Mutational Profile in Romanian Patients with Hemophilia A
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Samples/DNA Extraction
4.3. Next-Generation Sequencing (NGS)
4.4. Variant Interpretation
4.5. Bioinformatic Tools
4.6. MLPA Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mannucci, P.M.; Tuddenham, E.G. The Hemophilias—From royal genes to gene therapy. N. Engl. J. Med. 2001, 344, 1773–1779. [Google Scholar] [CrossRef]
- Pipe, S.W.; Morris, J.A.; Shah, J.; Kaufman, R.J. Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J. Biol. Chem. 1998, 273, 8537–8544. [Google Scholar] [CrossRef]
- Shahani, T.; Covens, K.; Lavend’homme, R.; Jazouli, N.; Sokal, É.; Peerlinck, K.; Jacquemin, M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J. Thromb. Haemost. 2014, 12, 36–42. [Google Scholar] [CrossRef]
- Vehar, G.A.; Keyt, B.; Eaton, D.; Rodriguez, H.; O’Brien, D.P.; Rotblat, F.; Oppermann, H.; Keck, R.; Wood, W.I.; Harkins, R.N.; et al. Structure of human factor VIII. Nature 1984, 312, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lollar, P.; Hill-Eubanks, D.C.; Parker, C.G. Association of the factor VIII light chain with von Willebrand factor. J. Biol. Chem. 1988, 263, 10451–10455. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Wasley, L.C.; Dorner, A.J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J. Biol. Chem. 1988, 263, 6352–6362. [Google Scholar] [PubMed]
- Eaton, D.; Rodriguez, H.; Vehar, G.A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry 1986, 25, 505–512. [Google Scholar] [CrossRef]
- Gitschier, J.; Wood, W.I.; Goralka, T.M.; Wion, K.L.; Chen, E.Y.; Eaton, D.H.; Vehar, G.A.; Capon, D.J.; Lawn, R.M. Characterization of the human factor VIII gene. Nature 1984, 312, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Rossiter, J.P.; Young, M.; Horst, J.; de Moerloose, P.; Sommer, S.S.; Ketterling, R.P.; Kazazian, H.H., Jr.; Negrier, C.; Vinciguerra, C.; et al. Factor VIII gene inversions in severe hemophilia A: Results of an international consortium study. Blood 1995, 86, 2206–2212. [Google Scholar] [CrossRef]
- Oldenburg, J.; Ananyeva, N.M.; Saenko, E.L. Molecular basis of haemophilia A. Haemophilia 2004, 10 (Suppl. S4), 133–139. [Google Scholar] [CrossRef]
- El-Maarri, O.; Olek, A.; Balaban, B.; Montag, M.; van der Ven, H.; Urman, B.; Olek, K.; Caglayan, S.H.; Walter, J.; Oldenburg, J. Methylation levels at selected CpG Sites in the factor VIII and FGFR3 genes, in mature female and male germ cells: Implications for male-driven evolution. Am. J. Hum. Genet. 1998, 63, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Millar, D.S.; Krawczak, M.; Cooper, D.N. Variation of site-specific methylation patterns in the factor VIII (F8C) gene in human sperm DNA. Hum. Genet. 1998, 103, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kemball-Cook, G.; Tuddenham, E.G.; Wacey, A.I. The factor VIII structure and mutation resource site: HAMSTeRS Version 4. Nucleic Acids Res. 1998, 26, 216–219. [Google Scholar] [CrossRef]
- EAHAD Factor VIII Variant Database. Available online: https://f8-db.eahad.org/ (accessed on 12 June 2024).
- Carcao, M.; Goudemand, J. Inhibitors in Hemophilia: A Primer, 5th ed.; World Federation of Hemophilia: Montreal, QC, Canada, 2018. [Google Scholar]
- Kreuz, W.; Becker, S.; Lenz, E.; Martinez-Saguer, I.; Escuriola-Ettingshausen, C.; Funk, M.; Ehrenforth, S.; Auerswald, G.; Kornhuber, B. Factor VIII inhibitors in patients with hemophilia A: Epidemiology of inhibitor development and induction of immune tolerance for factor VIII. Semin. Thromb. Hemost 1995, 21, 382–389. [Google Scholar] [CrossRef]
- Oldenburg, J.; Schröder, J.; Brackmann, H.H.; Müller-Reible, C.; Schwaab, R.; Tuddenham, E. Environmental and genetic factors influencing inhibitor development. Semin. Hematol. 2004, 41, 82–88. [Google Scholar] [CrossRef]
- World Federation of Hemophilia. World Federation of Hemophilia Report on the Annual Global Survey 2022; World Federation of Hemophilia: Montreal, QC, Canada, 2023. [Google Scholar]
- Brinza, M.; Grigore, A.; Dragomir, M.; Jardan, D.; Jardan, C.; Balanescu, P.; Tarniceriu, C.C.; Badulescu, O.V.; Blag, C.; Tomuleasa, C.; et al. Large intron inversions in Romanian patients with hemophilia A—First Report. Medicina 2023, 59, 1821. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; El-Maarri, O. New Insight into the Molecular Basis of Hemophilia A. Int. J. Hematol. 2006, 83, 96–102. [Google Scholar] [CrossRef]
- Bach, J.E.; Oldenburg, J.; Müller, C.R.; Rost, S. Mutational Spectrum and Deep Intronic Variants in the Factor VIII Gene of Haemophilia a Patients. Identification by next Generation Sequencing. Hamostaseologie 2016, 36, S25–S28. [Google Scholar]
- Gouw, S.C.; van den Berg, H.M.; Oldenburg, J.; Astermark, J.; de Groot, P.G.; Margaglione, M.; Thompson, A.R.; van Heerde, W.; Boekhorst, J.; Miller, C.H.; et al. F8 Gene Mutation Type and Inhibitor Development in Patients with Severe Hemophilia A: Systematic Review and Meta-Analysis. Blood 2012, 119, 2922–2934. [Google Scholar] [CrossRef]
- Johnsen, J.M.; Fletcher, S.N.; Huston, H.; Roberge, S.; Martin, B.K.; Kircher, M.; Josephson, N.C.; Shendure, J.; Ruuska, S.; Koerper, M.A.; et al. Novel approach to genetic analysis and results in 3000 hemophilia patients enrolled in the My Life, Our Future initiative. Blood Adv. 2017, 1, 824–834. [Google Scholar] [CrossRef]
- Oldenburg, J.; Pavlova, A. Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia 2006, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Graw, J.; Brackmann, H.H.; Oldenburg, J.; Schneppenheim, R.; Spannagl, M.; Schwaab, R. Haemophilia A: From mutation analysis to new therapies. Nat. Rev. Genet. 2005, 6, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.R. Structure and function of the factor VIII gene and protein. Semin. Thromb. Hemost. 2003, 29, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Selvaraj, S.R.; Miao, H.Z.; Pipe, S.W. Most factor VIII B domain missense mutations are unlikely to be causative mutations for severe hemophilia A: Implications for genotyping. J. Thromb. Haemost. 2011, 9, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, J.K.; Millar, D.S.; McVey, J.H.; Grundy, C.B.; Wieland, K.; Mibashan, R.S.; Martinowitz, U.; Tan-Un, K.; Vidaud, M.; Goossens, M.; et al. The molecular genetic analysis of hemophilia A: A directed search strategy for the detection of point mutations in the human factor VIII gene. Blood 1990, 76, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Castaman, G.; Giacomelli, S.H.; Mancuso, M.E.; D’Andrea, G.; Santacroce, R.; Sanna, S.; Santagostino, E.; Mannucci, P.M.; Goodeve, A.; Rodeghiero, F. Deep intronic variations may cause mild hemophilia A. J. Thromb. Haemost. 2011, 9, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Atik, T.; Işık, E.; Onay, H.; Akgün, B.; Shamsali, M.; Kavakli, K.; Evim, M.; Tüysüz, G.; Özbek, N.Y.; Şahin, F.; et al. Factor 8 gene mutation spectrum of 270 patients with hemophilia A: Identification of 36 novel mutations. Turk. J. Hematol. 2020, 37, 145–153. [Google Scholar] [CrossRef]
- Reitter, S.; Sturn, R.; Horvath, B.; Freitag, R.; Male, C.; Muntean, W.; Streif, W.; Pabinger, I.; Mannhalter, C.; Austrian Molecular Haemophilia Study Group. Spectrum of causative mutations in patients with haemophilia A in Austria. Thromb. Haemost. 2010, 104, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, R.; Acquila, M.; Belvini, D.; Castaldo, G.; Garagiola, I.; Giacomelli, S.H.; Lombardi, A.M.; Minuti, B.; Riccardi, F.; Salviato, R.; et al. AICE-Genetics Study Group. Identification of 217 unreported mutations in the F8 gene in a group of 1,410 unselected Italian patients with hemophilia A. J. Hum. Genet. 2008, 53, 275–284. [Google Scholar] [CrossRef]
- Klopp, N.; Oldenburg, J.; Uen, C.; Schneppenheim, R.; Graw, J. 11 hemophilia A patients without mutations in the factor VIII encoding gene. Thromb. Haemost. 2002, 88, 357–360. [Google Scholar] [CrossRef]
- Mazurier, C.; Dieval, J.; Jorieux, S.; Delobel, J.; Goudemand, M. A new von Willebrand factor (vWF) defect in a patient with factor VIII (FVIII) deficiency but with normal levels and multimeric patterns of both plasma and platelet vWF. Characterization of abnormal vWF/FVIII interaction. Blood 1990, 75, 20–26. [Google Scholar] [CrossRef]
- Gaucher, C.; Jorieux, S.; Mercier, B.; Oufkir, D.; Mazurier, C. The “Normandy” variant of von Willebrand disease: Characterization of a point mutation in the von Willebrand factor gene. Blood 1991, 77, 1937–1941. [Google Scholar] [CrossRef]
- Nichols, W.C.; Seligsohn, U.; Zivelin, A.; Terry, V.H.; Hertel, C.E.; Wheatley, M.A.; Moussalli, M.J.; Hauri, H.P.; Ciavarella, N.; Kaufman, R.J.; et al. Mutations in the ER–Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 1998, 93, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.C.; Terry, V.H.; Wheatley, M.A.; Yang, A.; Zivelin, A.; Ciavarella, N.; Stefanile, C.; Matsushita, T.; Saito, H.; de Bosch, N.B.; et al. ERGIC-53 gene structure and mutation analysis in 19 combined factors V and VIII deficiency families. Blood 1999, 93, 2261–2266. [Google Scholar] [PubMed]
- Zhang, B.; Cunningham, M.A.; Nichols, W.C.; Bernat, J.A.; Seligsohn, U.; Pipe, S.W.; McVey, J.H.; Schulte-Overberg, U.; de Bosch, N.B.; Ruiz-Saez, A.; et al. Bleeding due to disruption of a cargo-specific ER-To-Golgi transport complex. Nat. Genet. 2003, 34, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, R.; Brackmann, H.H.; Meyer, C.; Seehafer, J.; Kirchgesser, M.; Haack, A.; Olek, K.; Tuddenham, E.G.; Oldenburg, J. Haemophilia A: Mutation type determines risk of inhibitor formation. Thromb. Haemost. 1995, 74, 1402–1406. [Google Scholar] [CrossRef]
- Goodeve, A.C.; Williams, I.; Bray, G.L.; Peake, I.R. Relationship between Factor VIII mutation type and inhibitor development in a cohort of previously untreated patients treated with recombinant factor VIII (RecombinateTM). Recombinate PUP Study Group. Thromb. Haemost. 2000, 83, 844–848. [Google Scholar] [CrossRef]
- Hay, C.R. Factor VIII inhibitors in mild and moderate-severity haemophilia A. Haemophilia 1998, 4, 558–563. [Google Scholar] [CrossRef]
- Garagiola, I.; Palla, R.; Peyvandi, F. Risk factors for inhibitor development in severe hemophilia A. Thromb. Res. 2018, 168, 20–27. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]

| Exon/Intron | Protein Domain | Nucleotide Change | Codon Change | Amino Acid Change | Variant Type | Novel/Known | No of Patients | Phenotype | Inhibitor | Criteria | Classification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | A1 | c.71A>G | TAC>TGC | p.(Tyr24Cys) | missense | known | 1 | moderate | no | PM1 PM2 PP2 PP4 | LP |
| E1 | A1 | c.97T>C | TGG>CGG | p.(Trp33Arg) | missense | novel | 1 | severe | no | PM1 PM2 PP2 PP4 | LP |
| E2 | A1 | c.202A>G | ACT>GCT | p.(Thr68Ala) | missense | known | 1 | severe | no | PM1 PM2 PP2 PP4 | LP |
| E3 | A1 | c.292G>A | GAG>AAG | p.(Glu98Lys) | missense | known | 1 | severe | no | PM1 PM2 PP2 PP4 | LP |
| E3 | A1 | c.326A>G | AAC>AGC | p.(Asn109Ser) | missense | novel | 2 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E4 | A1 | c.541G>A | GTG>ATG | p.(Val181Met) | missense | known | 3 | mild x3 | no | PS3 PS4 PM1 PP2 | P |
| E5 | A1 | c.641T>G | TTT>TGT | p.(Phe214Cys) | missense | novel | 4 | mild x1/moderate x2/severe x1 | no | PM1 PM2 PM5 PP2 | LP |
| E7 | A1 | c. 871G>A | GAA>AAA | p.(Glu291Lys) | missense | known | 1 | moderate | no | PS3 PS4 PM1 PP2 | P |
| E7 | A1 | c.985T>G | TGT>GGT | p.(Cys329Gly) | missense | novel | 1 | severe | no | PM1 PM2 PM5 PP2 | LP |
| E8 | A1 | c.1016T>C | ATG>ACG | p.(Met339Thr) | missense | known | 1 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E8 | a1 | c.1171C>T | CGC>TGC | p.(Arg391Cys) | missense | known | 1 | moderate | no | PS3 PS4 PM1 PP2 | P |
| E8 | a1 | c.1172G>A | CGC>CAC | p.(Arg391His) | missense | known | 7 | moderatex3/severex4 | no | PS3 PS4 PM1 PP2 | P |
| E8 | A2 | c.1203G>T | TGG>TGT | p.(Trp401Cys) | missense | novel | 2 | moderate x2 | no | PM1 PM2 PP1 PP2 | LP |
| E8 | A2 | c.1244C>T | GCT>GTT | p.(Ala415Val) | missense | known | 1 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E9 | A2 | c.1315G>A | GGT>AGT | p.(Gly439Ser) | missense | known | 1 | severe | no | PM1 PM2 PM5 PP2 | LP |
| E10 | A2 | c.1492G>A | GGA>AGA | p.(Gly498Arg) | missense | known | 1 | moderate | no | PS3 PS4 PM1 PP2 | P |
| E11 | A2 | c.1595G>C | TGG>TCG | p.(Trp532Ser) | missense | novel | 1 | severe | no | PM1 PM2 PP2 PP4 | LP |
| E11 | A2 | c.1648C>T | CGC>TGC | p.(Arg550Cys) | missense | known | 1 | mild | no | PS3 PS4 PM1 PP2 | P |
| E11 | A2 | c.1683T>G | GAT>GAG | p.(Asp561Glu) | missense | novel | 1 | moderate | no | PM1 PM2 PM5 PP2 | LP |
| E13 | A2 | c.1909A>G | AAT>GAT | p.(Asn637Asp) | missense | known | 1 | severe | no | PM1 PM2 PM5 PP2 | LP |
| E13 | A2 | c.1990C>G | CAG>GAG | p.(Gln664Glu) | missense | novel | 1 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E13 | A2 | c.2048A>G | TAT>TGT | p.(Tyr683Cys) | missense | known | 3 | severe x3 | no | PS3 PS4 PM1 PP2 | P |
| E14 | A2 | c.2148T>A | TTT>TTA | p.(Phe716Leu) | missense | novel | 1 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E14 | A2 | c.2149C>T | CGG>TGG | p.(Arg717Trp) | missense | known | 1 | moderate | no | PS3 PS4 PM1 PP2 | P |
| E14 | A2 | c.2150G>A | CGG>CAG | p.(Arg717Gln) | missense | known | 1 | moderate | no | PS3 PS4 PM1 PP2 | P |
| E14 | A2 | c.2167G>A | GCC>ACC | p.(Ala723Thr) | missense | known | 1 | mild | no | PM1 PM2 PM5 PP2 | LP |
| E14 | A2 | c.2189G>C | TGT>TCT | p.(Cys730Ser) | missense | novel | 1 | severe | no | PM1 PM2 PP1 PP2 | LP |
| E14 | a3 | c.5122C>T | CGC>TGC | p.(Arg1708Cys) | missense | known | 2 | mild/moderate | no | PS3 PS4 PM1 PP2 | P |
| E16 | A3 | c.5398C>T | CGT>TGT | p.(Arg1800Cys) | missense | known | 1 | severe | no | PS3 PS4 PM1 PP2 | P |
| E17 | A3 | c.5593G>T | GAT>TAT | p.(Asp1865Tyr) | missense | known | 1 | severe | no | PS3 PS4 PM1 PP2 | P |
| E17 | A3 | c.5815G>A | GCA>ACA | p.(Ala1939Thr) | missense | known | 1 | severe | no | PM1 PM2 PM5 PP2 | LP |
| E18 | A3 | c.5824G>A | GGC>AGC | p.(Gly1942Ser) | missense | known | 1 | severe | no | PM1 PM2 PP2 PP4 | LP |
| E19 | A3 | c.6046C>T | CGG>TGG | p.(Arg2016Trp) | missense | known | 4 | moderate x3/severe x1 | yes (1 patient) | PS3 PS4 PM1 PP2 | P |
| E23 | C1 | c.6506G>A | CGT>CAT | p.(Arg2169His) | missense | known | 1 | mild | no | PS3 PS4 PM1 PP2 | P |
| E23 | C1 | c.6533G>A | CGC>CAC | p.(Arg2178His) | missense | known | 1 | moderate | no | PS1 PS3 PS4 PM1 PP2 | P |
| E23 | C1 | c.6544C>T | CGC>TGC | p.(Arg2182Cys) | missense | known | 1 | severe | no | PS3 PS4 PM1 PP2 | P |
| E23 | C1 | c.6545G>A | CGC>CAC | p.(Arg2182His) | missense | known | 2 | severe x2 | no | PS3 PS4 PM1 PP2 | P |
| E24 | C2 | c.6683G>A | GGA>CAA | p.(Arg2228Gln) | missense | known | 1 | severe | yes | PS1 PS3 PS4 PM1 PP2 | P |
| E1 | signal peptide | c.4C>T | CAA>TAA | p.(Gln2*) | nonsense | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P |
| E8 | a1 | c.1063C>T | CGA>TGA | p.(Arg355*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P |
| E8 | A2 | c.1203G>A | TGG>TGA | p.(Trp401*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P |
| E11 | A2 | c.1750C>T | CAG>TAG | p.(Gln584*) | nonsense | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 | P |
| E12 | A2 | c.1804C>T | CGA>TGA | p.(Arg602*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P |
| E14 | B | c.2440C>T | CGA>TGA | p.(Arg814*) | nonsense | known | 2 | severe x2 | no | PVS1 PM1 PM2 PM4 | P |
| E14 | B | c.3380G>A | TGG>TAG | p.(Trp1127*) | nonsense | novel | 2 | severe x2 | no | PVS1 PM1 PM2 PM4 PP1 | P |
| E14 | B | c.4128C>A | TAC>TAA | p.(Tyr1376*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P |
| E14 | B | c.4408G>T | GAG>TAG | p.(Glu1470*) | nonsense | known | 1 | severe | yes | PVS1 PM1 PM2 PM4 | P |
| E17 | A3 | c.5766C>A | TGC>TGA | p.(Cys1922*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P |
| E18 | A3 | c.5878C>T | CGA>TGA | p.(Arg1960*) | nonsense | known | 1 | severe | yes | PVS1 PM1 PM2 PM4 PP1 | P |
| E22 | C1 | c.6403C>T | CGA>TGA | p.(Arg2135*) | nonsense | known | 2 | severe x2 | no | PVS1 PM1 PM2 PM4 PP1 | P |
| E23 | C1 | c.6496C>T | CGA>TGA | p.(Arg2166*) | nonsense | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P |
| E25 | C2 | c.6743G>A | TGG>TAG | p.(Trp2248*) | nonsense | known | 1 | severe | yes | PVS1 PM1 PM2 PM4 PP1 | P |
| E2 | A1 | c.206_209delTGTT | p.(Leu69Leufs*2) | frameshift | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P | |
| E14 | a2 | c.2218delG | p.(Asp740Thrfs*11) | frameshift | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.3637delA | p.(Ile1213Phefs*5) | frameshift | known | 3 | severe x3 | no | PVS1 PM1 PM2 PM4 PP1 | P | |
| E14 | B | c.4085delA | p.(Asn1362Thrfs*1) | frameshift | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.4370delC | p.(Ala1457Alafs*8) | frameshift | novel | 1 | moderate | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.4372delA | p.(Lys1458Lysfs*7) | frameshift | known | 2 | severe x2 | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.4377_4380delAAAT | p.(Lys1459Lysfs*4) | frameshift | novel | 1 | severe | no | PVS1 PM1 PM2 PM4 | P | |
| E19 | A3 | c.6082delG | p.(Met2029*) | frameshift | known | 1 | severe | yes | PVS1 PM1 PM2 PM4 | P | |
| E25 | C2 | c.6876_6877delCT | p.(Phe2294Serfs*90) | frameshift | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.2945dupA | p.(Asn982Lysfs*9) | frameshift | known | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P | |
| E14 | B | c.3300dupA | p.(Glu1101Argfs*16) | frameshift | known | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P | |
| E14 | B | c.3637duplA | p.(Ile1213Asnfs*28) | frameshift | known | 1 | severe | no | PVS1 PM1 PM2 PM4 | P | |
| E14 | B | c.4379dupA | p.(Asn1460Lysfs*1) | frameshift | known | 1 | severe | no | PVS1 PM1 PM2 PM4 PP1 | P | |
| I6 | c.787+3A>G | splice | known | 3 | severe x3 | no | PPM1 PM2 PP1 PP4 PP5 | LP | |||
| I9 | c.1444-1G>A | splice | novel | 1 | severe | no | PM1 PM2 PP1 PP4 | LP | |||
| I11 | c.1753-2A>C | splice | known | 1 | severe | no | PM1 PM2 PP1 PP4 | LP | |||
| I11 | c.1753-12T>A | splice | novel | 2 | moderate x2 | no | PM1 PM2 PP1 PP4 | LP | |||
| I17 | c.5816-2A>G | splice | known | 1 | severe | no | PPM1 PM2 PP1 PP4 PP5 | LP | |||
| Del. Ex. 1-6 | deletion | known | 1 | severe | yes | ||||||
| Del. Ex. 1-10 | deletion | novel | 1 | severe | no | ||||||
| Del ex.5-6 | deletion | known | 1 | severe | no | ||||||
| Dupl. ex. 2-14 | duplication | novel | 1 | severe | no | ||||||
| Dupl. ex. 16-22 | duplication | novel | 1 | severe | yes |
| Mutation Type | Inversion 22 | Inversion 1 | Missense | Nonsense | Small Deletions | Small Duplications | Splice-Site Variants | Large Deletions | Large Duplications | Unknown | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 65 | 5 | 57 | 17 | 12 | 4 | 8 | 3 | 2 | 14 | 187 |
| Patients with inhibitors | 7 | 2 | 2 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 17 |
| Inhibitor frequency | 10.77% | 40% | 3.51% | 17.65% | 8.33% | 0% | 0% | 33.33% | 50% | 0% | 9.09% |
| HA-All Severities | Severe HA | Mild/Moderate HA | ||||||
|---|---|---|---|---|---|---|---|---|
| Mutation Type | Intron 22 Inversion | Intron 1 Inversion | Missense Variants | Nonsense Variants | Small Deletions and Insertions | Large Deletions | Large Duplications | Splice-Site Mutations |
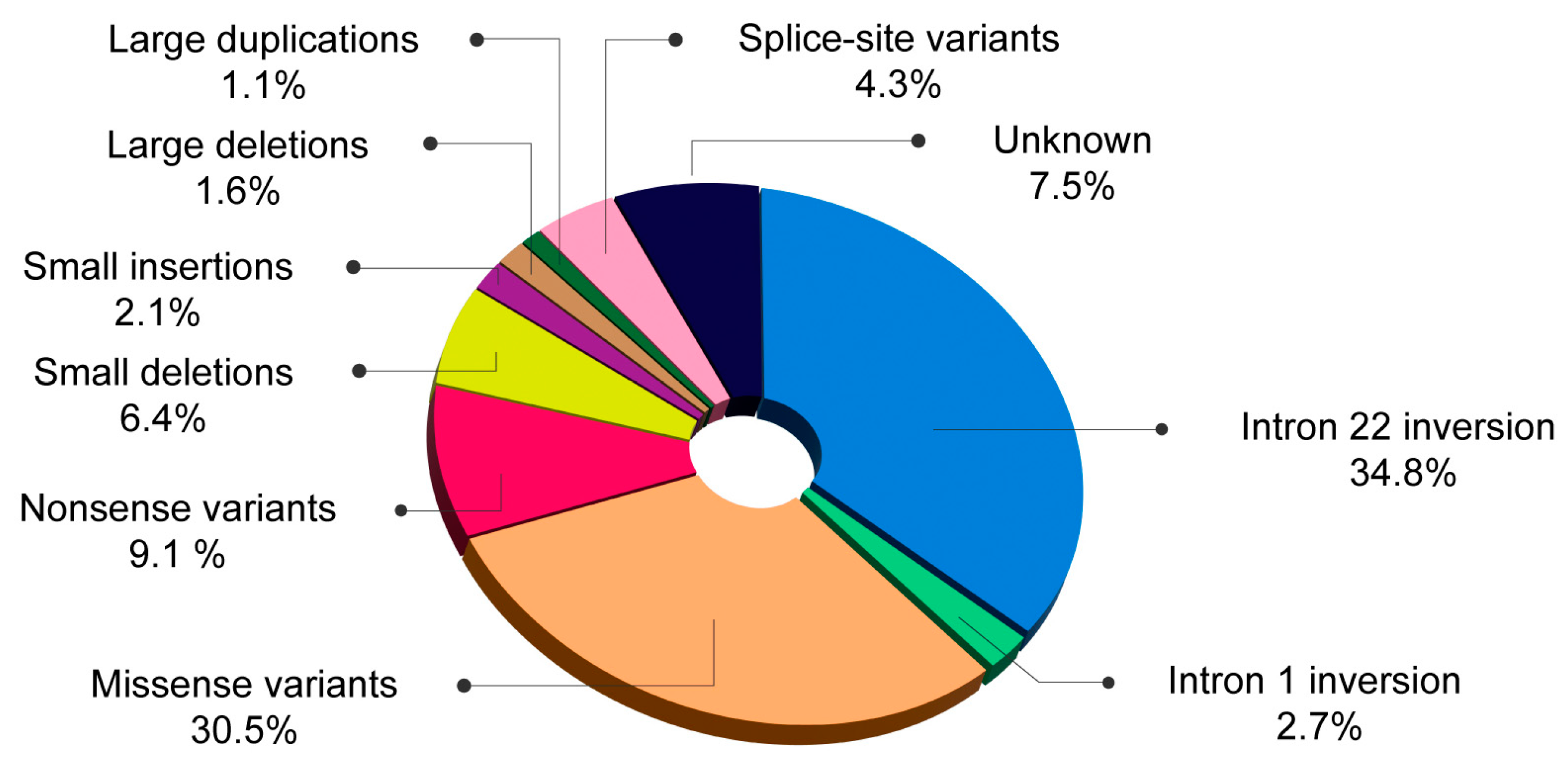
| Romanian patients (n = 187) | 34.8% | 2.7% | 30.5% | 9.1% | 8.5% | 1.6% | 1.1% | 4.3% |
| Oldenburg et al. [21] (n = 846) | 35.7% | 1% | 38.2% | 9.3% | 10.1% | 3% | 0% | 2.6% |
| Bach et al. [22] (n = 2671) | 25.38% | 1.12% | 43.77% | 7.08% | 10.71% | 2.81% | 0.64% | 3.59% |
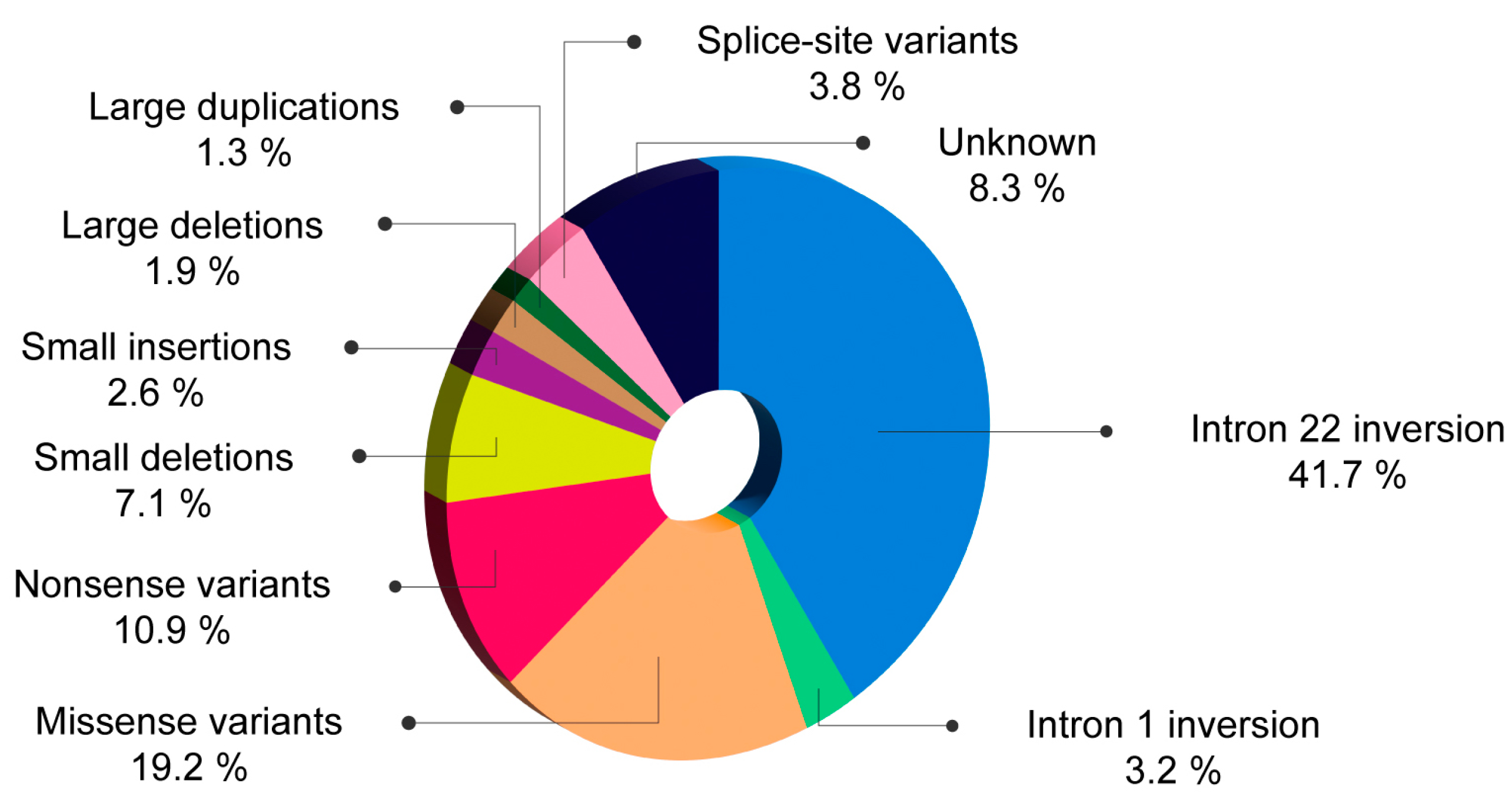
| Romanian patients (n = 156) | 41.7% | 3.2% | 19.2% | 10.9% | 9.7% | 1.9% | 1.3% | 3.8% |
| Gouw et al. [23] (n = 5383) | 45% | 2% | 15% | 10% | 16% | 3% | 0% | 3% |
| Johnsen et al. [24] (n = 1272) | 42% | 1.4% | 17.4% | 10.7% | 17.4% | NA | NA | 3.3% |
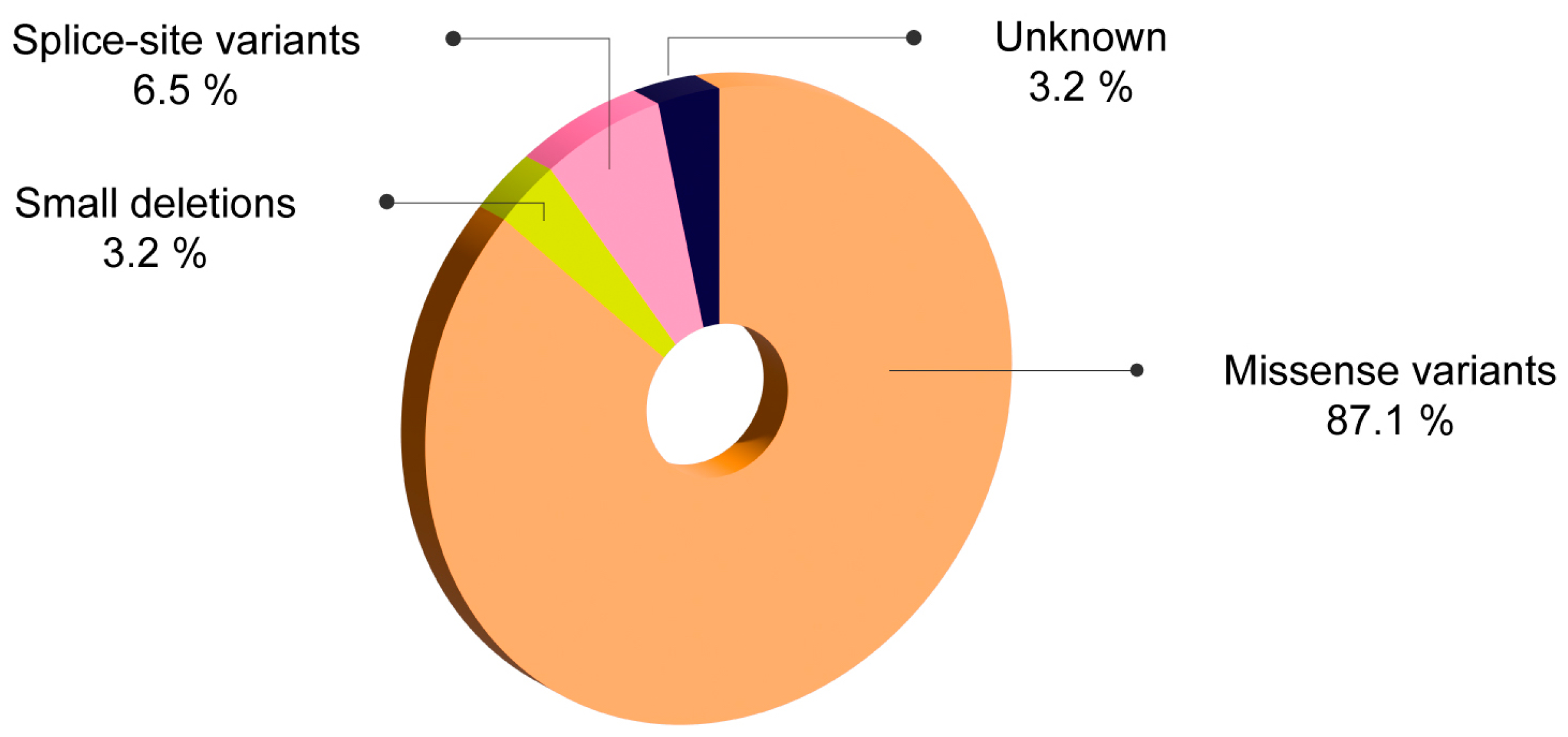
| Romanian patients (n = 31) | 0% | 0% | 87.1% | 0% | 3.2% | 0% | 0% | 6.5% |
| Johnsen et al. [24] (n = 1048) | 3.7% | 0.2% | 79.5% | 1.1% | 2% | NA | NA | 2.7% |
| Mutation Type | Inhibitor Frequency in Romanian Patients (n = 187) | Inhibitor Frequency-Gouw et al. [22] Meta-Analysis (n = 922) | Inhibitor Frequency-Oldenburg et al. [24] (Bonn Centre Study + HAMSTERS Databese) |
|---|---|---|---|
| Intron 22 inversion | 10.77% | NA | 21% |
| Intron 1 inversion | 40% | NA | 17% |
| Nonsense mutations in the light chain | 33.33% | 43% | 40% |
| Nonsense mutations outside the light chain | 9.09% | 12% | 17% |
| Missense mutations in the light chain | 14.3% | 11% | 10% |
| Missense mutations outside the light chain | 0% | 6% | 3% |
| Small deletions and insertions in poly-A runs | 0% | 6% | 3% |
| Small deletions and insertions outside poly-A runs | 16.67% | 19% | 21% |
| Large deletions > 1 exon | 33.33% | 67% | 88% |
| Splice-site mutations | 0% | 7% | 17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore, A.; Dragomir, M.; Călugăru, O.-T.; Jardan, D.; Jardan, C.; Brînză, M.; Bălănescu, P.; Coriu, D. Mutational Profile in Romanian Patients with Hemophilia A. Int. J. Mol. Sci. 2024, 25, 8366. https://doi.org/10.3390/ijms25158366
Grigore A, Dragomir M, Călugăru O-T, Jardan D, Jardan C, Brînză M, Bălănescu P, Coriu D. Mutational Profile in Romanian Patients with Hemophilia A. International Journal of Molecular Sciences. 2024; 25(15):8366. https://doi.org/10.3390/ijms25158366
Chicago/Turabian StyleGrigore, Andra, Mihaela Dragomir, Onda-Tabita Călugăru, Dumitru Jardan, Cerasela Jardan, Melen Brînză, Paul Bălănescu, and Daniel Coriu. 2024. "Mutational Profile in Romanian Patients with Hemophilia A" International Journal of Molecular Sciences 25, no. 15: 8366. https://doi.org/10.3390/ijms25158366
APA StyleGrigore, A., Dragomir, M., Călugăru, O.-T., Jardan, D., Jardan, C., Brînză, M., Bălănescu, P., & Coriu, D. (2024). Mutational Profile in Romanian Patients with Hemophilia A. International Journal of Molecular Sciences, 25(15), 8366. https://doi.org/10.3390/ijms25158366






