MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections
Abstract
1. Introduction
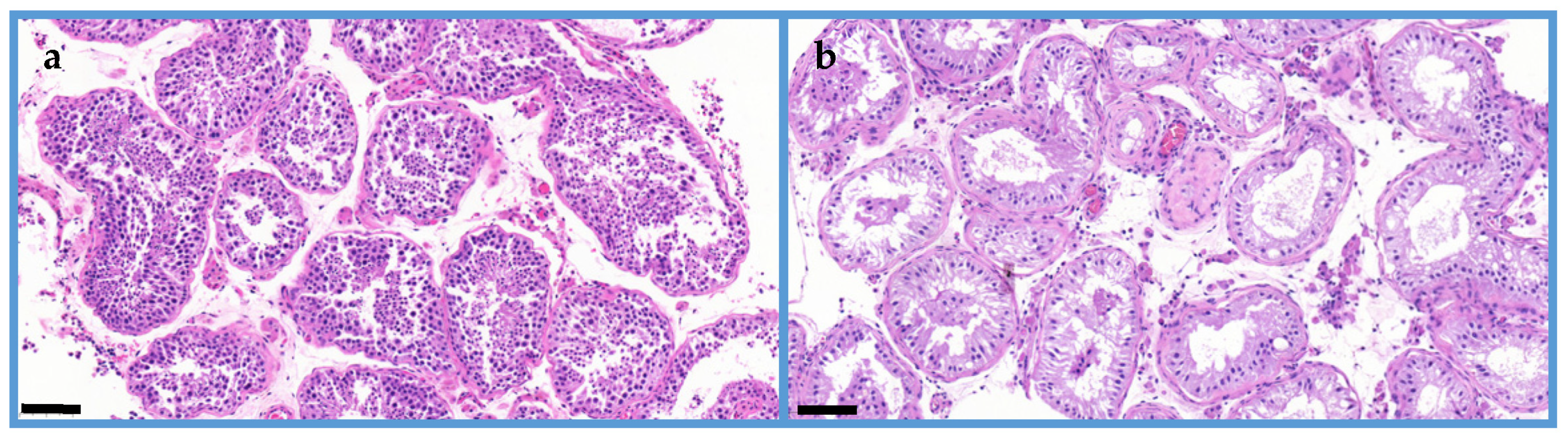
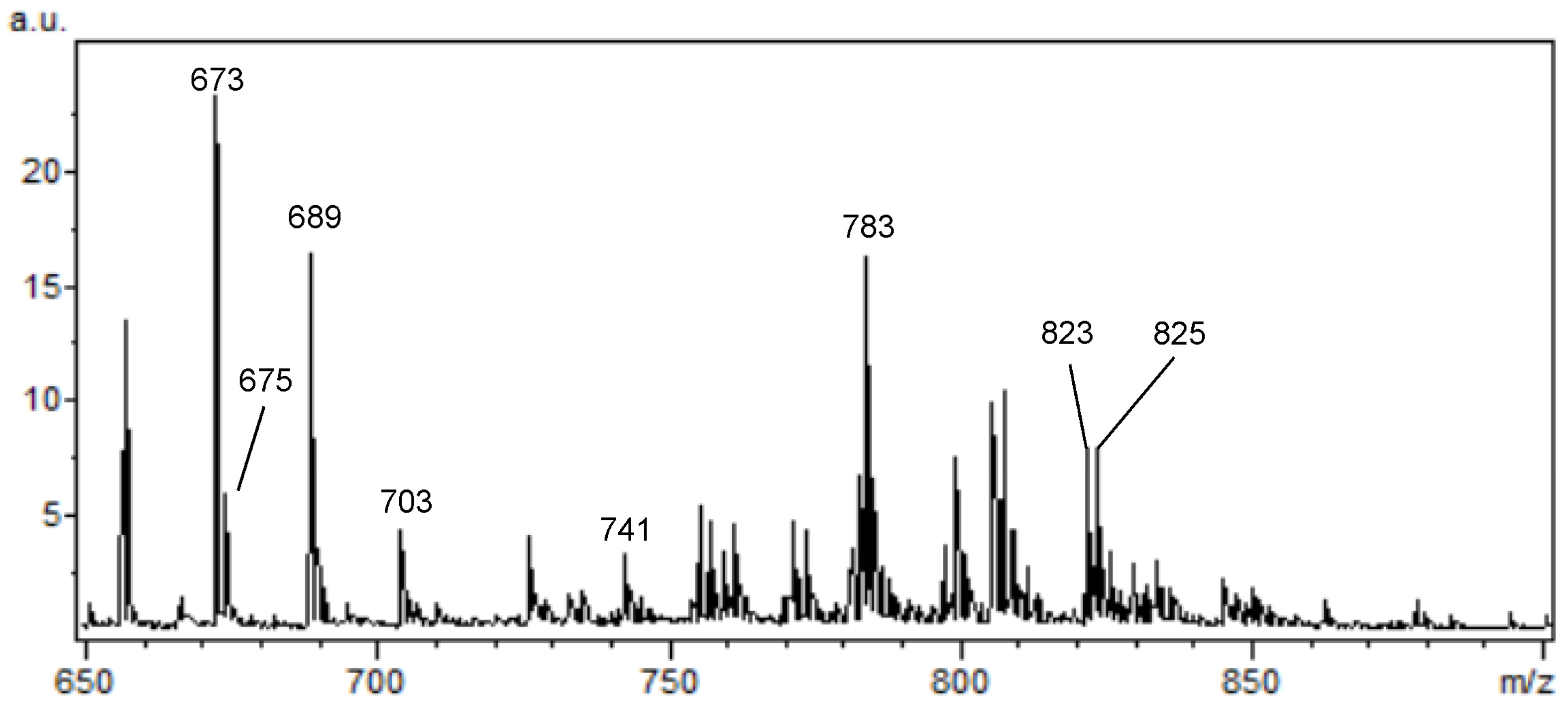
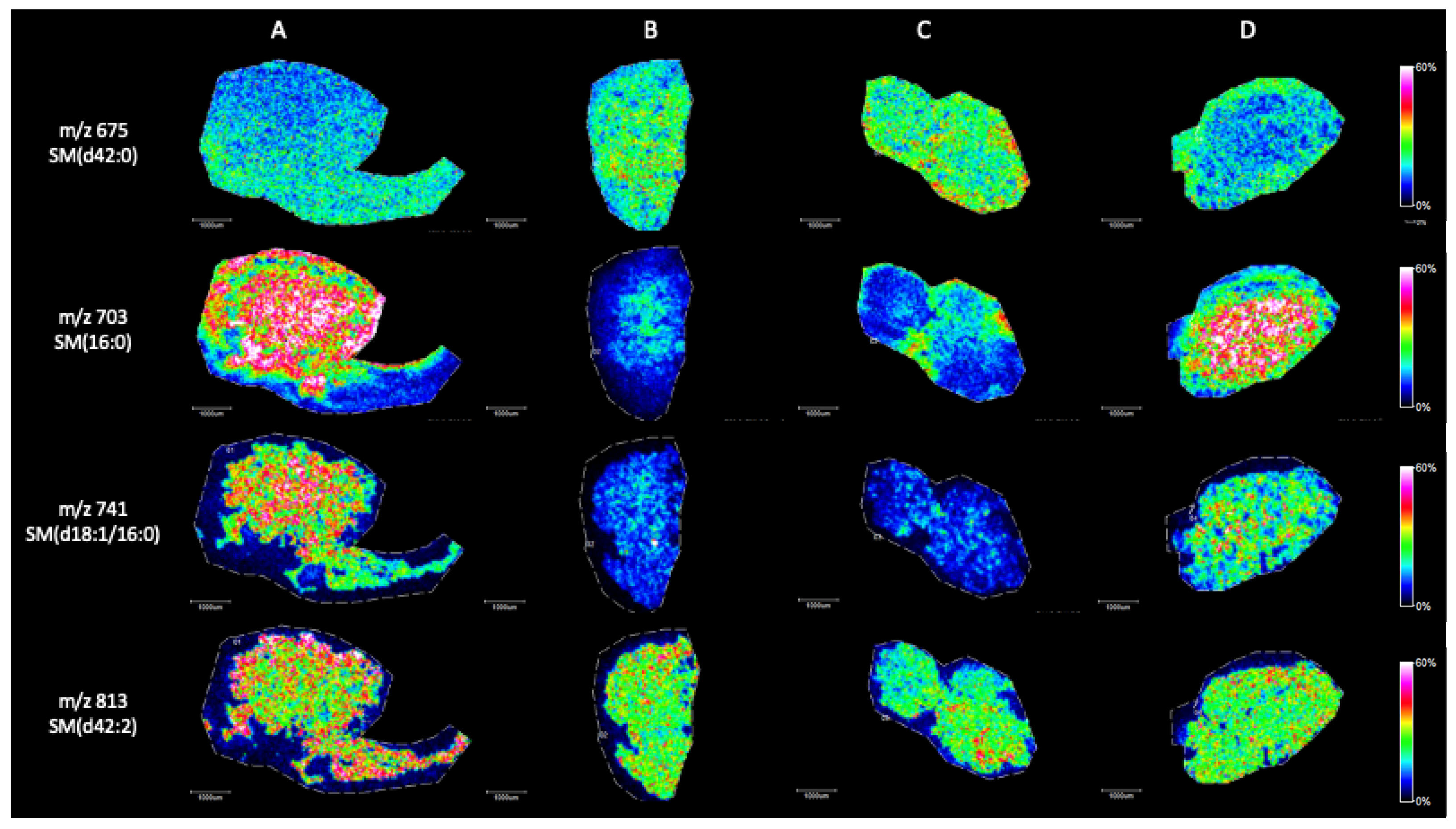
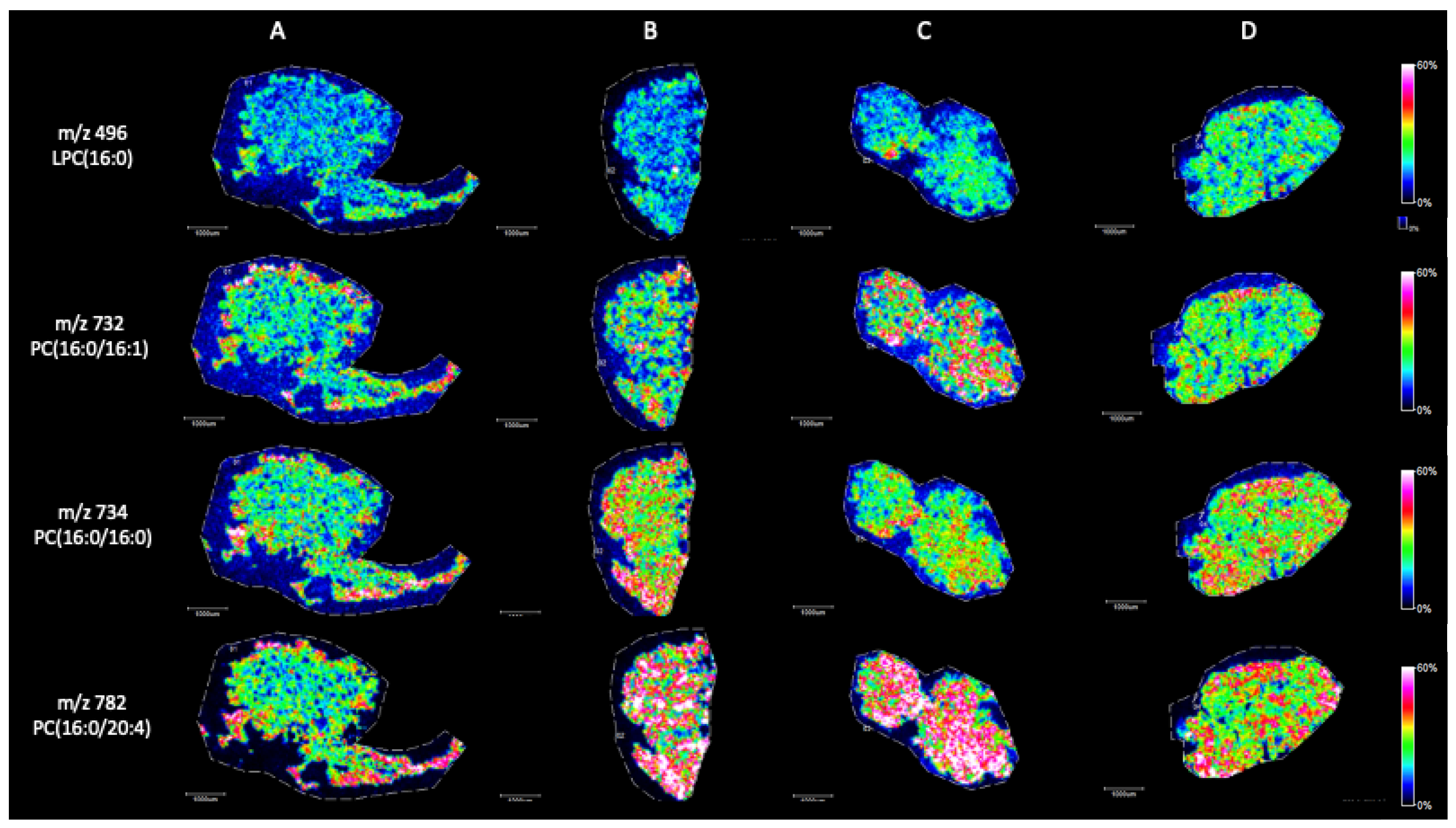
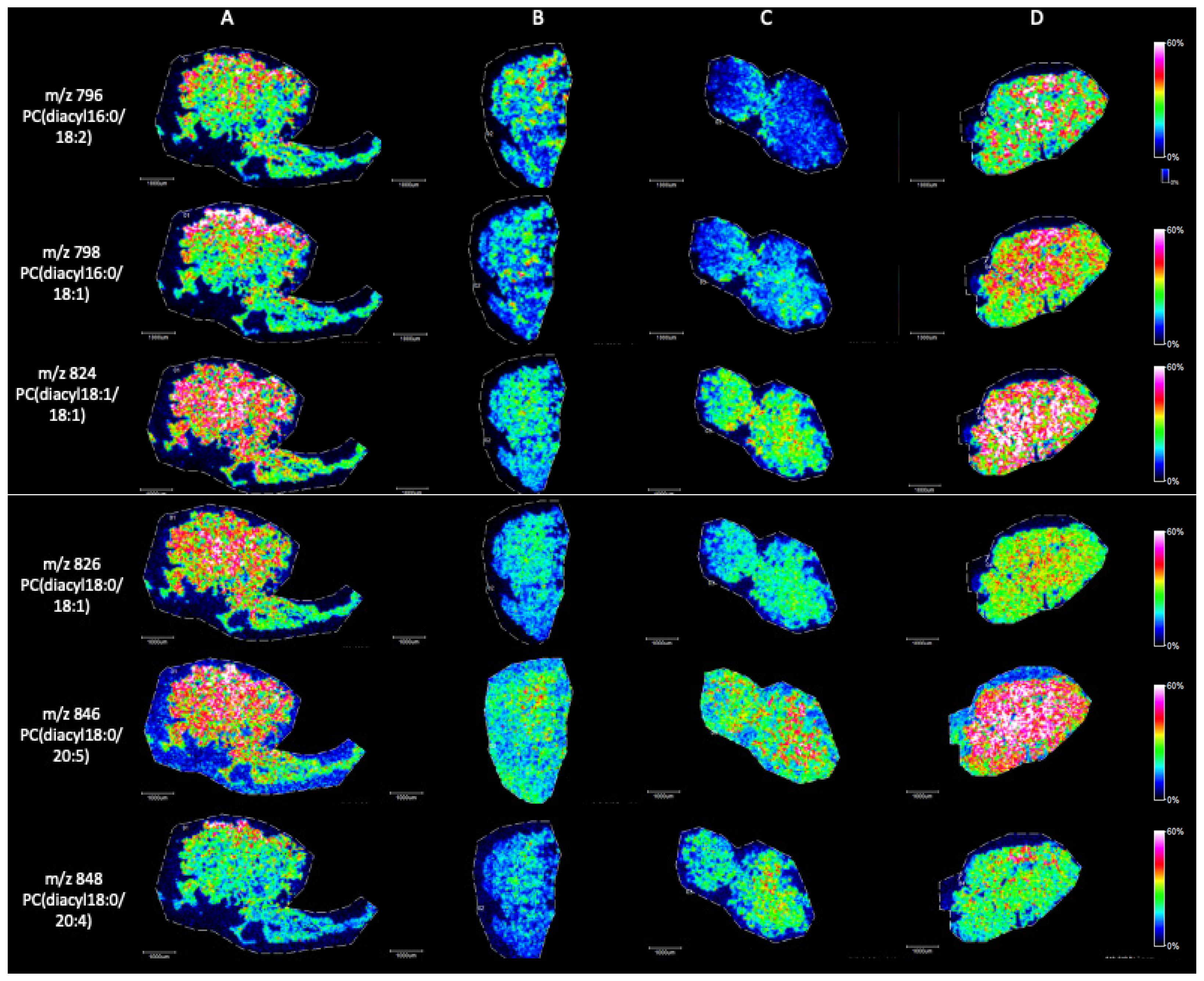
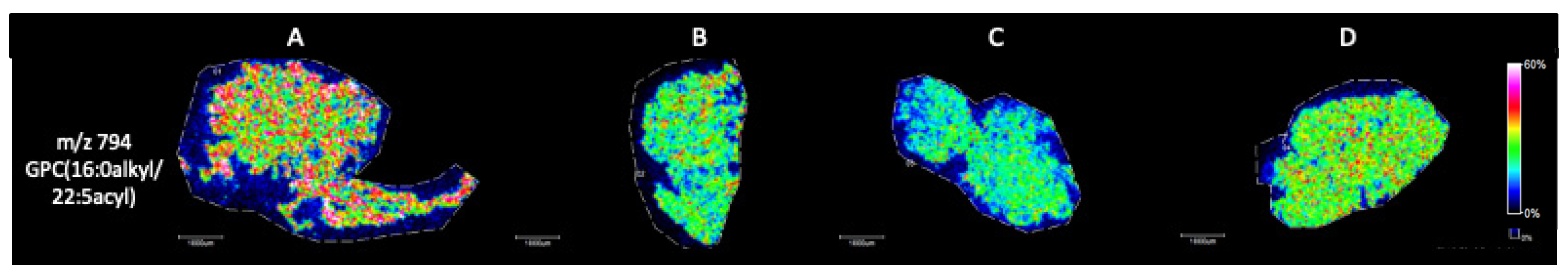
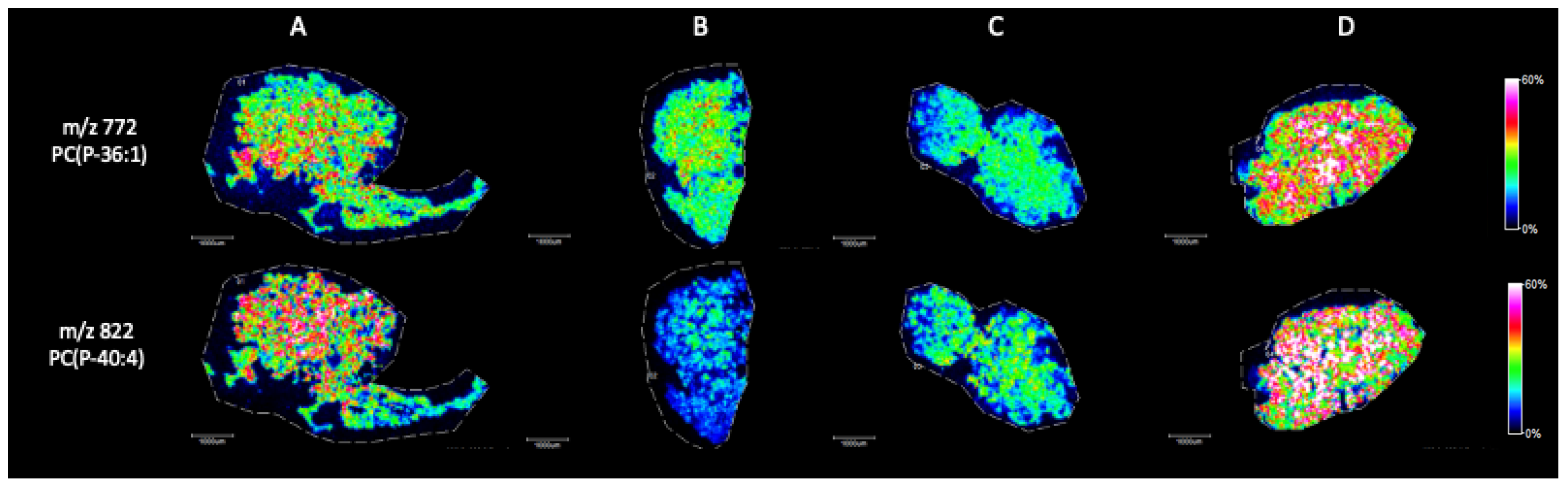
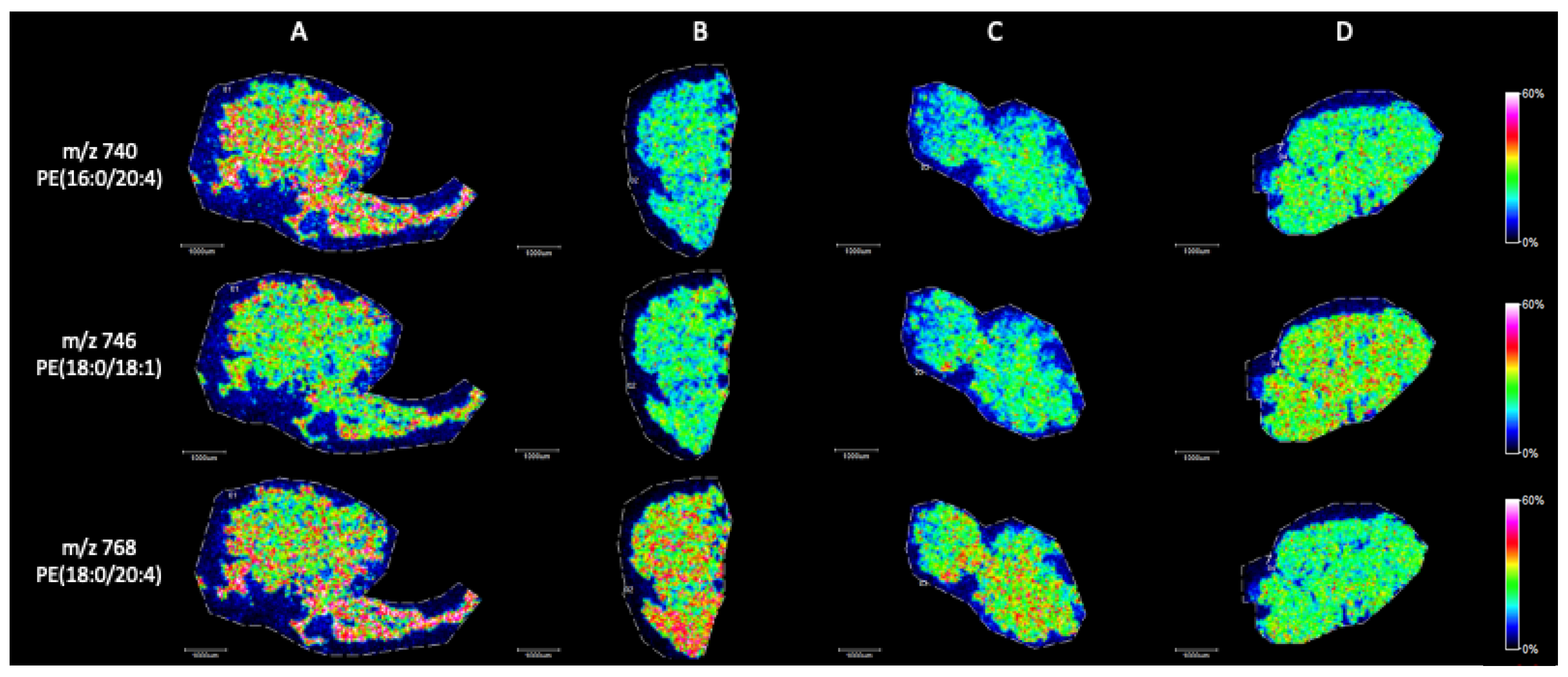
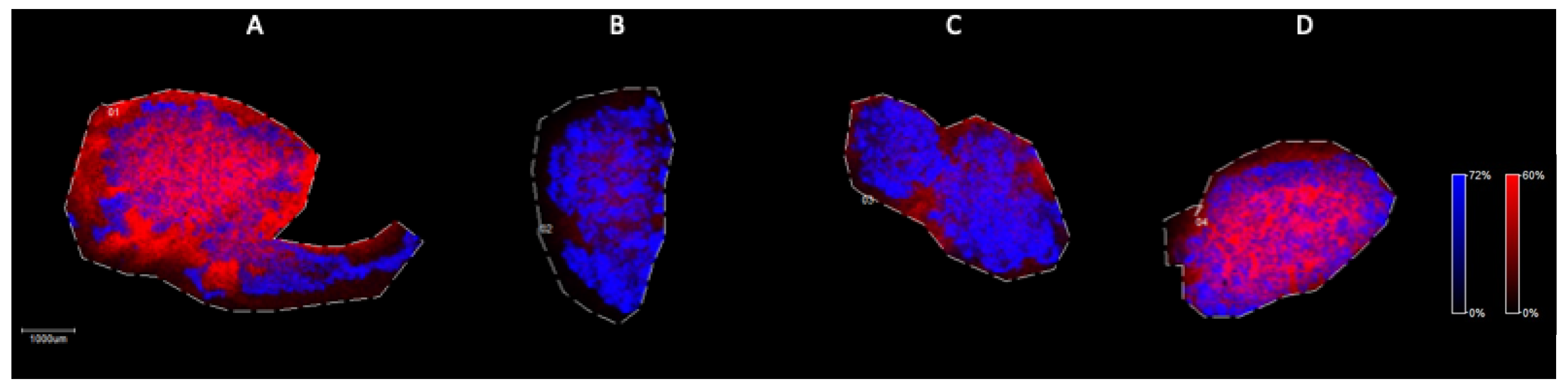
2. Results
MALDI Imaging Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Histological Tissue Sections
4.3. MALDI Imaging Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hanukoglu, A.; Hanukoglu, I. Localization of epithelial sodium channel (ENaC) and CFTR in the germinal epithelium of the testis, Sertoli cells, and spermatozoa. J. Mol. Hist. 2018, 49, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [PubMed]
- Alpatov, R.; Lesch, B.J.; Nakamoto-Kinoshita, M.; Blanco, A.; Chen, S.; Stützer, A.; Armache, K.J.; Simon, M.D.; Xu, C.; Ali, M.; et al. A Chromatin-Dependent Role of the Fragile X Mental Retardation Protein FMRP in the DNA Damage Response. Cell 2014, 157, 869–881. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Khire, A.; Fishman, E.L.; Kyoung, H. Atypical centrioles during sexual reproduction. Front. Cell Dev. Biol. 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Barrachina, F.; Soler-Ventura, A.; Jodar, M.; Mancini, F.; Marana, R.; Chiloiro, S.; Pontecorvi, A.; Oliva, R.; Milardi, D. The Role of Testosterone in Spermatogenesis: Lessons From Proteome Profiling of Human Spermatozoa in Testosterone Deficiency. Front. Endocrinol. 2022, 13, 852661. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Mruk, D.D.; Cheng, C.Y. Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum. Reprod. Update 2013, 19, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Goto-Inoue, N.; Hayasaka, T.; Zaima, N.; Setou, M. Imaging mass spectrometry reveals changes of metabolites distribution in mouse testis during testicular maturation. Surf. Interface Anal. 2012, 44, 749–754. [Google Scholar] [CrossRef]
- Goto-Inoue, N.; Hayasaka, T.; Zaima, N.; Setou, M. The specific localization of seminolipid molecular species on mouse testis during testicular maturation revealed by imaging mass spectrometry. Glycobiology 2009, 19, 950–957. [Google Scholar] [CrossRef]
- Fujimoto, H.; Tadano-Aritomi, K.; Tokumasu, A.; Ito, K.; Hikita, T.; Suzuki, K.; Ishizuka, I. Requirement of seminolipid in spermatogenesis revealed by UDP-galactose: Ceramide galactosyltransferase-deficient mice. J. Biol. Chem. 2000, 275, 22623–22626. [Google Scholar] [CrossRef]
- Ueno, K.; Ishizuka, I.; Yamakawa, T. Glycolipid composition of human testis at different ages and the stereochemical configuration of seminolipid. Biochim. Biophys. Acta 1977, 487, 61–73. [Google Scholar]
- Goto-Inoue, N.; Hayasaka, T.; Zaima, N.; Setou, M. Imaging mass spectrometry for lipidomics. Biochim. Biophys. Acta 2011, 1811, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Siangcham, T.; Chansela, P.; Hayasaka, T.; Masaki, N.; Sroyraya, M.; Poljaroen, J.; Suwansa-ard, S.; Engsusophon, A.; Hanna, P.J.; Sobhon, P.; et al. Changes of phosphatidylcholine and fatty acids in germ cells during testicular maturation in three developmental male morphotypes of Macrobrachium rosenbergii revealed by imaging mass spectrometry. PLoS ONE 2015, 10, e0120412. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, M.; Lavigne, R.; Guével, B.; Palmer, A.; Rondel, K.; Guillot, L.; Kobarg, J.H.; Pineau, C. Spatial segmentation and metabolite annotation involved in sperm maturation in the rat epididymis by MALDI imaging mass spectrometry. J. Mass Spectrom. 2020, 55, e4633. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, M.; Lavigne, R.; Guével, B.; Com, E.; Chaurand, P.; Pineau, C. Matrix-assisted laser desorption/ionization imaging mass spectrometry: A promising technique for reproductive research. Biol. Repro. 2012, 86, 74. [Google Scholar] [CrossRef]
- Wang, D.H.; Chen, Z.J.; Jiang, Y.Y.; Zhou, H.; Yang, W.X. Fatty acid composition and analysis of freshwater caridean shrimp Macrobrachium nipponense (De Haan) during spermiogenesis. Aquac. Res. 2010, 41, 1140–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Hayashi, Y.; Cheng, X.; Watanabe, T.; Wang, X.; Taniguchi, N.; Honke, K. Testis-specific sulfoglycolipid, seminolipid, is essential for germ cell function in spermatogenesis. Glycobiology 2005, 15, 649–654. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine, National Centre for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534293/ (accessed on 20 June 2023).
- Janosek-Albright, K.J.C.; Schlegel, P.N.; Dabaja, A.A. Testis sperm extraction. Asian J. Urol. 2015, 2, 79–84. [Google Scholar] [CrossRef]
- Ghanami, G.N.; Sadighi, G.M.A.; Abbasi, M. Sertoli cell-only syndrome: Etiology and clinical management. J. Assist. Reprod. Genet. 2021, 38, 559–572. [Google Scholar] [CrossRef]
- Adamczewska, D.; Slowikowska-Hilczer, J.; Marchlewska, K.; Walczak-Jedrzejowska, R. Features of gonadal dysgenesis and Leydig cell impairment in testes with Sertoli cell-only syndrome. Folia Histochem. Cytobiol. 2020, 58, 73–82. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Kongmanas, K.; Faull, K.F.; Whitelegge, J.; Compostella, F.; Goto-Inoue, N.; Linton, J.J.; Doyle, B.; Oko, R.; Xu, H.; et al. Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog. Lipid Res. 2018, 72, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Asadi, M.H.; Bahadoran, H.; Dashtnavard, H.; Imani, H.; Naghii, M.R. Cadmium Toxicity in Spermatogenesis and Protective Effects of L-Carnitine in Adult Male Rats. Biol. Trace Elem. Res. 2009, 137, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, R.; Tabet, J.C.; Ducoroy, P.; Hendra, J.B.; Salzet, M.; Fournier, I. Solid ionic matrixes for direct tissue analysis and MALDI imaging. Anal. Chem. 2006, 78, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Hashemitabar, M.; Sabbagh, S.; Orazizadeh, M.; Ghadiri, A.; Bahmanzadeh, M. A proteomic analysis on human sperm tail: Comparison between normozoospermia and asthenozoospermia. J. Assist. Reprod. Genet. 2015, 32, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.E.; Rolland, A.D.; Rajpert-De Meyts, E.; Janfelt, C.; Jørgensen, A.; Winge, S.B.; Kristensen, D.M.; Juul, A.; Chalmel, F.; Jégou, B.; et al. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci. Rep. 2019, 9, 12866. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.A.; Hankin, J.A.; Barkley, R.M.; Spraggins, J.M.; Caprioli, R.M.; Murphy, R.C. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem. Rev. 2011, 111, 6491–6512. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef] [PubMed]
- Pyttel, S.; Nimptsch, A.; Böttger, J.; Zschörnig, K.; Jakop, U.; Wegener, J.; Müller, K.; Paasch, U.; Schiller, J. Changes of murine sperm phospholipid composition during epididymal maturation determined by MALDI-TOF mass spectrometry. Theriogenology 2014, 82, 396–402. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Ma, Q.; Zhu, Y.; Zhao, W.; Yang, Y.; Xiao, W.; Huang, D.; Cai, F.; Chan, D.Y.L.; et al. Testis cell pyroptosis mediated by CASP1 and CASP4: Possible sertoli cell-only syndrome pathogenesis. Reprod. Biol. Endocrinol. 2023, 21, 53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulc, A.; Czétány, P.; Máté, G.; Balló, A.; Semjén, D.; Szántó, Á.; Márk, L. MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections. Int. J. Mol. Sci. 2024, 25, 8358. https://doi.org/10.3390/ijms25158358
Sulc A, Czétány P, Máté G, Balló A, Semjén D, Szántó Á, Márk L. MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections. International Journal of Molecular Sciences. 2024; 25(15):8358. https://doi.org/10.3390/ijms25158358
Chicago/Turabian StyleSulc, Alexandra, Péter Czétány, Gábor Máté, András Balló, Dávid Semjén, Árpád Szántó, and László Márk. 2024. "MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections" International Journal of Molecular Sciences 25, no. 15: 8358. https://doi.org/10.3390/ijms25158358
APA StyleSulc, A., Czétány, P., Máté, G., Balló, A., Semjén, D., Szántó, Á., & Márk, L. (2024). MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections. International Journal of Molecular Sciences, 25(15), 8358. https://doi.org/10.3390/ijms25158358



