Epigenetics of Skeletal Muscle Atrophy
Abstract
:1. Introduction
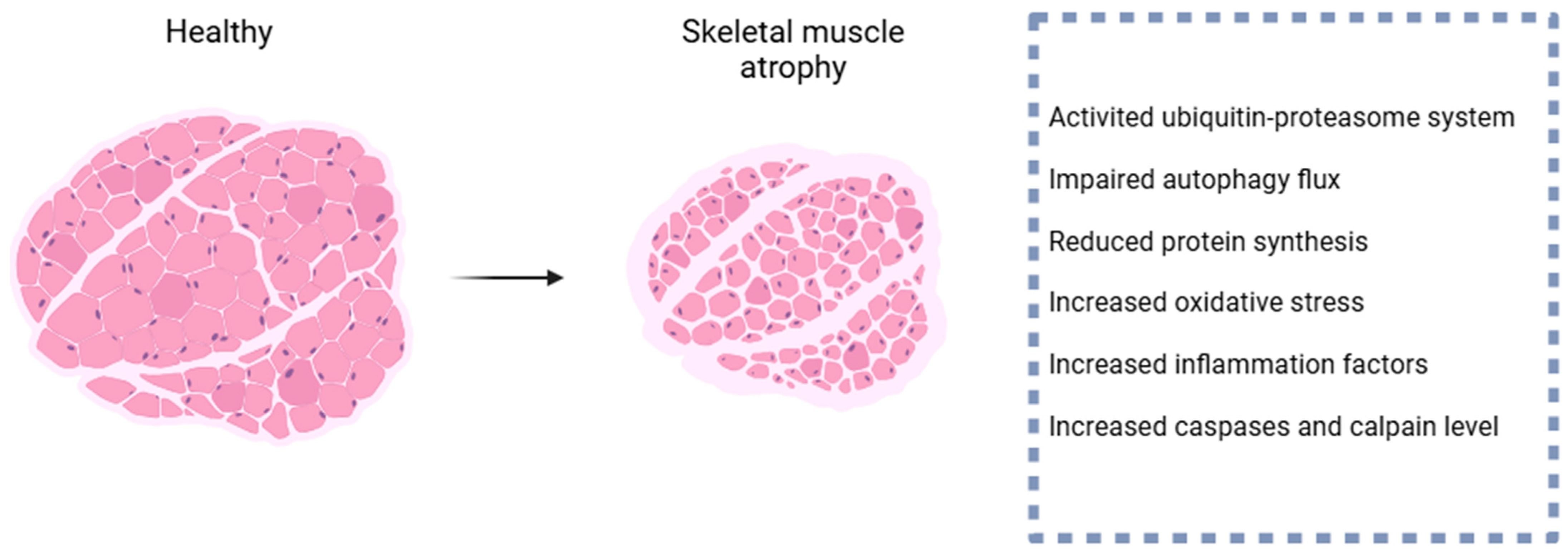
2. Pathogenesis of Skeletal Muscle Atrophy
2.1. Ubiquitin–Proteasome System
2.2. Autophagy–Lysosome System
2.3. Caspases
2.4. Calpain
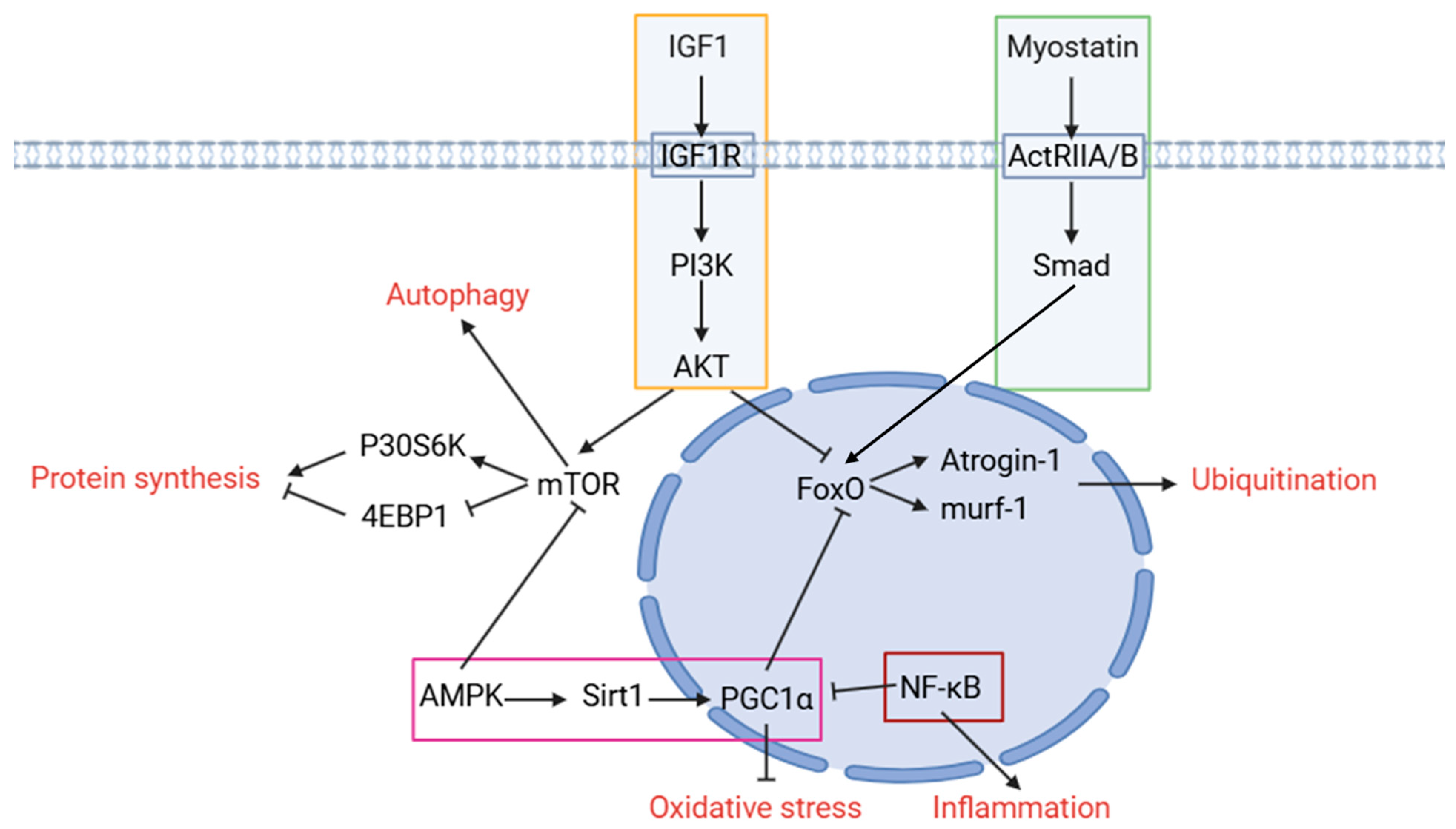
3. The Pathway Related to Skeletal Muscle Atrophy
4. Epigenetics in Different Kinds of Skeletal Muscle Atrophy
4.1. DNA Modification
4.1.1. The Role of DNA Modification in Skeletal Muscle Atrophy
4.1.2. The Protective Role of DNMT on Skeletal Muscle Atrophy
4.2. Histone Modifications
4.2.1. Histone Acetylation
The Role of Histone Acetylation in Skeletal Muscle Atrophy
HATs Play Different Roles in Different Types of Muscular Atrophy
HDACs Play Different Roles in Congenital and Acquired Muscular Atrophy
4.2.2. Histone Methylation
The Role of Histone Methylation in Skeletal Muscle Atrophy
HMTs Protects Skeletal Muscle from Atrophy
4.2.3. γ-H2AX (a Type of Histone Phosphorylation) Is Expressed More Frequently in Skeletal Muscle Atrophy
4.2.4. Histone Ubiquitination Reduced during Skeletal Muscle Atrophy
4.2.5. Histone Lactylation May Play a Critical Role in Skeletal Muscle Atrophy
4.3. RNA Modification
4.3.1. RNA Methylation
The Role of RNA Methylation in Skeletal Muscle Atrophy
The Role of Methyltransferases and Demethylases in Skeletal Muscle Atrophy
4.3.2. Other Kinds of RNA Modification
4.4. Noncoding RNA
4.4.1. miRNA
4.4.2. LncRNA
4.4.3. circRNA
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| ALS | amyotrophic lateral sclerosis |
| SMA | spinal muscular atrophy |
| DMD | Duchenne muscular dystrophy |
| CKD | chronic kidney disease |
| atrogin-1 | muscle atrophy F-box/MAFbx |
| murf-1 | muscle ring finger-1 |
| eIF3-f | eukaryotic translation initiation factor 3 subunit f |
| MyoD | myogenic differentiation antigen |
| Mib1 | mindbomb-1 |
| UPS | ubiquitin–proteasome system |
| EDL | extensor digitorum longus |
| Atg7 | autophagy related 7 |
| KO | knockout |
| Atg5 | autophagy related 5 |
| H2O2 | hydrogen peroxide |
| PI3K | phosphoinositide 3-kinase |
| AKT | serine/threonine-specific protein kinase |
| mTOR | mechanistic target of rapamycin kinase |
| P70S6K | p70 ribosomal protein S6 kinase |
| 4EBP1 | e IF4E-binding protein 1 |
| Foxo | forkhead box O |
| IGF-1 | insulin-like growth factor 1 |
| AMPK | AMP-activated protein kinase |
| mtDNA | mitochondrial DNA |
| IL-1 | interleukin-1 |
| TNF-α | tumor necrosis factor alpha |
| IL-6 | interleukin-6 |
| CRP | C-reactive protein |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nfkb1 | nuclear factor kappa B subunit 1 |
| GDF-8 | growth differentiation factor-8 |
| TGFβ | transforming growth factor β |
| 5mC | 5-methylcytosine |
| DNMT | DNA methyltransferase |
| 5hmC | 5-hydroxymethylcytosine |
| 5caC | 5-carboxylcytosine |
| 5fC | 5-formylcytosine |
| KEGG | kyoto encyclopedia of genes and genomes |
| DMR | differentially methylated region |
| DNMT | DNA methyltransferases |
| TET | ten-eleven translocation |
| HATs | histone acetyltransferases |
| HDACs | histone deacetylases |
| MLC3 | myosin light chains 3 |
| HMT | histone methyltransferase |
| me1 | monomethylation |
| me2 | dimethylation |
| me3 | trimethylation |
| PRMT | protein arginine methyltransferases |
| H2AX | H2A histone family member X |
| m6A | N6 adenylate methylation |
| METTL | methyltransferase like |
| FTO | fat mass and obesity-associated |
| ALKBH5 | alpha-ketoglutarate-dependent dioxygenase alkB homolog 5 |
| DAA | 3-Dezidenosine |
| R-2HG | R-2-HydroxyglutaTa |
| s2U | 2-thiouridine |
| Ψ | pseudouridine |
| ADARs | adenosine deaminases acting on RNA |
| ncRNA | noncoding RNA |
| lncRNA | long noncoding RNA |
| miRNA | microRNA |
| 3′UTR | 3′ untranslated region |
| siRNA | small interfering RNA |
| RNAi | RNA interference |
| piRNA | PIWI-interacting RNA |
| snoRNA | small nucleolar RNA |
| OSCC | oral squamous cell carcinoma |
| ceRNA | competing endogenous RNAs |
References
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Deans, C.; Maggert, K.A. What do you mean, “epigenetic”? Genetics 2015, 199, 887–896. [Google Scholar] [CrossRef]
- Kim, U.; Lee, D.S. Epigenetic Regulations in Mammalian Cells: Roles and Profiling Techniques. Mol. Cells 2023, 46, 86–98. [Google Scholar] [CrossRef]
- Hogg, S.J.; Beavis, P.A.; Dawson, M.A.; Johnstone, R.W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 2020, 19, 776–800. [Google Scholar] [CrossRef]
- Granic, A.; Suetterlin, K.; Shavlakadze, T.; Grounds, M.D.; Sayer, A.A. Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men. Clin. Sci. 2023, 137, 1721–1751. [Google Scholar]
- Agrawal, S.; Chakole, S.; Shetty, N.; Prasad, R.; Lohakare, T.; Wanjari, M. Exploring the Role of Oxidative Stress in Skeletal Muscle Atrophy: Mechanisms and Implications. Cureus 2023, 15, e42178. [Google Scholar]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Goodman, C.A.; Davey, J.R.; Hagg, A.; Parker, B.L.; Gregorevic, P. Dynamic Changes to the Skeletal Muscle Proteome and Ubiquitinome Induced by the E3 Ligase, ASB2beta. Mol. Cell. Proteom. 2021, 20, 100050. [Google Scholar] [CrossRef]
- Seo, J.Y.; Kang, J.S.; Kim, Y.L.; Jo, Y.W.; Kim, J.H.; Hann, S.H.; Park, J.; Park, I.; Park, H.; Yoo, K.; et al. Maintenance of type 2 glycolytic myofibers with age by Mib1-Actn3 axis. Nat. Commun. 2021, 12, 1294. [Google Scholar] [CrossRef]
- D’Cruz, R.; Plant, P.J.; Pablo, L.A.; Lin, S.; Chackowicz, J.; Correa, J.; Bain, J.; Batt, J. PDLIM7 is a novel target of the ubiquitin ligase Nedd4-1 in skeletal muscle. Biochem. J. 2016, 473, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cid-Diaz, T.; Leal-Lopez, S.; Fernandez-Barreiro, F.; Gonzalez-Sanchez, J.; Santos-Zas, I.; Andrade-Bulos, L.J.; Rodriguez-Fuentes, M.E.; Mosteiro, C.S.; Mouly, V.; Casabiell, X.; et al. Obestatin signalling counteracts glucocorticoid-induced skeletal muscle atrophy via NEDD4/KLF15 axis. J. Cachexia Sarcopenia Muscle 2021, 12, 493–505. [Google Scholar] [CrossRef]
- Pare, M.F.; Baechler, B.L.; Fajardo, V.A.; Earl, E.; Wong, E.; Campbell, T.L.; Tupling, A.R.; Quadrilatero, J. Effect of acute and chronic autophagy deficiency on skeletal muscle apoptotic signaling, morphology, and function. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 708–718. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Miguez, K.; Cefis, M.; Faitg, J.; Moamer, A.; Chaffer, T.J.; Reynaud, O.; Broering, F.E.; Shams, A.; Mayaki, D.; et al. Autophagy ablation in skeletal muscles worsens sepsis-induced muscle wasting, impairs whole-body metabolism, and decreases survival. iScience 2023, 26, 107475. [Google Scholar] [CrossRef]
- Doerr, V.; Montalvo, R.N.; Kwon, O.S.; Talbert, E.E.; Hain, B.A.; Houston, F.E.; Smuder, A.J. Prevention of Doxorubicin-Induced Autophagy Attenuates Oxidative Stress and Skeletal Muscle Dysfunction. Antioxidants 2020, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, C.; Zhang, A.; Cai, H.; Price, S.R.; Wang, X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J. Am. Soc. Nephrol. 2017, 28, 2631–2640. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Gu, X.; Miao, C.; Feng, L.; Shen, Q.; Liu, X.; Zhang, X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022, 8, 162. [Google Scholar]
- Zhu, S.; Nagashima, M.; Khan, M.A.; Yasuhara, S.; Kaneki, M.; Martyn, J.A. Lack of caspase-3 attenuates immobilization-induced muscle atrophy and loss of tension generation along with mitigation of apoptosis and inflammation. Muscle Nerve 2013, 47, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Plant, P.J.; Bain, J.R.; Correa, J.E.; Woo, M.; Batt, J. Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J. Appl. Physiol. 2009, 107, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Van Ba, H.; Hwang, I. Role of caspase-9 in the effector caspases and genome expressions, and growth of bovine skeletal myoblasts. Dev. Growth Differ. 2014, 56, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, C.; Pratt, E.P.S.; Anderson, L.; Bradley, K.; Latour, S.M.; Hashmi, M.N.; Urazaev, A.K.; Weilbaecher, R.; Davie, J.K.; Wang, W.H.; et al. The ERG1a potassium channel increases basal intracellular calcium concentration and calpain activity in skeletal muscle cells. Skelet. Muscle 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.M.; Judge, A.R.; Talbert, E.E.; Powers, S.K. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am. J. Physiol. Cell Physiol. 2009, 296, C363–C371. [Google Scholar] [CrossRef] [PubMed]
- Talbert, E.E.; Smuder, A.J.; Min, K.; Kwon, O.S.; Powers, S.K. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J. Appl. Physiol. 2013, 114, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dutt, V.; Kaur, N.; Mittal, A.; Dabur, R. Tinospora cordifolia protects from skeletal muscle atrophy by alleviating oxidative stress and inflammation induced by sciatic denervation. J. Ethnopharmacol. 2020, 254, 112720. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, A.; Phogat, J.; Dahuja, A.; Dabur, R. Magnoflorine prevent the skeletal muscle atrophy via Akt/mTOR/FoxO signal pathway and increase slow-MyHC production in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 267, 113510. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.; Saini, V.; Gupta, P.; Kaur, N.; Bala, M.; Gujar, R.; Grewal, A.; Gupta, S.; Dua, A.; Mittal, A. S-allyl cysteine inhibits TNFalpha-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 895–906. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Schmitt, T.L.; Martignoni, M.E.; Bachmann, J.; Fechtner, K.; Friess, H.; Kinscherf, R.; Hildebrandt, W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 2007, 85, 647–654. [Google Scholar] [CrossRef]
- Leger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163B–175B. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Pistilli, E.E.; Balduzzi, A.; Birnbaum, M.J.; Lachey, J.; Khurana, T.S.; Ahima, R.S. Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS ONE 2010, 5, e12707. [Google Scholar] [CrossRef]
- Chen, M.; Ji, C.; Yang, Q.; Gao, S.; Peng, Y.; Li, Z.; Gao, X.; Li, Y.; Jiang, N.; Zhang, Y.; et al. AKT2 regulates development and metabolic homeostasis via AMPK-depedent pathway in skeletal muscle. Clin. Sci. 2020, 134, 2381–2398. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef]
- Tao, W.; Ouyang, Z.; Liao, Z.; Li, L.; Zhang, Y.; Gao, J.; Ma, L.; Yu, S. Ursolic Acid Alleviates Cancer Cachexia and Prevents Muscle Wasting via Activating SIRT1. Cancers 2023, 15, 2378. [Google Scholar] [CrossRef]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-kappaB/TNF-alpha and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef]
- Baek, J.S.; Shin, Y.J.; Ma, X.; Park, H.S.; Hwang, Y.H.; Kim, D.H. Bifidobacterium bifidum and Lactobacillus paracasei alleviate sarcopenia and cognitive impairment in aged mice by regulating gut microbiota-mediated AKT, NF-kappaB, and FOXO3a signaling pathways. Immun. Ageing 2023, 20, 56. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Rhoads, M.G.; Kandarian, S.C.; Pacelli, F.; Doglietto, G.B.; Bossola, M. Expression of NF-kappaB and IkappaB proteins in skeletal muscle of gastric cancer patients. Eur. J. Cancer 2010, 46, 191–197. [Google Scholar] [CrossRef]
- Hunter, R.B.; Kandarian, S.C. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Investig. 2004, 114, 1504–1511. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Restoration of epigenetic impairment in the skeletal muscle and chronic inflammation resolution as a therapeutic approach in sarcopenia. Ageing Res. Rev. 2024, 96, 102267. [Google Scholar] [CrossRef]
- Raun, S.H.; Ali, M.S.; Han, X.; Henríquez-Olguín, C.; Pham, T.C.P.; Meneses-Valdés, R.; Knudsen, J.R.; Willemsen, A.C.H.; Larsen, S.; Jensen, T.E.; et al. Adenosine monophosphate-activated protein kinase is elevated in human cachectic muscle and prevents cancer-induced metabolic dysfunction in mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1631–1647. [Google Scholar] [CrossRef]
- Petrocelli, J.J.; Liu, J.; Yee, E.M.; Ferrara, P.J.; Bourrant, P.E.; de Hart, N.M.M.P.; Tatum, S.M.; Holland, W.J.; Funai, K. Skeletal muscle-specific inducible AMPKalpha1/alpha2 knockout mice develop muscle weakness, glycogen depletion, and fibrosis that persists during disuse atrophy. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E50–E60. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, J.; Tang, Y.; Wang, T.; Wei, B.; Feng, R.; Gong, B.; Wang, H.; Ji, G.; Lu, Z. AMP-activated kinase alpha2 deficiency protects mice from denervation-induced skeletal muscle atrophy. Arch. Biochem. Biophys. 2016, 600, 56–60. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Mitra, A.; Qaisar, R.; Bose, B.; Sudheer, S.P. The elusive role of myostatin signaling for muscle regeneration and maintenance of muscle and bone homeostasis. Osteoporos Sarcopenia 2023, 9, 1–7. [Google Scholar] [CrossRef]
- Aversa, Z.; Bonetto, A.; Penna, F.; Costelli, P.; Di Rienzo, G.; Lacitignola, A.; Baccino, F.M.; Ziparo, V.; Mercantini, P.; Rossi Fanelli, F.; et al. Changes in myostatin signaling in non-weight-losing cancer patients. Ann. Surg. Oncol. 2012, 19, 1350–1356. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Watanabe, A.; Shiratori, T.; Kaku, R.; Ueda, K.; Okamoto, K.; Kataoka, Y.; Ohshio, Y.; Hanaoka, J. Myostatin expression in lung cancer induces sarcopenia and promotes cancer progression. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 232–239. [Google Scholar] [CrossRef]
- Choi, K.; Jang, H.Y.; Ahn, J.M.; Hwang, S.H.; Chung, J.W.; Choi, Y.S.; Kim, J.W.; Jang, E.S.; Choi, G.H.; Jeong, S.H. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 492–505. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef]
- Ryan, A.S.; Li, G. Skeletal muscle myostatin gene expression and sarcopenia in overweight and obese middle-aged and older adults. JCSM Clin. Rep. 2021, 6, 137–142. [Google Scholar] [CrossRef]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.P.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef]
- Godala, M.; Gaszynska, E.; Walczak, K.; Malecka-Wojciesko, E. Myostatin and Activin A as Biomarkers of Sarcopenia in Inflammatory Bowel Disease Patients. Nutrients 2024, 16, 810. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef]
- Mendias, C.L.; Bakhurin, K.I.; Gumucio, J.P.; Shallal-Ayzin, M.V.; Davis, C.S.; Faulkner, J.A. Haploinsufficiency of myostatin protects against aging-related declines in muscle function and enhances the longevity of mice. Aging Cell 2015, 14, 704–706. [Google Scholar] [CrossRef]
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. Cell Physiol. 2009, 297, C1124–C1132. [Google Scholar] [CrossRef]
- Giannesini, B.; Vilmen, C.; Amthor, H.; Bernard, M.; Bendahan, D. Lack of myostatin impairs mechanical performance and ATP cost of contraction in exercising mouse gastrocnemius muscle In Vivo. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E33–E40. [Google Scholar] [CrossRef]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef]
- Nevi, L.; Pollanen, N.; Penna, F.; Caretti, G. Targeting Epigenetic Regulators with HDAC and BET Inhibitors to Modulate Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 16404. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Song, J.; Liu, Y.; Song, C.X.; Yi, C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell 2020, 11, 792–808. [Google Scholar]
- Li, J.W.; Shen, Z.K.; Lin, Y.S.; Wang, Z.Y.; Li, M.L.; Sun, H.X.; Wang, Q.; Zhao, C.; Xu, J.S.; Lu, X.; et al. DNA methylation of skeletal muscle function-related secretary factors identifies FGF2 as a potential biomarker for sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 1209–1217. [Google Scholar]
- He, L.; Khanal, P.; Morse, C.I.; Williams, A.; Thomis, M. Associations of combined genetic and epigenetic scores with muscle size and muscle strength: A pilot study in older women. J. Cachexia Sarcopenia Muscle 2020, 11, 1548–1561. [Google Scholar]
- He, L.; Khanal, P.; Morse, C.I.; Williams, A.; Thomis, M. Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women. J. Cachexia Sarcopenia Muscle 2019, 10, 1295–1306. [Google Scholar]
- Jin, L.; Jiang, Z.; Xia, Y.; Lou, P.; Chen, L.; Wang, H.; Bai, L.; Xie, Y.; Liu, Y.; Li, W.; et al. Genome-wide DNA methylation changes in skeletal muscle between young and middle-aged pigs. BMC Genom. 2014, 15, 653. [Google Scholar]
- Fisher, A.G.; Seaborne, R.A.; Hughes, T.M.; Gutteridge, A.; Stewart, C.; Coulson, J.M.; Sharples, A.P.; Jarvis, J.C. Transcriptomic and epigenetic regulation of disuse atrophy and the return to activity in skeletal muscle. FASEB J. 2017, 31, 5268–5282. [Google Scholar]
- Tomiga, Y.; Ito, A.; Sudo, M.; Ando, S.; Eshima, H.; Sakai, K.; Nakashima, S.; Uehara, Y.; Tanaka, H.; Soejima, H.; et al. One week, but not 12 hours, of cast immobilization alters promotor DNA methylation patterns in the nNOS gene in mouse skeletal muscle. J. Physiol. 2019, 597, 5145–5159. [Google Scholar] [CrossRef]
- Seaborne, R.A.; Hughes, D.C.; Turner, D.C.; Owens, D.J.; Baehr, L.M.; Gorski, P.; Semenova, E.A.; Borisov, O.V.; Larin, A.K.; Popov, D.V.; et al. UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J. Physiol. 2019, 597, 3727–3749. [Google Scholar] [CrossRef]
- Tajrishi, M.M.; Shin, J.; Hetman, M.; Kumar, A. DNA methyltransferase 3a and mitogen-activated protein kinase signaling regulate the expression of fibroblast growth factor-inducible 14 (Fn14) during denervation-induced skeletal muscle atrophy. J. Biol. Chem. 2014, 289, 19985–19999. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Ono, Y.; Hirose, Y.; Kanai, S.; Fujii, N.L.; Machida, S.; Nishino, I.; Shimizu, T.; Okano, M.; Kamei, Y.; et al. Reduced Dnmt3a increases Gdf5 expression with suppressed satellite cell differentiation and impaired skeletal muscle regeneration. FASEB J. 2018, 32, 1452–1467. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Q. Histone Modifications Represent a Key Epigenetic Feature of Epithelial-to-Mesenchyme Transition in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 4820. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, B.; Xu, Z.Y. Dynamic regulation of DNA methylation and histone modifications in response to abiotic stresses in plants. J. Integr. Plant Biol. 2022, 64, 2252–2274. [Google Scholar] [CrossRef]
- Tang, J.; Zhuang, S. Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin. Sci. 2019, 133, 597–609. [Google Scholar] [CrossRef]
- Chelladurai, P.; Boucherat, O.; Stenmark, K.; Kracht, M.; Seeger, W.; Bauer, U.M.; Bonnet, S.; Pullamsetti, S.S. Targeting histone acetylation in pulmonary hypertension and right ventricular hypertrophy. Br. J. Pharmacol. 2021, 178, 54–71. [Google Scholar] [CrossRef]
- Burns, A.M.; Graff, J. Cognitive epigenetic priming: Leveraging histone acetylation for memory amelioration. Curr. Opin. Neurobiol. 2021, 67, 75–84. [Google Scholar] [CrossRef]
- Ohsawa, I.; Konno, R.; Masuzawa, R.; Kawano, F. Amount of daily exercise is an essential stimulation to alter the epigenome of skeletal muscle in rats. J. Appl. Physiol. 2018, 125, 1097–1104. [Google Scholar] [CrossRef]
- Ryder, D.J.; Judge, S.M.; Beharry, A.W.; Farnsworth, C.L.; Silva, J.C.; Judge, A.R. Identification of the Acetylation and Ubiquitin-Modified Proteome during the Progression of Skeletal Muscle Atrophy. PLoS ONE 2015, 10, e0136247. [Google Scholar] [CrossRef]
- Kawano, F.; Nimura, K.; Ishino, S.; Nakai, N.; Nakata, K.; Ohira, Y. Differences in histone modifications between slow- and fast-twitch muscle of adult rats and following overload, denervation, or valproic acid administration. J. Appl. Physiol. 2015, 119, 1042–1052. [Google Scholar] [CrossRef]
- Yoshihara, T.; Machida, S.; Tsuzuki, T.; Kakigi, R.; Chang, S.W.; Sugiura, T.; Naito, H. Age-related changes in histone modification in rat gastrocnemius muscle. Exp. Gerontol. 2019, 125, 110658. [Google Scholar] [CrossRef]
- Wang, J.; Feng, S.; Zhang, Q.; Qin, H.; Xu, C.; Fu, X.; Yan, L.; Zhao, Y.; Yao, K. Roles of Histone Acetyltransferases and Deacetylases in the Retinal Development and Diseases. Mol. Neurobiol. 2023, 60, 2330–2354. [Google Scholar] [CrossRef]
- Beharry, A.W.; Judge, A.R. Differential expression of HDAC and HAT genes in atrophying skeletal muscle. Muscle Nerve 2015, 52, 1098–1101. [Google Scholar] [CrossRef]
- Addicks, G.C.; Zhang, H.; Ryu, D.; Vasam, G.; Green, A.E.; Marshall, P.L.; Patel, S.; Kang, B.E.; Kim, D.; Katsyuba, E.; et al. GCN5 maintains muscle integrity by acetylating YY1 to promote dystrophin expression. J. Cell Biol. 2022, 221, e202104022. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. Muscle Wasting in Fasting Requires Activation of NF-kappaB and Inhibition of AKT/Mechanistic Target of Rapamycin (mTOR) by the Protein Acetylase, GCN5. J. Biol. Chem. 2015, 290, 30269–30279. [Google Scholar] [CrossRef]
- Chamberlain, W.; Gonnella, P.; Alamdari, N.; Aversa, Z.; Hasselgren, P.O. Multiple muscle wasting-related transcription factors are acetylated in dexamethasone-treated muscle cells. Biochem. Cell Biol. 2012, 90, 200–208. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, J.; Chen, Q.N.; Lyu, A.K.; Chen, J.L.; Sun, Y.; Lyu, Q.; Zhao, Y.X.; Guo, A.; Liao, Z.Y.; et al. Type 2 diabetes-induced overactivation of P300 contributes to skeletal muscle atrophy by inhibiting autophagic flux. Life Sci. 2020, 258, 118243. [Google Scholar] [CrossRef]
- Alamdari, N.; Smith, I.J.; Aversa, Z.; Hasselgren, P.O. Sepsis and glucocorticoids upregulate p300 and downregulate HDAC6 expression and activity in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R509–R520. [Google Scholar] [CrossRef]
- Senf, S.M.; Sandesara, P.B.; Reed, S.A.; Judge, A.R. p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 300, C1490–C1501. [Google Scholar] [CrossRef]
- Sin, T.K.; Zhang, G.; Zhang, Z.; Zhu, J.Z.; Zuo, Y.; Frost, J.A.; Li, M.; Li, Y.P. Cancer-Induced Muscle Wasting Requires p38beta MAPK Activation of p300. Cancer Res. 2021, 81, 885–897. [Google Scholar] [CrossRef]
- Sin, T.K.; Zhu, J.Z.; Zhang, G.; Li, Y.P. p300 Mediates Muscle Wasting in Lewis Lung Carcinoma. Cancer Res. 2019, 79, 1331–1342. [Google Scholar] [CrossRef]
- Wang, X.; Waschke, B.C.; Woolaver, R.A.; Chen, S.M.Y.; Chen, Z.; Wang, J.H. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell 2020, 11, 472–482. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef]
- Luo, L.; Martin, S.C.; Parkington, J.; Cadena, S.M.; Zhu, J.; Ibebunjo, C.; Summermatter, S.; Londraville, N.; Patora-Komisarska, K.; Widler, L.; et al. HDAC4 Controls Muscle Homeostasis through Deacetylation of Myosin Heavy Chain, PGC-1alpha, and Hsc70. Cell Rep. 2019, 29, 749–763 e712. [Google Scholar]
- Pigna, E.; Simonazzi, E.; Sanna, K.; Bernadzki, K.M.; Proszynski, T.; Heil, C.; Palacios, D.; Adamo, S.; Moresi, V. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine 2019, 40, 717–732. [Google Scholar] [CrossRef]
- Beharry, A.W.; Sandesara, P.B.; Roberts, B.M.; Ferreira, L.F.; Senf, S.M.; Judge, A.R. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J. Cell Sci. 2014, 127 Pt 7, 1441–1453. [Google Scholar] [CrossRef]
- Ratti, F.; Ramond, F.; Moncollin, V.; Simonet, T.; Milan, G.; Mejat, A.; Thomas, J.L.; Streichenberger, N.; Gilquin, B.; Matthias, P.; et al. Histone deacetylase 6 is a FoxO transcription factor-dependent effector in skeletal muscle atrophy. J. Biol. Chem. 2015, 290, 4215–4224. [Google Scholar] [CrossRef]
- Ding, J.; Li, F.; Cong, Y.; Miao, J.; Wu, D.; Liu, B.; Wang, L. Trichostatin A inhibits skeletal muscle atrophy induced by cigarette smoke exposure in mice. Life Sci. 2019, 235, 116800. [Google Scholar] [CrossRef]
- Yoo, Y.E.; Ko, C.P. Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2011, 231, 147–159. [Google Scholar] [CrossRef]
- Dupre-Aucouturier, S.; Castells, J.; Freyssenet, D.; Desplanches, D. Trichostatin A, a histone deacetylase inhibitor, modulates unloaded-induced skeletal muscle atrophy. J. Appl. Physiol. 2015, 119, 342–351. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Mochalova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Differences in the Role of HDACs 4 and 5 in the Modulation of Processes Regulating MAFbx and MuRF1 Expression during Muscle Unloading. Int. J. Mol. Sci. 2020, 21, 4815. [Google Scholar] [CrossRef] [PubMed]
- Osseni, A.; Ravel-Chapuis, A.; Belotti, E.; Scionti, I.; Gangloff, Y.G.; Moncollin, V.; Mazelin, L.; Mounier, R.; Leblanc, P.; Jasmin, B.J.; et al. Pharmacological inhibition of HDAC6 improves muscle phenotypes in dystrophin-deficient mice by downregulating TGF-beta via Smad3 acetylation. Nat. Commun. 2022, 13, 7108. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, S.; Hu, W.; Lu, X.; Lou, N.; Yang, Z.; Chen, S.; Zhang, X.; Yang, H. Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPbeta-regulated atrogin1 expression in cancer cachexia. Am. J. Physiol. Cell Physiol. 2016, 311, C101–C115. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Sim, A.Y.; Huang, S.L.; Leng, Y.; Long, Y.C. HC toxin (a HDAC inhibitor) enhances IRS1-Akt signalling and metabolism in mouse myotubes. J. Mol. Endocrinol. 2015, 55, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Habibian, J.S.; Bolino, M.; Qian, A.; Woolsey, R.; Quilici, D.; Petereit, J.; Ferguson, B.S. Class I HDAC inhibitors attenuate dexamethasone-induced muscle atrophy via increased protein kinase C (PKC) delta phosphorylation. Cell. Signal. 2023, 110, 110815. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Reversing histone methylation. Nature 2005, 436, 1103–1106. [Google Scholar] [CrossRef]
- Kooistra, S.M.; Helin, K. Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol. 2012, 13, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, F.; Wu, J. Targeting histone methylation for cancer therapy: Enzymes, inhibitors, biological activity and perspectives. J. Hematol. Oncol. 2016, 9, 49. [Google Scholar] [CrossRef]
- Stouth, D.W.; Manta, A.; Ljubicic, V. Protein arginine methyltransferase expression, localization, and activity during disuse-induced skeletal muscle plasticity. Am. J. Physiol. Cell Physiol. 2018, 314, C177–C190. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Brown, T.; Zheng, Y.G. Mechanisms and Inhibitors of Histone Arginine Methylation. Chem. Rec. 2018, 18, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jeong, H.J.; Kim, H.; Choi, D.; Cho, S.C.; Seong, J.K.; Koo, S.H.; Kang, J.S. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy 2019, 15, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Stouth, D.W.; vanLieshout, T.L.; Mikhail, A.I.; Ng, S.Y.; Raziee, R.; Edgett, B.A.; Vasam, G.; Webb, E.K.; Gilotra, K.S.; Markou, M.; et al. CARM1 drives mitophagy and autophagy flux during fasting-induced skeletal muscle atrophy. Autophagy 2024, 20, 1247–1269. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.K.; Ng, S.Y.; Mikhail, A.I.; Stouth, D.W.; vanLieshout, T.L.; Syroid, A.L.; Ljubicic, V. Impact of short-term, pharmacological CARM1 inhibition on skeletal muscle mass, function, and atrophy in mice. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E252–E266. [Google Scholar] [CrossRef] [PubMed]
- Kealy, L.; Runting, J.; Thiele, D.; Scheer, S. An emerging maestro of immune regulation: How DOT1L orchestrates the harmonies of the immune system. Front. Immunol. 2024, 15, 1385319. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, R.; Drost, E.M.; MacNee, W.; Bastos, R.; Rabinovich, R.A. 2D-DIGE proteomic analysis of vastus lateralis from COPD patients with low and normal fat free mass index and healthy controls. Respir. Res. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Chakravarti, D. A peek into the complex realm of histone phosphorylation. Mol. Cell Biol. 2011, 31, 4858–4873. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, D.; Avvakumov, N.; Cote, J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef]
- Alsharidah, M.; Lazarus, N.R.; George, T.E.; Agley, C.C.; Velloso, C.P.; Harridge, S.D. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell 2013, 12, 333–344. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, B.; Hassounah, F.; Price, S.R.; Klein, J.; Mohamed, T.M.A.; Wang, Y.; Park, J.; Cai, H.; Zhang, X.; et al. The impact of senescence on muscle wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2023, 14, 126–141. [Google Scholar] [CrossRef]
- Mutsaers, C.A.; Wishart, T.M.; Lamont, D.J.; Riessland, M.; Schreml, J.; Comley, L.H.; Murray, L.M.; Parson, S.H.; Lochmuller, H.; Wirth, B.; et al. Reversible molecular pathology of skeletal muscle in spinal muscular atrophy. Hum. Mol. Genet. 2011, 20, 4334–4344. [Google Scholar] [CrossRef]
- Gerosa, L.; Malvandi, A.M.; Gomarasca, M.; Verdelli, C.; Sansoni, V.; Faraldi, M.; Ziemann, E.; Olivieri, F.; Banfi, G.; Lombardi, G. Murine Myoblasts Exposed to SYUIQ-5 Acquire Senescence Phenotype and Differentiate into Sarcopenic-Like Myotubes, an In Vitro Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae022. [Google Scholar] [CrossRef]
- Mattiroli, F.; Penengo, L. Histone Ubiquitination: An Integrative Signaling Platform in Genome Stability. Trends Genet. 2021, 37, 566–581. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Xu, J.; Pan, H.; Wang, W.; Yu, X.; Shi, S. Histone lactylation: From tumor lactate metabolism to epigenetic regulation. Int. J. Biol. Sci. 2024, 20, 1833–1854. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, H.; Liu, M.; Zhou, T.; Cheng, X.; Huang, W.; Cao, L. The role and mechanism of histone lactylation in health and diseases. Front. Genet. 2022, 13, 949252. [Google Scholar] [CrossRef]
- Tonkin, J.; Villarroya, F.; Puri, P.L.; Vinciguerra, M. SIRT1 signaling as potential modulator of skeletal muscle diseases. Curr. Opin. Pharmacol. 2012, 12, 372–376. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, M.M.; Park, S.; Jeong, K.S. Sirt2 positively regulates muscle regeneration after Notexin-induced muscle injury. Exp. Mol. Pathol. 2022, 127, 104798. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1alpha/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef]
- Orsolic, I.; Carrier, A.; Esteller, M. Genetic and epigenetic defects of the RNA modification machinery in cancer. Trends Genet. 2023, 39, 74–88. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.; Guan, Q.; Zhou, H.; Zhou, L.; Liu, L.; Liu, J.; Li, F.; Li, W.; Liu, H. RNA modification in cardiovascular disease: Implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 412. [Google Scholar] [CrossRef]
- An, Y.; Duan, H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.Q.; Tan, Y.; Yuan, R.; Chen, Z.S.; Zou, C. RNA methylation and cancer treatment. Pharmacol. Res. 2021, 174, 105937. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Han, J.; Yang, Y.; Chen, Y.; Tang, Z.; Gao, F. Longitudinal epitranscriptome profiling reveals the crucial role of N(6)-methyladenosine methylation in porcine prenatal skeletal muscle development. J. Genet. Genom. 2020, 47, 466–476. [Google Scholar] [CrossRef]
- Gheller, B.J.; Blum, J.E.; Fong, E.H.H.; Malysheva, O.V.; Cosgrove, B.D.; Thalacker-Mercer, A.E. A defined N6-methyladenosine (m(6)A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov. 2020, 6, 95. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Chen, Z.; Zhang, H.; Cao, Y.; Yao, X.; Chen, X.; Liu, B.; Gao, Z.; Shen, Y.; et al. Altered m6A RNA methylation governs denervation-induced muscle atrophy by regulating ubiquitin proteasome pathway. J. Transl. Med. 2023, 21, 845. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Wang, Q.; Fu, R.; Zhang, Z.; Chen, N.; Li, Z.; Gao, G.; Peng, S.; Yang, D. m(6) A demethylase ALKBH5 drives denervation-induced muscle atrophy by targeting HDAC4 to activate FoxO3 signalling. J. Cachexia Sarcopenia Muscle 2022, 13, 1210–1223. [Google Scholar] [CrossRef]
- Kung, M.L.; Yang, T.H.; Lin, C.C.; Ho, J.Y.; Hung, T.C.; Chang, C.H.; Huang, K.W.; Chen, C.C.; Chen, Y.W. ADAR2 deficiency ameliorates non-alcoholic fatty liver disease and muscle atrophy through modulating serum amyloid A1. J. Cachexia Sarcopenia Muscle 2024, 15, 949–962. [Google Scholar] [CrossRef]
- Deng, L.; Han, X.; Wang, Z.; Nie, X.; Bian, J. The Landscape of Noncoding RNA in Pulmonary Hypertension. Biomolecules 2022, 12, 796. [Google Scholar] [CrossRef]
- van der Werf, J.; Chin, C.V.; Fleming, N.I. SnoRNA in Cancer Progression, Metastasis and Immunotherapy Response. Biology 2021, 10, 809. [Google Scholar] [CrossRef]
- Gagan, J.; Dey, B.K.; Layer, R.; Yan, Z.; Dutta, A. MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation. J. Biol. Chem. 2011, 286, 19431–19438. [Google Scholar] [CrossRef]
- Iwasaki, H.; Ichihara, Y.; Morino, K.; Lemecha, M.; Sugawara, L.; Sawano, T.; Miake, J.; Sakurai, H.; Nishi, E.; Maegawa, H.; et al. MicroRNA-494-3p inhibits formation of fast oxidative muscle fibres by targeting E1A-binding protein p300 in human-induced pluripotent stem cells. Sci. Rep. 2021, 11, 1161. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Chen, H.; Yuan, M.; Kang, Y.; Duscher, D.; Machens, H.G.; Chen, Z. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis. Theranostics 2020, 10, 1415–1432. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Ge, M.; Gu, L.; Zhang, K.; Su, Y.; Zhang, Y.; Liu, C.; Lan, M.; Yu, Y.; et al. MiR-29ab1 Cluster Resists Muscle Atrophy Through Inhibiting MuRF1. DNA Cell Biol. 2021, 40, 1167–1176. [Google Scholar] [CrossRef]
- Liang, R.; Shen, X.; Wang, F.; Wang, X.; DesJarlais, A.; Syed, A.; Saba, R.; Tan, Z.; Yu, F.; Ji, X.; et al. H19X-encoded miR-322(424)/miR-503 regulates muscle mass by targeting translation initiation factors. J. Cachexia Sarcopenia Muscle 2021, 12, 2174–2186. [Google Scholar] [CrossRef]
- Shao, X.; Gong, W.; Wang, Q.; Wang, P.; Shi, T.; Mahmut, A.; Qin, J.; Yao, Y.; Yan, W.; Chen, D.; et al. Atrophic skeletal muscle fibre-derived small extracellular vesicle miR-690 inhibits satellite cell differentiation during ageing. J. Cachexia Sarcopenia Muscle 2022, 13, 3163–3180. [Google Scholar] [CrossRef]
- Goljanek-Whysall, K.; Soriano-Arroquia, A.; McCormick, R.; Chinda, C.; McDonagh, B. miR-181a regulates p62/SQSTM1, parkin, and protein DJ-1 promoting mitochondrial dynamics in skeletal muscle aging. Aging Cell 2020, 19, e13140. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; House, L.; Tregilgas, L.; Canty-Laird, E.; Goljanek-Whysall, K. The functional consequences of age-related changes in microRNA expression in skeletal muscle. Biogerontology 2016, 17, 641–654. [Google Scholar] [CrossRef]
- Patricia, S.P.; Ameena, H.; Aladin, M.B.; Mohamed, J.S. MicroRNA-434-3p regulates age-related apoptosis through eIF5A1 in the skeletal muscle. Aging 2017, 9, 1012–1029. [Google Scholar]
- Hu, Z.; Klein, J.D.; Mitch, W.E.; Zhang, L.; Martinez, I.; Wang, X.H. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging 2014, 6, 160–175. [Google Scholar] [CrossRef]
- Chen, Z.L.; Guo, C.; Zou, Y.Y.; Feng, C.; Yang, D.X.; Sun, C.C.; Wen, W.; Jian, Z.J.; Zhao, Z.; Xiao, Q.; et al. Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish. Exp. Gerontol. 2023, 180, 112265. [Google Scholar] [CrossRef]
- Chen, F.X.; Shen, Y.; Liu, Y.; Wang, H.F.; Liang, C.Y.; Luo, M. Inflammation-dependent downregulation of miR-532-3p mediates apoptotic signaling in human sarcopenia through targeting BAK1. Int. J. Biol. Sci. 2020, 16, 1481–1494. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Li, S.; Li, T.; Li, Y.; Wang, N.; Bao, X.; Xue, P.; Liu, S. BMSC-Derived Exosomes Inhibit Dexamethasone-Induced Muscle Atrophy via the miR-486-5p/FoxO1 Axis. Front. Endocrinol. 2021, 12, 681267. [Google Scholar] [CrossRef]
- Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Woodworth-Hobbs, M.E.; Franch, H.A.; Price, S.R. miR-182 attenuates atrophy-related gene expression by targeting FoxO3 in skeletal muscle. Am. J. Physiol. Cell Physiol. 2014, 307, C314–C319. [Google Scholar] [CrossRef]
- van de Worp, W.; Schols, A.; Dingemans, A.C.; Op den Kamp, C.M.H.; Degens, J.; Kelders, M.; Coort, S.; Woodruff, H.C.; Kratassiouk, G.; Harel-Bellan, A.; et al. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 452–463. [Google Scholar] [CrossRef]
- Chang, S.Y.; Han, S.Z.; Choe, H.M.; Gao, K.; Jin, Z.Y.; Liu, X.Y.; Yang, L.H.; Lv, S.T.; Yin, X.J.; Quan, L.H.; et al. miR-320 regulates myogenesis by targeting growth factor receptor-bound protein-2 and ameliorates myotubes atrophy. Int. J. Biochem. Cell Biol. 2022, 147, 106212. [Google Scholar] [CrossRef]
- Li, J.; Chan, M.C.; Yu, Y.; Bei, Y.; Chen, P.; Zhou, Q.; Cheng, L.; Chen, L.; Ziegler, O.; Rowe, G.C.; et al. miR-29b contributes to multiple types of muscle atrophy. Nat. Commun. 2017, 8, 15201. [Google Scholar] [CrossRef]
- Wada, S.; Kato, Y.; Okutsu, M.; Miyaki, S.; Suzuki, K.; Yan, Z.; Schiaffino, S.; Asahara, H.; Ushida, T.; Akimoto, T. Translational suppression of atrophic regulators by microRNA-23a integrates resistance to skeletal muscle atrophy. J. Biol. Chem. 2011, 286, 38456–38465. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Wang, Z.; Yu, J.; Ma, M.; Nie, Q. miR-27b-3p Attenuates Muscle Atrophy by Targeting Cbl-b in Skeletal Muscles. Biomolecules 2022, 12, 191. [Google Scholar] [CrossRef]
- Xie, K.; Xiong, H.; Xiao, W.; Xiong, Z.; Hu, W.; Ye, J.; Xu, N.; Shi, J.; Yuan, C.; Chen, Z.; et al. Downregulation of miR-29c promotes muscle wasting by modulating the activity of leukemia inhibitory factor in lung cancer cachexia. Cancer Cell Int. 2021, 21, 627. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, W.; Wu, C.; Yuan, Y.; Li, Y. Exosomes of oral squamous cell carcinoma cells containing miR-181a-3p induce muscle cell atrophy and apoptosis by transmissible endoplasmic reticulum stress signaling. Biochem. Biophys. Res. Commun. 2020, 533, 831–837. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Chen, M.; Zhang, K.; Gu, L.; Li, Q.; Yu, Z.; Li, N.; Meng, Q. miR-18a induces myotubes atrophy by down-regulating IgfI. Int. J. Biochem. Cell Biol. 2017, 90, 145–154. [Google Scholar] [CrossRef]
- Cho, K.A.; Choi, D.W.; Kim, Y.H.; Kim, J.; Ryu, K.H.; Woo, S.Y. Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors. Cells 2021, 10, 2169. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, H.; Wang, B.; Yuan, Y.; Klein, J.D.; Wang, X.H. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J. 2019, 33, 13590–13601. [Google Scholar] [CrossRef]
- Wang, X.H.; Hu, Z.; Klein, J.D.; Zhang, L.; Fang, F.; Mitch, W.E. Decreased miR-29 suppresses myogenesis in CKD. J. Am. Soc. Nephrol. 2011, 22, 2068–2076. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; He, W.; Zhao, Y.; Zhang, A.; Liu, Y.; Hassounah, F.; Ma, F.; Klein, J.D.; Wang, X.H.; et al. Exogenous miR-29a Attenuates Muscle Atrophy and Kidney Fibrosis in Unilateral Ureteral Obstruction Mice. Hum. Gene Ther. 2020, 31, 367–375. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef]
- McFarlane, C.; Vajjala, A.; Arigela, H.; Lokireddy, S.; Ge, X.; Bonala, S.; Manickam, R.; Kambadur, R.; Sharma, M. Negative auto-regulation of myostatin expression is mediated by Smad3 and microRNA-27. PLoS ONE 2014, 9, e87687. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.; Workeneh, B.; Dong, Y.; Wang, X.; Hu, Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012, 82, 401–411. [Google Scholar] [CrossRef]
- Hudson, M.B.; Woodworth-Hobbs, M.E.; Zheng, B.; Rahnert, J.A.; Blount, M.A.; Gooch, J.L.; Searles, C.D.; Price, S.R. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am. J. Physiol. Cell Physiol. 2014, 306, C551–C558. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Wang, B.; Klein, J.D.; Price, S.R.; Wang, X.H. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J. Cachexia Sarcopenia Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef]
- Yang, S.; Yang, G.; Wu, H.; Kang, L.; Xiang, J.; Zheng, P.; Qiu, S.; Liang, Z.; Lu, Y.; Jia, L. MicroRNA-193b impairs muscle growth in mouse models of type 2 diabetes by targeting the PDK1/Akt signalling pathway. Diabetologia 2022, 65, 563–581. [Google Scholar] [CrossRef]
- Ikenaka, A.; Kitagawa, Y.; Yoshida, M.; Lin, C.Y.; Niwa, A.; Nakahata, T.; Saito, M.K. SMN promotes mitochondrial metabolic maturation during myogenesis by regulating the MYOD-miRNA axis. Life Sci. Alliance 2023, 6, e202201457. [Google Scholar] [CrossRef]
- Pourshafie, N.; Lee, P.R.; Chen, K.L.; Harmison, G.G.; Bott, L.C.; Fischbeck, K.H.; Rinaldi, C. Systemic Delivery of MicroRNA Using Recombinant Adeno-associated Virus Serotype 9 to Treat Neuromuscular Diseases in Rodents. J. Vis. Exp. 2018, 138, e55724. [Google Scholar]
- Boon, H.; Sjogren, R.J.; Massart, J.; Egan, B.; Kostovski, E.; Iversen, P.O.; Hjeltnes, N.; Chibalin, A.V.; Widegren, U.; Zierath, J.R. MicroRNA-208b progressively declines after spinal cord injury in humans and is inversely related to myostatin expression. Physiol. Rep. 2015, 3, e12622. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Vechetti, I.J., Jr.; Lawrence, M.M.; Van Pelt, K.L.; Patel, P.; Miller, B.F.; Butterfield, T.A.; Dupont-Versteegden, E.E. Serum extracellular vesicle miR-203a-3p content is associated with skeletal muscle mass and protein turnover during disuse atrophy and regrowth. Am. J. Physiol. Cell Physiol. 2020, 319, C419–C431. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A.; Peterson, C.A.; Dupont-Versteegden, E.E. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol. Genom. 2009, 39, 219–226. [Google Scholar] [CrossRef]
- Jung, W.; Juang, U.; Gwon, S.; Nguyen, H.; Huang, Q.; Lee, S.; Lee, B.; Kim, S.H.; Ryu, S.; Park, J.; et al. Identifying the potential therapeutic effects of miR-6516 on muscle disuse atrophy. Mol. Med. Rep. 2024, 30, 119. [Google Scholar] [CrossRef]
- Khayrullin, A.; Smith, L.; Mistry, D.; Dukes, A.; Pan, Y.A.; Hamrick, M.W. Chronic alcohol exposure induces muscle atrophy (myopathy) in zebrafish and alters the expression of microRNAs targeting the Notch pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 479, 590–595. [Google Scholar] [CrossRef]
- Shin, J.; Miyaki, S.; Asahara, H.; Akimoto, T. MicroRNA-140 is not involved in sepsis-induced muscle atrophy. Am. J. Physiol. Cell Physiol. 2023, 325, C509–C518. [Google Scholar] [CrossRef]
- Borja-Gonzalez, M.; Casas-Martinez, J.C.; McDonagh, B.; Goljanek-Whysall, K. Inflamma-miR-21 Negatively Regulates Myogenesis during Ageing. Antioxidants 2020, 9, 345. [Google Scholar] [CrossRef]
- Freire, P.P.; Cury, S.S.; Lopes, L.O.; Fernandez, G.J.; Liu, J.; de Moraes, L.N.; de Oliveira, G.; Oliveira, J.S.; de Moraes, D.; Cabral-Marques, O.; et al. Decreased miR-497-5p Suppresses IL-6 Induced Atrophy in Muscle Cells. Cells 2021, 10, 3527. [Google Scholar] [CrossRef]
- Che, J.; Xu, C.; Wu, Y.; Jia, P.; Han, Q.; Ma, Y.; Wang, X.; Zheng, Y. MiR-1290 promotes myoblast differentiation and protects against myotube atrophy via Akt/p70/FoxO3 pathway regulation. Skelet Muscle 2021, 11, 6. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Ausin, P.; Martinez-Llorens, J.; Gea, J.; Barreiro, E. Do epigenetic events take place in the vastus lateralis of patients with mild chronic obstructive pulmonary disease? PLoS ONE 2014, 9, e102296. [Google Scholar]
- Farre-Garros, R.; Lee, J.Y.; Natanek, S.A.; Connolly, M.; Sayer, A.A.; Patel, H.; Cooper, C.; Polkey, M.I.; Kemp, P.R. Quadriceps miR-542-3p and -5p are elevated in COPD and reduce function by inhibiting ribosomal and protein synthesis. J. Appl. Physiol. 2019, 126, 1514–1524. [Google Scholar] [CrossRef]
- Garros, R.F.; Paul, R.; Connolly, M.; Lewis, A.; Garfield, B.E.; Natanek, S.A.; Bloch, S.; Mouly, V.; Griffiths, M.J.; Polkey, M.I.; et al. MicroRNA-542 Promotes Mitochondrial Dysfunction and SMAD Activity and Is Elevated in Intensive Care Unit-acquired Weakness. Am. J. Respir. Crit. Care Med. 2017, 196, 1422–1433. [Google Scholar] [CrossRef]
- Connolly, M.; Paul, R.; Farre-Garros, R.; Natanek, S.A.; Bloch, S.; Lee, J.; Lorenzo, J.P.; Patel, H.; Cooper, C.; Sayer, A.A.; et al. miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J. Cachexia Sarcopenia Muscle 2018, 9, 400–416. [Google Scholar] [CrossRef]
- Jin, J.; Li, F.; Fan, C.; Wu, Y.; He, C. Elevated mir-145-5p is associated with skeletal muscle dysfunction and triggers apoptotic cell death in C2C12 myotubes. J. Muscle Res. Cell Motil. 2022, 43, 135–145. [Google Scholar] [CrossRef]
- Lewis, A.; Riddoch-Contreras, J.; Natanek, S.A.; Donaldson, A.; Man, W.D.; Moxham, J.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Downregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPD. Thorax 2012, 67, 26–34. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef]
- Guess, M.G.; Barthel, K.K.; Harrison, B.C.; Leinwand, L.A. miR-30 family microRNAs regulate myogenic differentiation and provide negative feedback on the microRNA pathway. PLoS ONE 2015, 10, e0118229. [Google Scholar] [CrossRef]
- Klockner, I.; Schutt, C.; Gerhardt, T.; Boettger, T.; Braun, T. Control of CRK-RAC1 activity by the miR-1/206/133 miRNA family is essential for neuromuscular junction function. Nat. Commun. 2022, 13, 3180. [Google Scholar] [CrossRef]
- Schutt, C.; Hallmann, A.; Hachim, S.; Klockner, I.; Valussi, M.; Atzberger, A.; Graumann, J.; Braun, T.; Boettger, T. Linc-MYH configures INO80 to regulate muscle stem cell numbers and skeletal muscle hypertrophy. EMBO J. 2020, 39, e105098. [Google Scholar] [CrossRef]
- Lv, W.; Jiang, W.; Luo, H.; Tong, Q.; Niu, X.; Liu, X.; Miao, Y.; Wang, J.; Guo, Y.; Li, J.; et al. Long noncoding RNA lncMREF promotes myogenic differentiation and muscle regeneration by interacting with the Smarca5/p300 complex. Nucleic Acids Res. 2022, 50, 10733–10755. [Google Scholar] [CrossRef]
- Cabianca, D.S.; Casa, V.; Bodega, B.; Xynos, A.; Ginelli, E.; Tanaka, Y.; Gabellini, D. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell 2012, 149, 819–831. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Han, N.; Kwak, S.; Lee, H.T.; Kim, J.H.; Kang, K.; Youn, B.H.; Yang, J.H.; Jeong, H.J.; et al. Long non-coding RNA ChRO1 facilitates ATRX/DAXX-dependent H3.3 deposition for transcription-associated heterochromatin reorganization. Nucleic Acids Res. 2018, 46, 11759–11775. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Bao, X.; Zhu, X.; Kwok, Y.K.; Sun, K.; Chen, X.; Huang, Y.; Jauch, R.; Esteban, M.A.; et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 2015, 25, 335–350. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, C.; Deng, K.; Zhang, Z.; Li, J.; Deng, M.; Zhang, Y.; Wang, F. The regulation of LncRNA GTL2 expression by DNA methylation during sheep skeletal muscle development. Genomics 2022, 114, 110453. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Yuan, R.; Zhou, Z.; Zhang, J.; Kong, S.; Lin, D.; Lian, L.; Li, J.; Zhang, X.; et al. MYH1G-AS is a chromatin-associated lncRNA that regulates skeletal muscle development in chicken. Cell. Mol. Biol. Lett. 2024, 29, 9. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Li, Q.; Li, J. Integrated analysis of long non-coding RNAs (lncRNAs) and mRNA expression profiles identifies lncRNA PRKG1-AS1 playing important roles in skeletal muscle aging. Aging 2021, 13, 15044–15060. [Google Scholar] [CrossRef]
- Ruan, L.; Mendhe, B.; Parker, E.; Kent, A.; Isales, C.M.; Hill, W.D.; McGee-Lawrence, M.; Fulzele, S.; Hamrick, M.W. Long Non-coding RNA MALAT1 Is Depleted with Age in Skeletal Muscle in vivo and MALAT1 Silencing Increases Expression of TGF-beta1 In Vitro. Front. Physiol. 2021, 12, 742004. [Google Scholar] [CrossRef]
- Neppl, R.L.; Wu, C.L.; Walsh, K. lncRNA Chronos is an aging-induced inhibitor of muscle hypertrophy. J. Cell Biol. 2017, 216, 3497–3507. [Google Scholar] [CrossRef]
- Yu, M.; He, X.; Liu, T.; Li, J. lncRNA GPRC5D-AS1 as a ceRNA inhibits skeletal muscle aging by regulating miR-520d-5p. Aging 2023, 15, 13980–13997. [Google Scholar] [CrossRef]
- Li, Y.; Shi, H.; Chen, R.; Zhou, S.; Lei, S.; She, Y. Role of miRNAs and lncRNAs in dexamethasone-induced myotube atrophy In Vitro. Exp. Ther. Med. 2021, 21, 146. [Google Scholar] [CrossRef]
- Jin, J.; Du, M.; Wang, J.; Guo, Y.; Zhang, J.; Zuo, H.; Hou, Y.; Wang, S.; Lv, W.; Bai, W.; et al. Conservative analysis of Synaptopodin-2 intron sense-overlapping lncRNA reveals its novel function in promoting muscle atrophy. J. Cachexia Sarcopenia Muscle 2022, 13, 2017–2030. [Google Scholar] [CrossRef]
- Li, Z.; Cai, B.; Abdalla, B.A.; Zhu, X.; Zheng, M.; Han, P.; Nie, Q.; Zhang, X. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 2019, 10, 391–410. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Tang, H.; Sha, Z.; Chen, R.; Chen, L.; Yu, Y.; Rowe, G.C.; Das, S.; Xiao, J. Inhibition of lncRNA MAAT Controls Multiple Types of Muscle Atrophy by cis- and trans-Regulatory Actions. Mol. Ther. 2021, 29, 1102–1119. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Kiyofuji, Y.; Inagaki, H.; Kurahashi, H.; Tsuchida, K. An Analysis of Differentially Expressed Coding and Long Non-Coding RNAs in Multiple Models of Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2021, 22, 2558. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B.T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Funasaki, S.; Hijikata, I.; Maekawa, M.; Honda, M.; Tsuchida, K. Expression Levels of Long Non-Coding RNAs Change in Models of Altered Muscle Activity and Muscle Mass. Int. J. Mol. Sci. 2020, 21, 1628. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B.T. Long Noncoding RNA lncMUMA Reverses Established Skeletal Muscle Atrophy following Mechanical Unloading. Mol. Ther. 2018, 26, 2669–2680. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Pang, X.; Zhou, Y.; Li, S.; Li, W.; Zhang, P.; Chen, X. Decreased expression of H19/miR-675 ameliorates muscle atrophy by regulating the IGF1R/Akt/FoxO signaling pathway. Mol. Med. 2023, 29, 78. [Google Scholar] [CrossRef]
- Lei, S.; She, Y.; Zeng, J.; Chen, R.; Zhou, S.; Shi, H. Expression patterns of regulatory lncRNAs and miRNAs in muscular atrophy models induced by starvation In Vitro and In Vivo. Mol. Med. Rep. 2019, 20, 4175–4185. [Google Scholar] [CrossRef]
- Alessio, E.; Buson, L.; Chemello, F.; Peggion, C.; Grespi, F.; Martini, P.; Massimino, M.L.; Pacchioni, B.; Millino, C.; Romualdi, C.; et al. Single cell analysis reveals the involvement of the long non-coding RNA Pvt1 in the modulation of muscle atrophy and mitochondrial network. Nucleic Acids Res. 2019, 47, 1653–1670. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, J.; Xiao, J.; Yang, L.; Cai, M.; Shen, H.; Chen, X.; Ma, Y.; Hu, S.; Wang, Z.; et al. Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 2017, 8, 14718. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Takasaki, A.; Ouchi, Y.; Uezumi, A.; Ageta, H.; Inagaki, H.; Kurahashi, H.; Tsuchida, K. Myogenin promoter-associated lncRNA Myoparr is essential for myogenic differentiation. EMBO Rep. 2019, 20, e47468. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Long Non-Coding RNA Myoparr Regulates GDF5 Expression in Denervated Mouse Skeletal Muscle. Noncoding RNA 2019, 5, 33. [Google Scholar] [CrossRef]
- Yu, J.; Loh, K.; Yang, H.Q.; Du, M.R.; Wu, Y.X.; Liao, Z.Y.; Guo, A.; Yang, Y.F.; Chen, B.; Zhao, Y.X.; et al. The Whole-transcriptome Landscape of Diabetes-related Sarcopenia Reveals the Specific Function of Novel lncRNA Gm20743. Commun. Biol. 2022, 5, 774. [Google Scholar] [CrossRef]
- Chen, R.; Yang, T.; Jin, B.; Xu, W.; Yan, Y.; Wood, N.; Lehmann, H.I.; Wang, S.; Zhu, X.; Yuan, W.; et al. CircTmeff1 Promotes Muscle Atrophy by Interacting with TDP-43 and Encoding A Novel TMEFF1-339aa Protein. Adv. Sci. 2023, 10, e2206732. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Shen, X.; Zhang, Y.; Zhang, Y.; Ye, L.; Li, D.; Zhu, Q.; Yin, H. CircCCDC91 regulates chicken skeletal muscle development by sponging miR-15 family via activating IGF1-PI3K/AKT signaling pathway. Poult. Sci. 2022, 101, 101803. [Google Scholar] [CrossRef] [PubMed]
| Name | Mechanism | Results | Reference |
|---|---|---|---|
| Trichostatin A | Inhibiting HDAC activity | Promoting atrogin-1 mRNA level | Alamdari et al. (2010) [87] |
| Improving amyotrophic lateral sclerosis | Yoo et al. (2011) [100] | ||
| Improving unloaded-induced skeletal muscle atrophy | Aucouturier et al. (2015) [101] | ||
| Inhibiting HDAC1/2 | Improving muscle atrophy induced by cigarette smoke exposure | Ding et al. (2019) [99] | |
| MS-275 | Inhibiting class I HDAC | Improving fasting and denervation-induced skeletal muscle atrophy | Beharry et al. (2014) [97] |
| Butyrate | Improving sarcopenia | Walsh et al. (2015) [102] | |
| HC toxin | Improving starvation-induced muscle atrophy | Tan et al. (2015) [106] | |
| Valproic acid | Attenuating cancer cachexia- induced skeletal muscle atrophy | Sun et al. (2016) [105] | |
| NVS-HD1 | Inhibiting HDAC4 | Improving dexamethasone-induced skeletal muscle atrophy | Luo et al. (2019) [95] |
| LMK-235 | Inhibiting HDAC5 | Improving unloading-induced muscle atrophy | Mochalova et al. (2020) [103] |
| Tubastatin A | Inhibiting HDAC6 | Improving Duchenne muscular dystrophy | Osseni et al. (2022) [104] |
| trichostatin A, apicidin, romidepsin | Inhibiting HDAC1/2 | attenuating dexamethasone-induced muscle atrophy | Habibian et al. (2023) [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wu, Q.; Bae, E.J. Epigenetics of Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2024, 25, 8362. https://doi.org/10.3390/ijms25158362
Du J, Wu Q, Bae EJ. Epigenetics of Skeletal Muscle Atrophy. International Journal of Molecular Sciences. 2024; 25(15):8362. https://doi.org/10.3390/ijms25158362
Chicago/Turabian StyleDu, Jiacheng, Qian Wu, and Eun Ju Bae. 2024. "Epigenetics of Skeletal Muscle Atrophy" International Journal of Molecular Sciences 25, no. 15: 8362. https://doi.org/10.3390/ijms25158362
APA StyleDu, J., Wu, Q., & Bae, E. J. (2024). Epigenetics of Skeletal Muscle Atrophy. International Journal of Molecular Sciences, 25(15), 8362. https://doi.org/10.3390/ijms25158362






