Functional Insights into the Sphingolipids C1P, S1P, and SPC in Human Fibroblast-like Synoviocytes by Proteomic Analysis
Abstract
1. Introduction
2. Results
2.1. Regulated Proteins Identified by MS
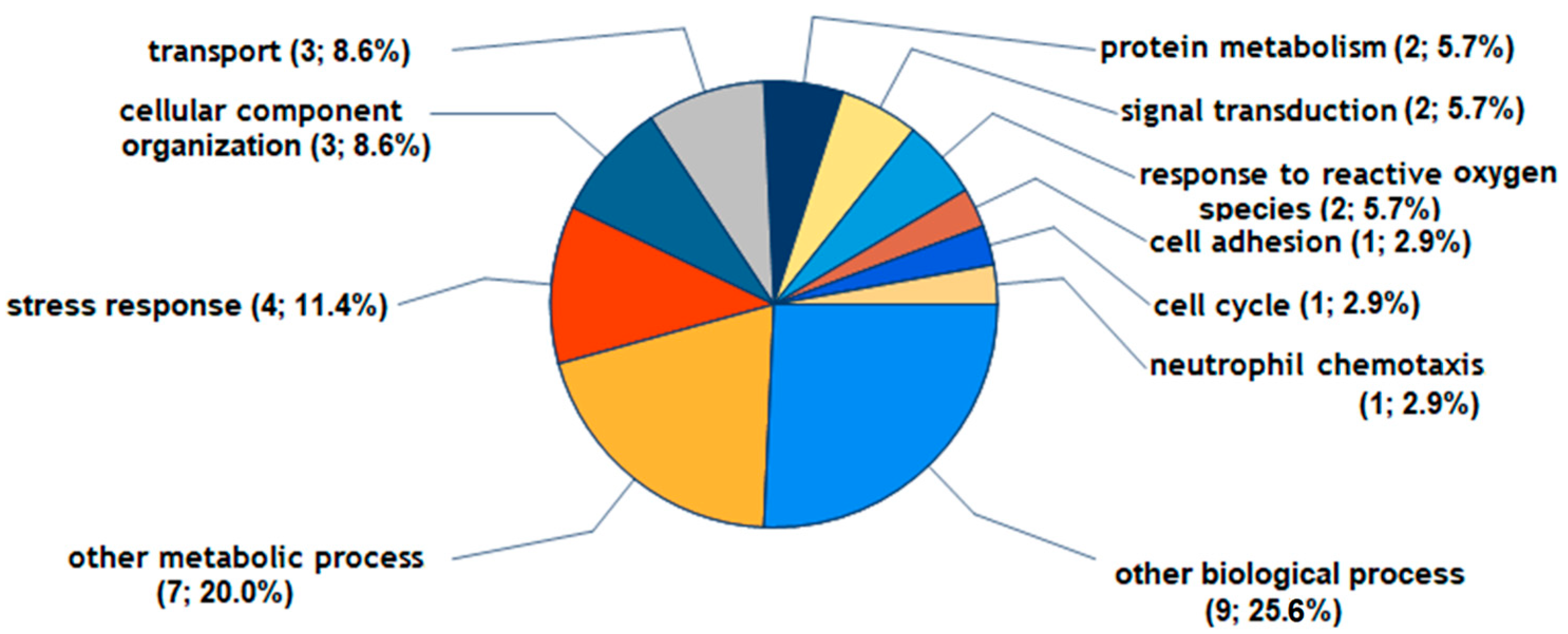
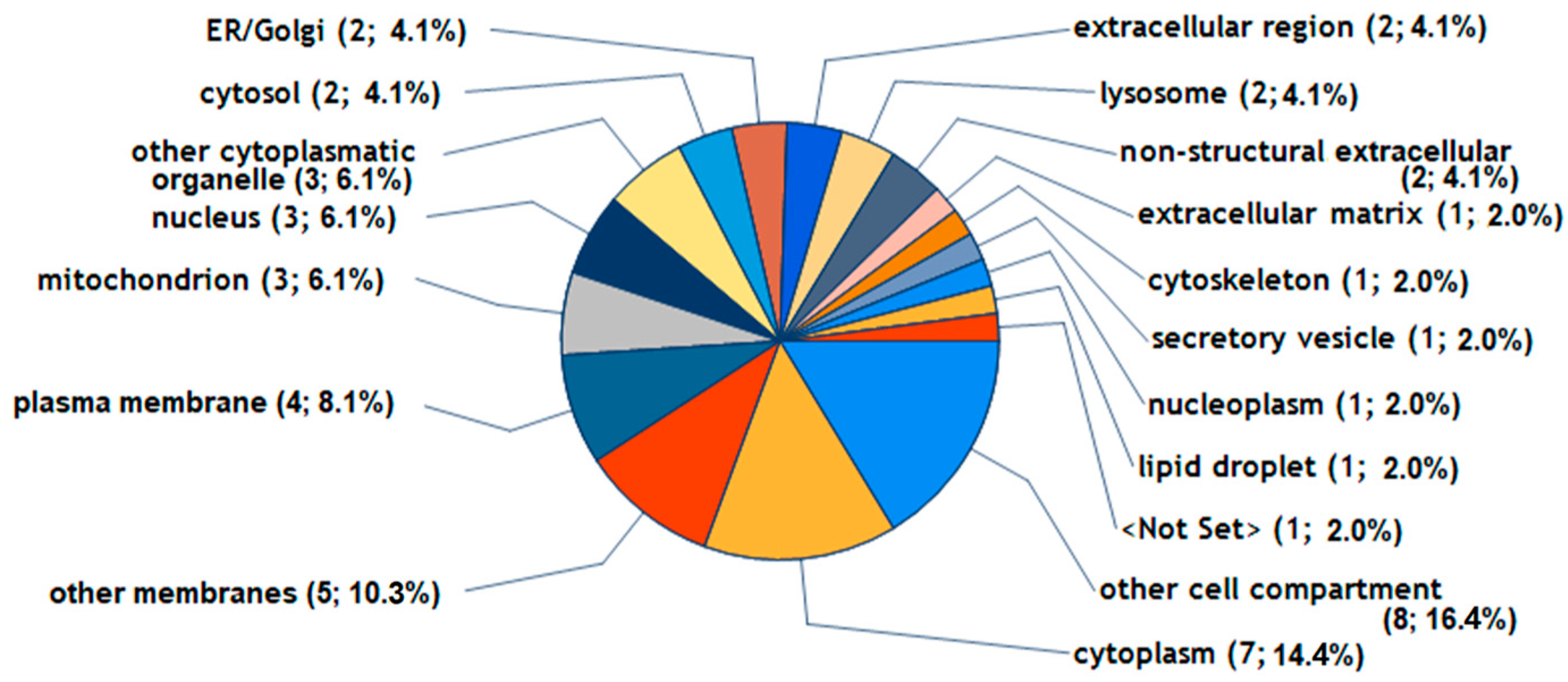
2.2. Regulated Proteins and Their Potential Biological Functions in FLS
2.3. RT-PCR on Several Regulated Proteins
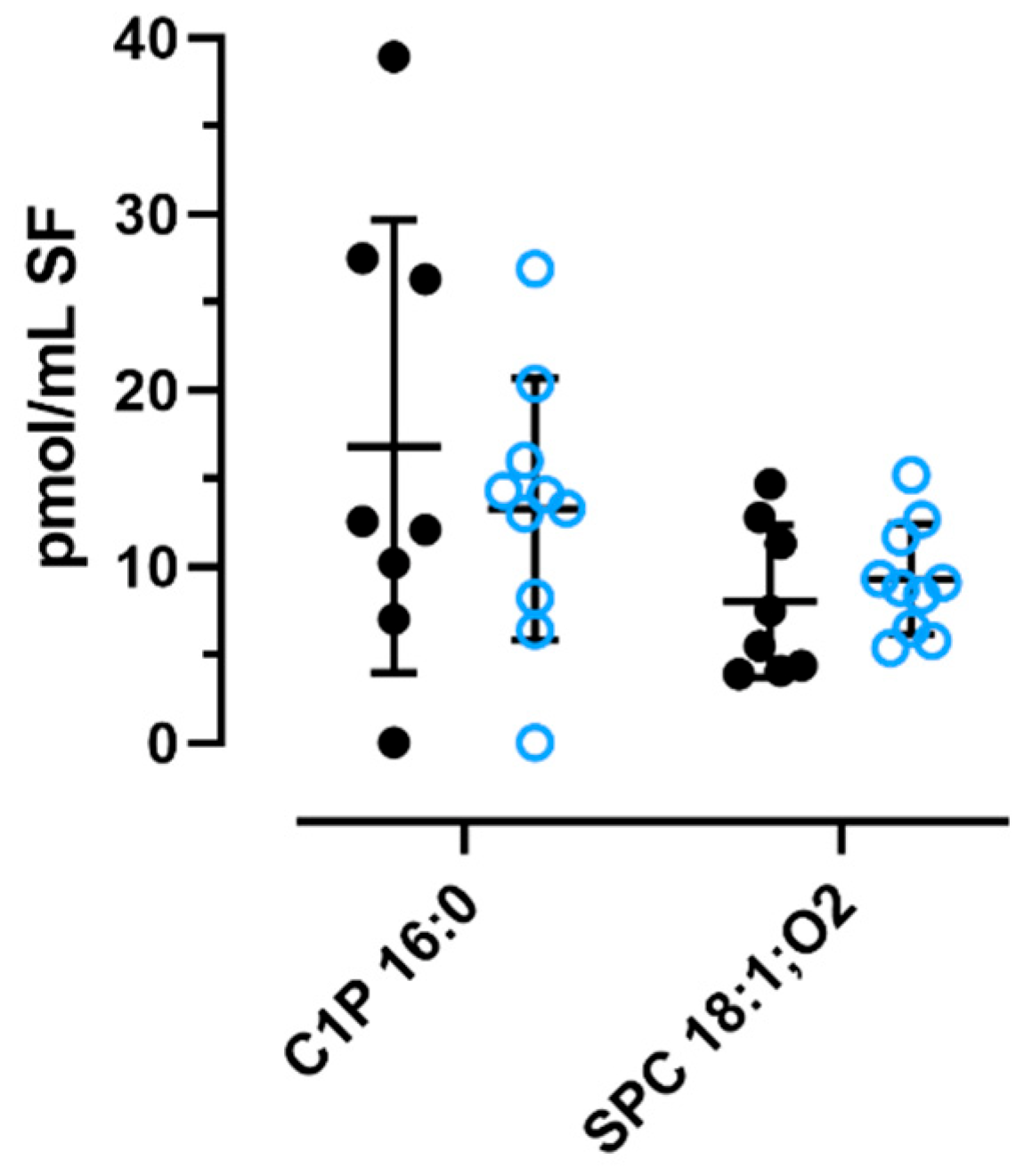
2.4. Quantification of C1P and SPC in OA Knee Synovial Fluid
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Specimen Selection for Isolation of FLSs
4.3. Culture of FLSs
4.4. Treatment of Cultured FLSs for Proteomic and mRNA Analysis
4.5. Isolation, TMT Labeling, and Mass Spectrometry of Cellular Proteins
4.6. Protein Identification and Quantitation
4.7. Relative mRNA Expression
4.8. Sampling of Human Synovial Fluid
4.9. MS Analysis of Sphingolipids in Knee Synovial Fluid of OA Patients
4.10. Bioinformatics
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. Sphingolipids in human synovial fluid—A lipidomic study. PLoS ONE 2014, 9, e91769. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.-C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PLoS ONE 2015, 10, e0125192. [Google Scholar] [CrossRef] [PubMed]
- Thottakkattumana Parameswaran, V.; Hild, C.; Eichner, G.; Ishaque, B.; Rickert, M.; Steinmeyer, J. Interleukin-1 Induces the Release of Lubricating Phospholipids from Human Osteoarthritic Fibroblast-like Synoviocytes. Int. J. Mol. Sci. 2022, 23, 2409. [Google Scholar] [CrossRef] [PubMed]
- Sluzalska, K.D.; Liebisch, G.; Wilhelm, J.; Ishaque, B.; Hackstein, H.; Schmitz, G.; Rickert, M.; Steinmeyer, J. Growth factors regulate phospholipid biosynthesis in human fibroblast-like synoviocytes obtained from osteoarthritic knees. Sci. Rep. 2017, 7, 13469. [Google Scholar] [CrossRef] [PubMed]
- Sluzalska, K.D.; Liebisch, G.; Lochnit, G.; Ishaque, B.; Hackstein, H.; Schmitz, G.; Rickert, M.; Steinmeyer, J. Interleukin-1β affects the phospholipid biosynthesis of fibroblast-like synoviocytes from human osteoarthritic knee joints. Osteoarthr. Cartil. 2017, 25, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Ingale, D.; Kulkarni, P.; Electricwala, A.; Moghe, A.; Kamyab, S.; Jagtap, S.; Martson, A.; Koks, S.; Harsulkar, A. Synovium-Synovial Fluid Axis in Osteoarthritis Pathology: A Key Regulator of the Cartilage Degradation Process. Genes 2021, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Ishijima, M.; Kaneko, H.; Kurihara, H.; Arikawa-Hirasawa, E.; Kubota, M.; Liu, L.; Xu, Z.; Futami, I.; Yusup, A.; et al. Correlations between both the expression levels of inflammatory mediators and growth factor in medial perimeniscal synovial tissue and the severity of medial knee osteoarthritis. Int. Orthop. 2011, 35, 831–838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Timm, T.; Hild, C.; Liebisch, G.; Rickert, M.; Lochnit, G.; Steinmeyer, J. Functional Characterization of Lysophospholipids by Proteomic and Lipidomic Analysis of Fibroblast-like Synoviocytes. Cells 2023, 12, 1743. [Google Scholar] [CrossRef]
- Christie, W.W. Ceramide-1-Phosphate. The LipidWeb. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=lipids/sphingo/cer-1-p/index.htm (accessed on 15 February 2024).
- Graf, C.; Zemann, B.; Rovina, P.; Urtz, N.; Schanzer, A.; Reuschel, R.; Mechtcheriakova, D.; Müller, M.; Fischer, E.; Reichel, C.; et al. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J. Immunol. 2008, 180, 3457–3466. [Google Scholar] [CrossRef]
- Scherer, M.; Böttcher, A.; Schmitz, G.; Liebisch, G. Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.H.; Gangoiti, P.; Ouro, A.; Arana, L.; González, M.; Trueba, M.; Gómez-Muñoz, A. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell. Signal. 2009, 21, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Presa, N.; Gomez-Larrauri, A.; Rivera, I.-G.; Ordoñez, M.; Trueba, M.; Gomez-Muñoz, A. Regulation of cell migration and inflammation by ceramide 1-phosphate. Biochim. Biophys. Acta 2016, 1861, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.-G.; Trueba, M.; Ordoñez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Grösch, S.; Alessenko, A.V.; Albi, E. The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response. Mediat. Inflamm. 2018, 2018, 5378284. [Google Scholar] [CrossRef]
- Pettus, B.J.; Bielawska, A.; Subramanian, P.; Wijesinghe, D.S.; Maceyka, M.; Leslie, C.C.; Evans, J.H.; Freiberg, J.; Roddy, P.; Hannun, Y.A.; et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004, 279, 11320–11326. [Google Scholar] [CrossRef]
- Lamour, N.F.; Chalfant, C.E. Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target. Curr. Drug Targets 2008, 9, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Lamour, N.F.; Subramanian, P.; Wijesinghe, D.S.; Stahelin, R.V.; Bonventre, J.V.; Chalfant, C.E. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J. Biol. Chem. 2009, 284, 26897–26907. [Google Scholar] [CrossRef]
- Hankins, J.L.; Fox, T.E.; Barth, B.M.; Unrath, K.A.; Kester, M. Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J. Biol. Chem. 2011, 286, 44357–44366. [Google Scholar] [CrossRef]
- Christie, W.W. Sphingosine-1-Phosphate. The LipidWeb. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=lipids/sphingo/sph-1-p/index.htm (accessed on 15 February 2024).
- Kitano, M.; Hla, T.; Sekiguchi, M.; Kawahito, Y.; Yoshimura, R.; Miyazawa, K.; Iwasaki, T.; Sano, H.; Saba, J.D.; Tam, Y.Y. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: Regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006, 54, 742–753. [Google Scholar] [CrossRef]
- Lucaciu, A.; Brunkhorst, R.; Pfeilschifter, J.M.; Pfeilschifter, W.; Subburayalu, J. The S1P–S1PR Axis in Neurological Disorders—Insights into Current and Future Therapeutic Perspectives. Cells 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Weske, S. Targeting sphingosine-1-phosphate lyase as an anabolic therapy for bone loss. Nat. Med. 2018, 24, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.; Wegner, D.; Groß, S.; Bahls, M.; Dörr, M.; Felix, S.B.; Ittermann, T.; Oswald, S.; Nauck, M.; Friedrich, N.; et al. Reference intervals for serum sphingosine-1-phosphate in the population-based Study of Health in Pomerania. Clin. Chim. Acta 2017, 468, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.; Jedlitschky, G.; Negnal, J.; Tzvetkov, M.V.; Daum, G.; Dörr, M.; Felix, S.B.; Völzke, H.; Nauck, M.; Schwedhelm, E.; et al. Increased Sphingosine-1-Phosphate Serum Concentrations in Subjects with Periodontitis: A Matter of Inflammation. J. Inflamm. Res. 2021, 14, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Green, C.; Maines, L.W.; Smith, C.D. Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology 2011, 87, 135–143. [Google Scholar] [CrossRef] [PubMed]
- El Jamal, A.; Bougault, C.; Mebarek, S.; Magne, D.; Cuvillier, O.; Brizuela, L. The role of sphingosine 1-phosphate metabolism in bone and joint pathologies and ectopic calcification. Bone 2020, 130, 115087. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Murata, M.; Beppu, M.; Nakamura, H.; Kato, T.; Yudoh, K. Sphingosine-1-phosphate modulates expression of vascular endothelial growth factor in human articular chondrocytes: A possible new role in arthritis. Int. J. Rheum. Dis. 2012, 15, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Murata, M.; Nakamura, H.; Yudoh, K.; Nishioka, K.; Kato, T. Sphingosine-1-phosphate attenuates proteoglycan aggrecan expression via production of prostaglandin E2 from human articular chondrocytes. BMC Musculoskelet. Disord. 2007, 8, 29. [Google Scholar] [CrossRef]
- Stradner, M.H.; Gruber, G.; Angerer, H.; Huber, V.; Setznagl, D.; Kremser, M.-L.; Moazedi-Fürst, F.C.; Windhager, R.; Graninger, W.B. Sphingosine 1-phosphate counteracts the effects of interleukin-1β in human chondrocytes. Arthritis Rheum. 2013, 65, 2113–2122. [Google Scholar] [CrossRef]
- Cherifi, C.; Latourte, A.; Vettorazzi, S.; Tuckermann, J.; Provot, S.; Ea, H.-K.; Ledoux, A.; Casas, J.; Cuvillier, O.; Richette, P.; et al. Inhibition of sphingosine 1-phosphate protects mice against chondrocyte catabolism and osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Lee, H.Y.; Kwak, J.-Y.; Park, J.-I.; Yun, J.; Bae, Y.-S. Sphingosine-1-phosphate stimulates rat primary chondrocyte proliferation. Biochem. Biophys. Res. Commun. 2006, 345, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Meyer zu Heringdorf, D.; Himmel, H.M.; Jakobs, K.H. Sphingosylphosphorylcholine-biological functions and mechanisms of action. Biochim. Biophys. Acta 2002, 1582, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wirrig, C.; Hunter, I.; Mathieson, F.A.; Nixon, G.F. Sphingosylphosphorylcholine is a proinflammatory mediator in cerebral arteries. J. Cereb. Blood Flow Metab. 2011, 31, 212–221. [Google Scholar] [CrossRef]
- Christie, W.W. Sphingomyelin and Related Sphingophospholipids. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=lipids/sphingo/sph/index.htm (accessed on 22 February 2024).
- Park, M.K.; Lee, C.H. Role of Sphingosylphosphorylcholine in Tumor and Tumor Microenvironment. Cancers 2019, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Liliom, K.; Sun, G.; Bünemann, M.; Virág, T.; Nusser, N.; Baker, D.L.; Wang, D.A.; Fabian, M.J.; Brandts, B.; Bender, K.; et al. Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: A possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 2001, 355, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like synoviocytes: Role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell. Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis—Chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Shen, S.; Guo, J.; Luo, Y.; Zhang, W.; Cui, Y.; Wang, Q.; Zhang, Z.; Wang, T. Functional proteomics revealed IL-1β amplifies TNF downstream protein signals in human synoviocytes in a TNF-independent manner. Biochem. Biophys. Res. Commun. 2014, 450, 538–544. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, M.; Zhang, Y.-Y.; Zhao, S.-Z.; Gu, S. The endosomal sorting complex required for transport repairs the membrane to delay cell death. Front. Oncol. 2022, 12, 1007446. [Google Scholar] [CrossRef]
- Adler, D.H.; Cogan, J.D.; Phillips, J.A.; Schnetz-Boutaud, N.; Milne, G.L.; Iverson, T.; Stein, J.A.; Brenner, D.A.; Morrow, J.D.; Boutaud, O.; et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J. Clin. Investig. 2008, 118, 2121–2131. [Google Scholar] [CrossRef]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef] [PubMed]
- Leistad, L.; Feuerherm, A.J.; Faxvaag, A.; Johansen, B. Multiple phospholipase A2 enzymes participate in the inflammatory process in osteoarthritic cartilage. Scand. J. Rheumatol. 2011, 40, 308–316. [Google Scholar] [CrossRef]
- Golej, D.L.; Askari, B.; Kramer, F.; Barnhart, S.; Vivekanandan-Giri, A.; Pennathur, S.; Bornfeldt, K.E. Long-chain acyl-CoA synthetase 4 modulates prostaglandin E₂ release from human arterial smooth muscle cells. J. Lipid Res. 2011, 52, 782–793. [Google Scholar] [CrossRef]
- Silva, R.L.; Lopes, A.H.; Guimarães, R.M.; Cunha, T.M. CXCL1/CXCR2 signaling in pathological pain: Role in peripheral and central sensitization. Neurobiol. Dis. 2017, 105, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.-M.; Chen, P.-C.; Lin, C.-M.; Fang, M.-L.; Chi, M.-C.; Liu, J.-F. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 2020, 22, 251. [Google Scholar] [CrossRef]
- Merz, D.; Liu, R.; Johnson, K.; Terkeltaub, R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J. Immunol. 2003, 171, 4406–4415. [Google Scholar] [CrossRef]
- Olivotto, E.; Vitellozzi, R.; Fernandez, P.; Falcieri, E.; Battistelli, M.; Burattini, S.; Facchini, A.; Flamigni, F.; Santi, S.; Facchini, A.; et al. Chondrocyte hypertrophy and apoptosis induced by GROalpha require three-dimensional interaction with the extracellular matrix and a co-receptor role of chondroitin sulfate and are associated with the mitochondrial splicing variant of cathepsin B. J. Cell. Physiol. 2007, 210, 417–427. [Google Scholar] [CrossRef]
- Sayadi, A.; Nguyen, A.-T.; Bard, F.A.; Bard-Chapeau, E.A. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflamm. Res. 2013, 62, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, P.; Benderdour, M.; Shi, Q.; Lajeunesse, D.; Fernandes, J.C. Involvement of ICAM-1 in bone metabolism: A potential target in the treatment of bone diseases? Expert Opin. Biol. Ther. 2005, 5, 313–320. [Google Scholar] [CrossRef]
- Kroef, V.; Ruegenberg, S.; Horn, M.; Allmeroth, K.; Ebert, L.; Bozkus, S.; Miethe, S.; Elling, U.; Schermer, B.; Baumann, U.; et al. GFPT2/GFAT2 and AMDHD2 act in tandem to control the hexosamine pathway. Elife 2022, 11, e69223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Schnoor, M.; Yin, L.-M. Metallothionein-2: An emerging target in inflammatory diseases and cancers. Pharmacol. Ther. 2023, 244, 108374. [Google Scholar] [CrossRef]
- Dai, H.; Wang, L.; Li, L.; Huang, Z.; Ye, L. Metallothionein 1: A New Spotlight on Inflammatory Diseases. Front. Immunol. 2021, 12, 739918. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Hiraiwa, M.; O’Brien, J.S. Saposins: Structure, function, distribution, and molecular genetics. J. Lipid Res. 1992, 33, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Riepl, B.; Knedla, A.; Lefèvre, S.; Tarner, I.H.; Grifka, J.; Steinmeyer, J.; Schölmerich, J.; Gay, S.; Müller-Ladner, U. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res. Ther. 2010, 12, R83. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Wen, G.; Gessner, D.K.; Ringseis, R.; Lochnit, G.; Eder, K.; Zorn, H.; Timm, T. Tandem mass tag-based proteomics for studying the effects of a biotechnologically produced oyster mushroom against hepatic steatosis in obese Zucker rats. J. Proteom. 2021, 242, 104255. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Schmitz, G.; Liebisch, G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2009, 55, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Leuthäuser-Jaschinski, K.; Ecker, J.; Schmitz, G.; Liebisch, G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J. Lipid Res. 2010, 51, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timm, T.; Hild, C.; Liebisch, G.; Rickert, M.; Lochnit, G.; Steinmeyer, J. Functional Insights into the Sphingolipids C1P, S1P, and SPC in Human Fibroblast-like Synoviocytes by Proteomic Analysis. Int. J. Mol. Sci. 2024, 25, 8363. https://doi.org/10.3390/ijms25158363
Timm T, Hild C, Liebisch G, Rickert M, Lochnit G, Steinmeyer J. Functional Insights into the Sphingolipids C1P, S1P, and SPC in Human Fibroblast-like Synoviocytes by Proteomic Analysis. International Journal of Molecular Sciences. 2024; 25(15):8363. https://doi.org/10.3390/ijms25158363
Chicago/Turabian StyleTimm, Thomas, Christiane Hild, Gerhard Liebisch, Markus Rickert, Guenter Lochnit, and Juergen Steinmeyer. 2024. "Functional Insights into the Sphingolipids C1P, S1P, and SPC in Human Fibroblast-like Synoviocytes by Proteomic Analysis" International Journal of Molecular Sciences 25, no. 15: 8363. https://doi.org/10.3390/ijms25158363
APA StyleTimm, T., Hild, C., Liebisch, G., Rickert, M., Lochnit, G., & Steinmeyer, J. (2024). Functional Insights into the Sphingolipids C1P, S1P, and SPC in Human Fibroblast-like Synoviocytes by Proteomic Analysis. International Journal of Molecular Sciences, 25(15), 8363. https://doi.org/10.3390/ijms25158363




