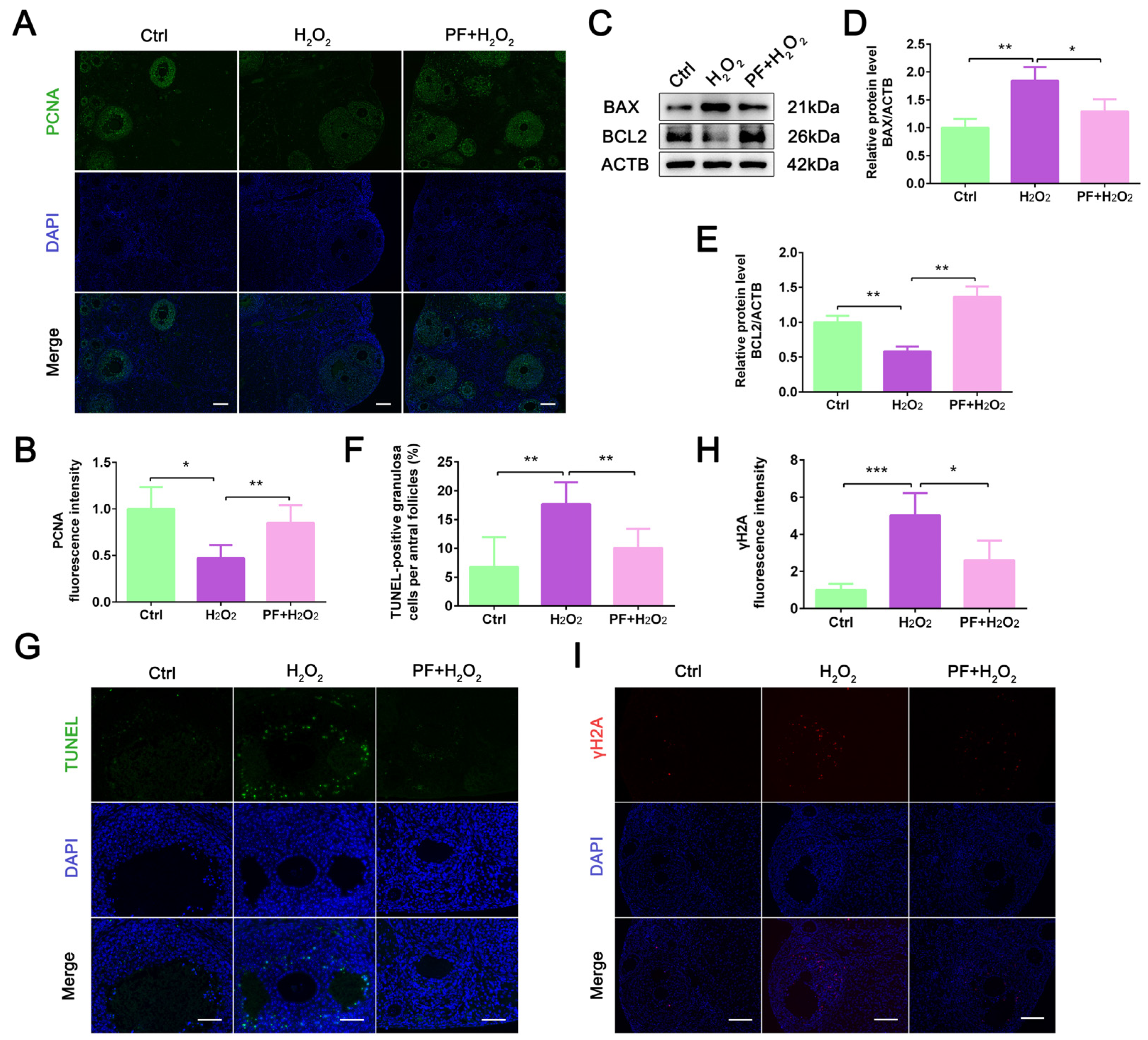
2.2. PF Inhibits H2O2-Induced Cell Apoptosis and Promotes Granulosa Cell Proliferation
2.3. PF Prevents Oxidative Stress and Inhibits Ferroptosis in Mouse Ovaries
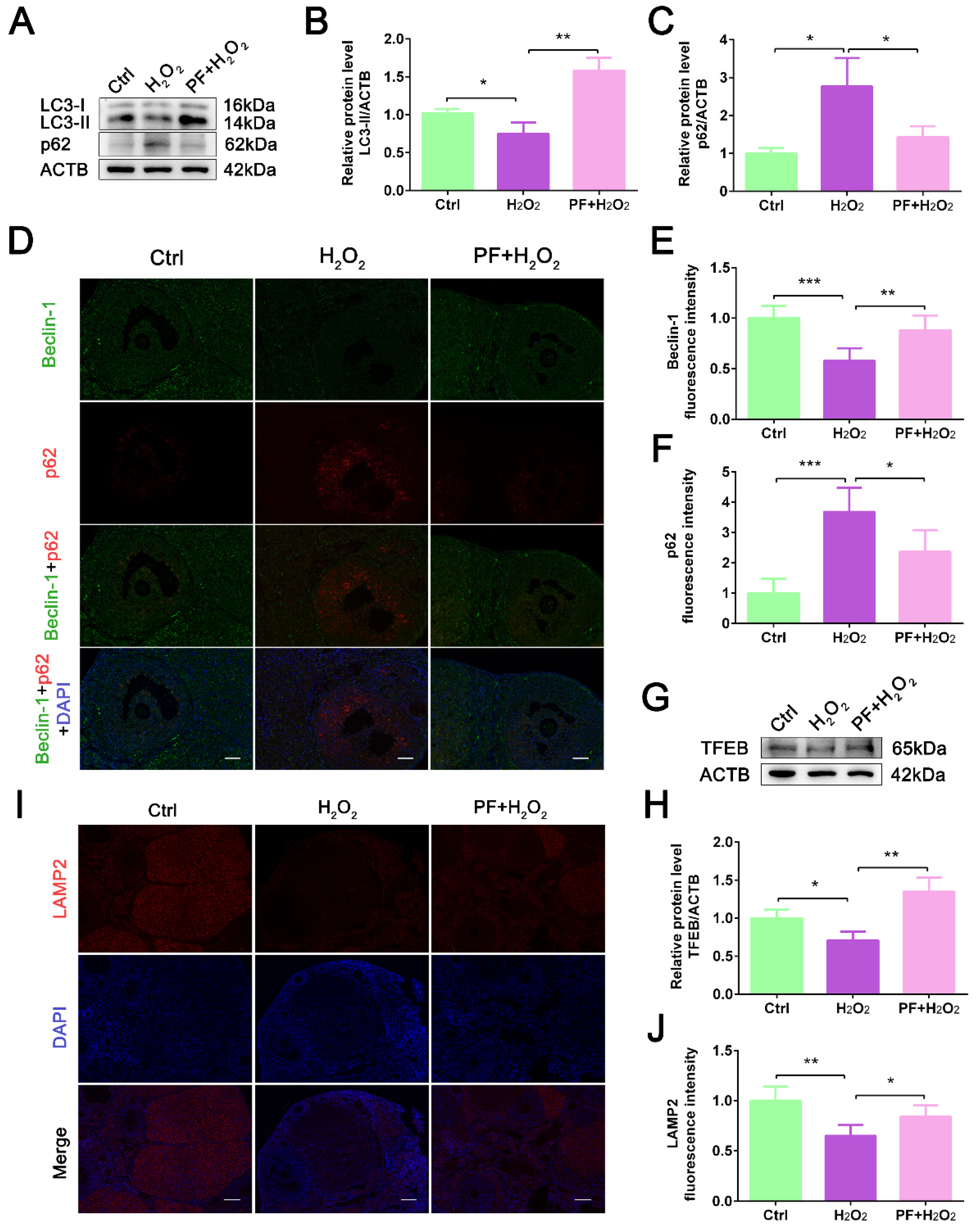
2.4. PF Activates Autophagy Activity and Lysosomal Biogenesis
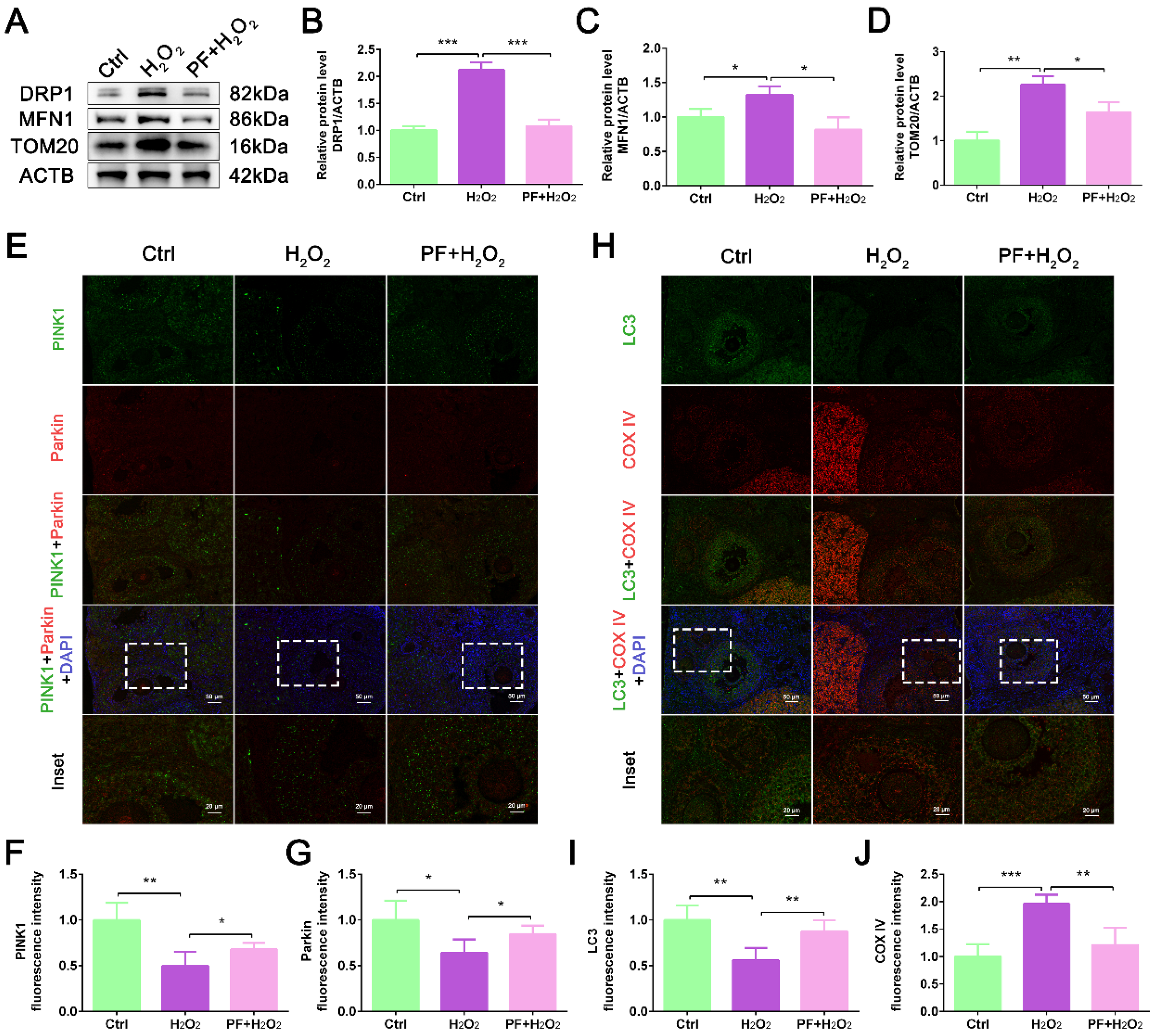
2.5. PF Promotes Mitophagy to Improve Mitochondrial Quality
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Histology and Follicle Count
4.4. TUNEL Assay
4.5. Immunofluorescence (IF)
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Pan, Y.; Wang, Q.; Lu, S.; Li, Y.; Liu, W.; Zheng, T.; Wang, B.; Qiang, J.; Xu, P. Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil. Antioxidants 2023, 12, 1524. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, L.G.; Paulino, L.R.; Silva, B.R.; Barbalho, E.C.; Nascimento, D.R.; Neto, M.F.L.; Silva, J.R. N-acetyl-cysteine and the control of oxidative stress during in vitro ovarian follicle growth, oocyte maturation, embryo development and cryopreservation. Anim. Reprod. Sci. 2021, 231, 106801. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020, 37, 101726. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.K.; Liu, H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, M.; Chen, Y.; Wei, Y.; Tao, J.; Liu, H. Melatonin Represses Mitophagy to Protect Mouse Granulosa Cells from Oxidative Damage. Biomolecules 2021, 11, 968. [Google Scholar] [CrossRef]
- Hou, L.; Gu, T.; Weng, K.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effects of Oxidative Stress on the Autophagy and Apoptosis of Granulosa Cells in Broody Geese. Int. J. Mol. Sci. 2023, 24, 2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Q.; Shen, M.; Zhu, C.-C.; Yu, F.-X.; Liu, Z.-Q.; Ally, N.; Sun, S.-C.; Li, K.; Liu, H.-L. 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS ONE 2014, 9, e86589. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 2014, 147, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gawriluk, T.R.; Hale, A.N.; Flaws, J.A.; Dillon, C.P.; Green, D.R.; Rucker, E.B., 3rd. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 2011, 141, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Gioia, L.; Festuccia, C.; Colapietro, A.; Gloria, A.; Contri, A.; Valbonetti, L. Abundances of autophagy-related protein LC3B in granulosa cells, cumulus cells, and oocytes during atresia of pig antral follicles. Anim. Reprod. Sci. 2019, 211, 106225. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, Y.; Zhang, Y.; Yan, P.; Xiao, D.; Zhao, Y.; Jia, W.; Ding, L.; Dong, H.; Wei, C.; et al. Paeoniflorin alleviates AngII-induced cardiac hypertrophy in H9c2 cells by regulating oxidative stress and Nrf2 signaling pathway. Biomed. Pharmacother. 2023, 165, 115253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, J.; Ban, Y.; Wan, T.T.; Song, W.; Zhao, W.; Teng, Y. Synergistic effect of paeoniflorin combined with luteolin in alleviating Lipopolysaccharides-induced acute lung injury. J. Ethnopharmacol. 2024, 327, 118022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dong, C.; Zhai, L.; Lou, J.; Jin, J.; Cheng, S.; Chen, Z.; Guo, X.; Lin, D.; Ding, J.; et al. Paeoniflorin Suppresses TBHP-Induced Oxidative Stress and Apoptosis in Human Umbilical Vein Endothelial Cells via the Nrf2/HO-1 Signaling Pathway and Improves Skin Flap Survival. Front. Pharmacol. 2021, 12, 735530. [Google Scholar] [CrossRef]
- Wang, L.; An, H.; Yu, F.; Yang, J.; Ding, H.; Bao, Y.; Xie, H.; Huang, D. The neuroprotective effects of paeoniflorin against MPP(+)-induced damage to dopaminergic neurons via the Akt/Nrf2/GPX4 pathway. J. Chem. Neuroanat. 2022, 122, 102103. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, Y.; Wang, X.; Zhu, M. Paeoniflorin attenuates DHEA-induced polycystic ovary syndrome via inactivation of TGF-β1/Smads signaling pathway in vivo. Aging 2021, 13, 7084–7095. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, M.; Li, Y.; Zhao, X.; Fan, C.; Dai, Y. Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway. Molecules 2023, 28, 8123. [Google Scholar] [CrossRef]
- Park, M.J.; Han, S.-E.; Kim, H.J.; Heo, J.D.; Choi, H.-J.; Ha, K.-T.; Yang, S.W.; Lee, K.S.; Kim, S.C.; Kim, C.W.; et al. Paeonia lactiflora improves ovarian function and oocyte quality in aged female mice. Anim. Reprod. 2020, 17, e20200013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, H.; Mu, H.; Zeng, L.; Cai, S.; Su, P.; Li, H.; Zhang, L.; Xiang, W. miR-484 mediates oxidative stress-induced ovarian dysfunction and promotes granulosa cell apoptosis via SESN2 downregulation. Redox Biol. 2023, 62, 102684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Q.; Chang, M.; Pan, Y.; Yahaya, B.H.; Liu, Y.; Lin, J. Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis. 2023, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Jiang, D.; Guo, Y.; Wang, Z.; Sun, Q.; Wang, X.; Ling, W.; An, X.; Ji, C.; Li, S.; et al. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023, 332, 122109. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; He, T.; Yang, H.; Dai, W.; Liu, L.; Ma, X.; Ma, J.; Yang, G.; Si, R.; Du, X.; et al. Activation of the p62-Keap1-Nrf2 pathway protects against oxidative stress and excessive autophagy in ovarian granulosa cells to attenuate DEHP-induced ovarian impairment in mice. Ecotoxicol. Environ. Saf. 2023, 265, 115534. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Q.L.; Li, Z.H.; Ji, R.; Wang, J.Y.; Cao, M.L.; Mu, X.F.; Zhang, Y.; Guo, D.Y.; Yang, J. Pterostilbene ameliorates oxidative damage and ferroptosis in human ovarian granulosa cells by regulating the Nrf2/HO-1 pathway. Arch. Biochem. Biophys. 2023, 738, 109561. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Deng, F.; Wang, P.; Yang, L.; Hu, L.; Huang, K.; He, J. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022, 29, 1982–1995. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Zhang, M.; Zhou, L.; Jiang, L.; Gao, L.; Wang, X.; Huang, Y.; Zeng, H.; Wu, Y. Paeoniflorin alleviates ischemia/reperfusion induced acute kidney injury by inhibiting Slc7a11-mediated ferroptosis. Int. Immunopharmacol. 2023, 116, 109754. [Google Scholar]
- Zeng, Q.; Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008, 13, 188–192. [Google Scholar] [CrossRef]
- Hussein, M.R. Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Update 2005, 11, 162–177. [Google Scholar] [CrossRef]
- Zhou, H.-Q.; Liu, W.; Wang, J.; Huang, Y.-Q.; Li, P.-Y.; Zhu, Y.; Wang, J.-B.; Ma, X.; Li, R.-S.; Wei, S.-Z.; et al. Paeoniflorin attenuates ANIT-induced cholestasis by inhibiting apoptosis in vivo via mitochondria-dependent pathway. Biomed. Pharmacother. 2017, 89, 696–704. [Google Scholar] [CrossRef]
- Hua, X.; Feng, X.; Hua, Y.; Wang, D. Paeoniflorin attenuates polystyrene nanoparticle-induced reduction in reproductive capacity and increase in germline apoptosis through suppressing DNA damage checkpoints in Caenorhabditis elegans. Sci. Total Environ. 2023, 871, 162189. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Yang, J.-L.; Zhang, J.; Li, L.; Huang, L.; Ji, S.-Y.; Hu, Z.-Y.; Gao, F.; Liu, Y.-X. Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology 2011, 152, 2437–2447. [Google Scholar] [CrossRef]
- Lv, X.; He, C.; Huang, C.; Wang, H.; Hua, G.; Wang, Z.; Zhou, J.; Chen, X.; Ma, B.; Timm, B.K.; et al. Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development. FASEB J. 2019, 33, 10049–10064. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cui, J.; Xiao, T.; Jiang, D. Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway. Biomed. Pharmacother. 2016, 78, 197–203. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Lin, H.; Chen, H.; Wang, S. Paeoniflorin Inhibits the Proliferation and Metastasis of Ulcerative Colitis-Associated Colon Cancer by Targeting EGFL7. J. Oncol. 2022, 2022, 7498771. [Google Scholar] [CrossRef]
- Hułas-Stasiak, M.; Gawron, A. Follicular atresia in the prepubertal spiny mouse (Acomys cahirinus) ovary. Apoptosis Int. J. Program. Cell Death 2011, 16, 967–975. [Google Scholar] [CrossRef]
- Inoue, N.; Matsuda, F.; Goto, Y.; Manabe, N. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J. Reprod. Dev. 2011, 57, 169–175. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, J.; Guan, X.; Yin, S.; Chen, J.; Yuan, S.; Liu, H.; Lin, S.; Zhou, Y.; Qiu, J.; et al. Paeoniflorin suppresses kidney inflammation by regulating macrophage polarization via KLF4-mediated mitophagy. Phytomedicine 2023, 116, 154901. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Liu, X.-Q.; Huang, Y.-B.; Wang, A.-L.; Zeng, H.-X.; Gao, L.; Zhu, Q.-J.; Xia, L.-L.; Wu, Y.-G. Paeoniflorin binds to VEGFR2 to restore autophagy and inhibit apoptosis for podocyte protection in diabetic kidney disease through PI3K-AKT signaling pathway. Phytomedicine 2022, 106, 154400. [Google Scholar] [CrossRef]
- Zhao, F.; Peng, C.; Li, H.; Chen, H.; Yang, Y.; Ai, Q.; Chen, N.; Liu, F. Paeoniae Radix Rubra extract attenuates cerebral ischemia injury by inhibiting ferroptosis and activating autophagy through the PI3K/Akt signalling pathway. J. Ethnopharmacol. 2023, 315, 116567. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Cheng, X.; Lin, L.; Quan, S.; Li, S.; Zhan, R.; Wu, Q.; Liu, S. Paeoniflorin, ferulic acid, and atractylenolide III improved LPS-induced neuroinflammation of BV2 microglia cells by enhancing autophagy. J. Pharmacol. Sci. 2023, 152, 151–161. [Google Scholar] [CrossRef]
- Sun, R.; Liu, J.; Yu, M.; Xia, M.; Zhang, Y.; Sun, X.; Xu, Y.; Cui, X. Paeoniflorin Ameliorates BiPN by Reducing IL6 Levels and Regulating PARKIN-Mediated Mitochondrial Autophagy. Drug Des. Dev. Ther. 2022, 16, 2241–2259. [Google Scholar] [CrossRef]
- Albano, G.D.; Montalbano, A.M.; Gagliardo, R.; Profita, M. Autophagy/Mitophagy in Airway Diseases: Impact of Oxidative Stress on Epithelial Cells. Biomolecules 2023, 13, 1217. [Google Scholar] [CrossRef]
- Talebi, M.; Vadoud, S.A.M.; Haratian, A.; Talebi, M.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The interplay between oxidative stress and autophagy: Focus on the development of neurological diseases. Behav. Brain Funct. BBF 2022, 18, 3. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef]
- Yang, Q.E.; Gwost, I.; Oatley, M.J.; Oatley, J.M. Retinoblastoma protein (RB1) controls fate determination in stem cells and progenitors of the mouse male germline. Biol. Reprod. 2013, 89, 113. [Google Scholar] [CrossRef] [PubMed]

| For Immunofluorescence Assay | |||
|---|---|---|---|
| Antibody | Dilution | Cat No. | Company |
| PCNA | 1:500 | GB11010 | Servicebio, Wuhan, China |
| HO-1 | 1:400 | GB12104 | Servicebio, Wuhan, China |
| Nrf2 | 1:300 | 16396-1-AP | Proteintech, Rosemont, IL, USA |
| Beclin-1 | 1:300 | 66665-1-Ig | Proteintech, Rosemont, IL, USA |
| p62 | 1:500 | 18420-1-AP | Proteintech, Rosemont, IL, USA |
| LAMP2 | 1:400 | GB11330 | Servicebio, Wuhan, China |
| PINK1 | 1:300 | GB114934 | Servicebio, Wuhan, China |
| Parkin | 1:200 | 66674-1-Ig | Proteintech, Rosemont, IL, USA |
| LC3 | 1:100 | A19665 | Abclonal, Wuhan, China |
| COX IV | 1:500 | GB12250 | Servicebio, Wuhan, China |
| γH2A | 1:500 | GB111841 | Servicebio, Wuhan, China |
| For Western blot assay | |||
| TOM20 | 1:3000 | A19403 | Abclonal, Wuhan, China |
| MFN1 | 1:4500 | 13798-1-AP | Proteintech, Rosemont, IL, USA |
| DRP1 | 1:1000 | 8570S | Cell Signaling Technology, Danvers, MA, USA |
| TFEB | 1:1000 | ER65144 | HUABIO, Hangzhou, China |
| p62 | 1:10,000 | 18420-1-AP | Proteintech, Rosemont, IL, USA |
| LC3 | 1:1000 | A19665 | Abclonal, Wuhan, China |
| GPX4 | 1:1000 | 30388-1-AP | Proteintech, Rosemont, IL, USA |
| BCL2 | 1:4000 | 12789-1-AP | Proteintech, Rosemont, IL, USA |
| BAX | 1:6000 | 50599-2-Ig | Proteintech, Rosemont, IL, USA |
| ACTB | 1:5000 | 20536-1-AP | Proteintech, Rosemont, IL, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, H.; Wang, Z.; Li, M.; Duan, X.; Li, Y. Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 8355. https://doi.org/10.3390/ijms25158355
Xi H, Wang Z, Li M, Duan X, Li Y. Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress. International Journal of Molecular Sciences. 2024; 25(15):8355. https://doi.org/10.3390/ijms25158355
Chicago/Turabian StyleXi, Huaming, Ziqian Wang, Minghui Li, Xing Duan, and Yuan Li. 2024. "Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress" International Journal of Molecular Sciences 25, no. 15: 8355. https://doi.org/10.3390/ijms25158355
APA StyleXi, H., Wang, Z., Li, M., Duan, X., & Li, Y. (2024). Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress. International Journal of Molecular Sciences, 25(15), 8355. https://doi.org/10.3390/ijms25158355






