Concurrent Analysis of Tiafenacil and Its Transformation Products in Soil by Using Newly Developed UHPLC-QTOF-MS/MS-Based Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Instrument Conditions
2.1.1. MS/MS Parameters
2.1.2. Extraction and Clean-Up Pretreatment
2.2. Method Performance
2.2.1. Specificity, Linearity, LOQ, and ME
2.2.2. Precision, Accuracy, and Measurement Uncertainty
2.2.3. Comparison of Established Methods
2.3. Dissipation Dynamics of TFA in Soil
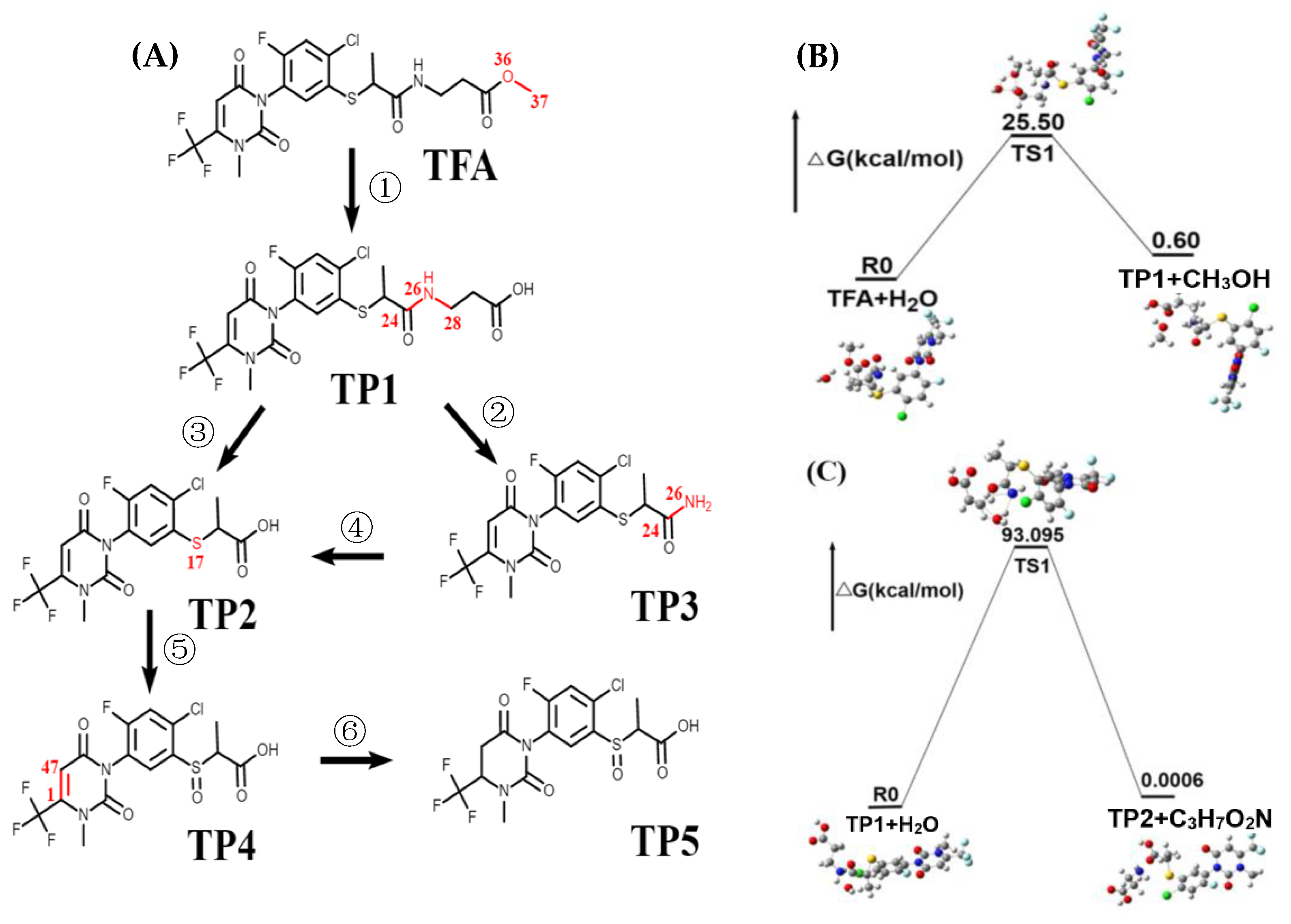
2.4. DFT-Based Prediction of TFA Transformants
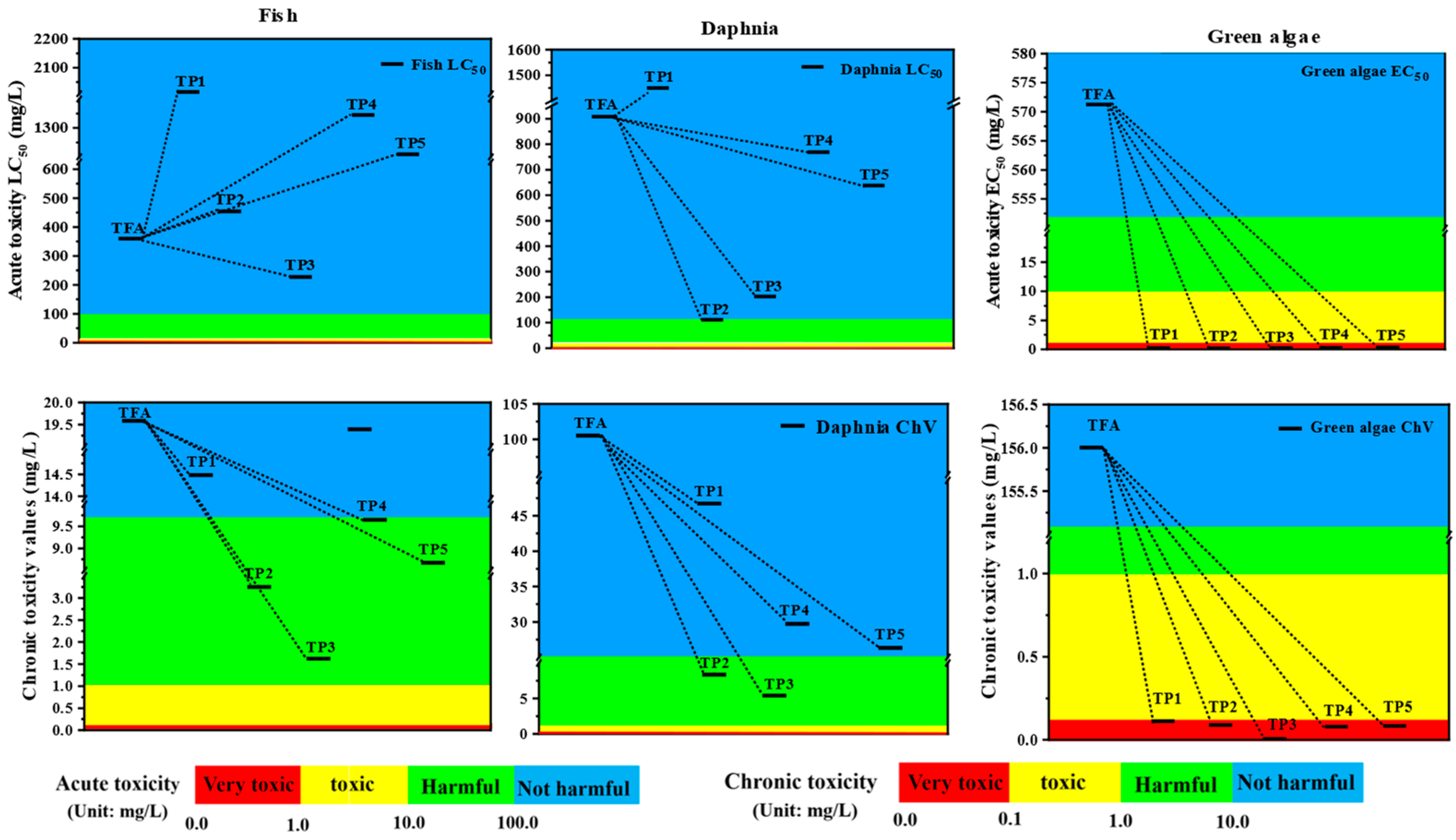
2.5. Risk Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Instrumental Parameters
3.4. Dissipation Experiments
3.5. Theoretical Calculation of TFA Dissipation Reactions
3.6. Toxicity Prediction by ECOSAR
3.7. Actual Field Samples
3.8. Date Processing
3.8.1. Dissipation Kinetics Study
3.8.2. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Chang, J.; Fang, W.; Chen, L.; Zhang, P.; Zhang, G.; Zhang, H.; Liang, J.; Wang, Q.; Ma, W. Toxicological effects, environmental behaviors and remediation technologies of herbicide atrazine in soil and sediment: A comprehensive review. Chemosphere 2022, 307, 136006. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Annamalai, P.; Parven, A.; Megharaj, M. Degradation of four pesticides in five urban landscape soils: Human and environmental health risk assessment. Environ. Geochem. Health 2023, 45, 1599–1614. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Bi, G.; Ward, T.J.; Li, L. Adsorption and degradation of neonicotinoid insecticides in agricultural soils. Environ. Sci. Pollut. Res. 2023, 30, 47516–47526. [Google Scholar] [CrossRef]
- Kandil, M.; Moustafa, M.; Saleh, M. Dissipation kinetics and degradation products of cyantraniliprole in tomato plants and soil in the open field. Egypt. J. Chem. 2023, 66, 483–493. [Google Scholar]
- Isin, E.M.; Elmore, C.S.; Nilsson, G.N.; Thompson, R.A.; Weidolf, L. Use of Radiolabeled Compounds in Drug Metabolism and Pharmacokinetic Studies. Chem. Res. Toxicol. 2012, 25, 532–542. [Google Scholar] [CrossRef]
- Mutlib, A.E. Application of Stable Isotope-Labeled Compounds in Metabolism and in Metabolism-Mediated Toxicity Studies. Chem. Res. Toxicol. 2008, 21, 1672–1689. [Google Scholar] [CrossRef]
- Tang, Y.N.; Pang, Y.X.; He, X.C.; Zhang, Y.Z.; Zhang, J.Y.; Zhao, Z.Z.; Yi, T.; Chen, H.B. UPLC-QTOF-MS identification of metabolites in rat biosamples after oral administration of Dioscorea saponins: A comparative study. J. Ethnopharmacol. 2015, 165, 127–140. [Google Scholar] [CrossRef]
- Nam, M.; Jo, S.R.; Kim, Y.C.; Kim, M.S. UPLC-QTOF-MS-Based Metabolomics and Antioxidant Capacity of Codonopsis lanceolata from Different Geographical Origins. Foods 2023, 12, 267. [Google Scholar] [CrossRef]
- Moretti, M.L. Tiafenacil—A new herbicide in hops. In Proceedings of the Scientific-Technical Commission, Lugo, Spain, 3–7 July 2022; pp. 67–70. [Google Scholar]
- Hu, M.; Tan, H.; Li, Y.; Qiu, J.; Liu, L.; Zeng, D. Simultaneous determination of tiafenacil and its six metabolites in fruits using ultra-high-performance liquid chromatography/tandem mass spectrometry. Food Chem. 2020, 327, 127015. [Google Scholar] [CrossRef]
- Soltani, N.; Shropshire, C.; Sikkema, P.H. Response of dry beans to tiafenacil applied preemergence. Weed Technol. 2021, 35, 991–994. [Google Scholar] [CrossRef]
- Park, J.; Ahn, Y.O.; Nam, J.W.; Hong, M.K.; Song, N.; Kim, T.; Yu, G.H.; Sung, S.K. Biochemical and physiological mode of action of tiafenacil, a new protoporphyrinogen IX oxidase-inhibiting herbicide. Pestic. Biochem. Physiol. 2018, 152, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Shin, G.; Ahn, G.; Jeong, S.Y.; Ji, M.G.; Alimzhan, A.; Kim, M.G.; Kim, W.Y. Loss-of-function in GIGANTEA confers resistance to PPO-inhibiting herbicide tiafenacil through transcriptional activation of antioxidant genes in Arabidopsis. Appl. Biol. Chem. 2022, 65, 66. [Google Scholar] [CrossRef]
- Ivantsova, E.; Konig, I.; Lopez-Scarim, V.; English, C.; Charnas, S.R.; Souders, C.L.; Martyniuk, C.J. Molecular and behavioral toxicity assessment of tiafenacil, a novel PPO-inhibiting herbicide, in zebrafish embryos/larvae. Environ. Toxicol. Pharmacol. 2023, 98, 104084. [Google Scholar] [CrossRef]
- Buerge, I.J.; Bächli, A.; Kasteel, R.; Portmann, R.; López-Cabeza, R.; Schwab, L.F.; Poiger, T. Behavior of the Chiral Herbicide Imazamox in Soils: pH-Dependent, Enantioselective Degradation, Formation and Degradation of Several Chiral Metabolites. Environ. Sci. Technol. 2019, 53, 5725–5732. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Bhattacharya, B.; Agarwal, T.; Paul, V.; Maurya, V.K.; Chakkaravarthi, S.; Simal-Gandara, J. Method development and validation for acrylamide in potato cutlet by UHPLC-MS/MS. Food Control 2023, 151, 109817. [Google Scholar] [CrossRef]
- Liu, L.; Rao, L.; Li, W.; Zhou, W.; Li, B.; Tang, L. Detection of Glyamifop residues in rice and its environment by the QuEChERS method combined with HPLC–MS. Microchem. J. 2020, 158, 105157. [Google Scholar] [CrossRef]
- Gao, M.; Bian, C.; Zhou, W.; Liu, L.; Li, B.; Tang, L. Dissipation of tiafenacil in five types of citrus orchard soils using the HPLC-MS coupled with the quick, easy, cheap, effective, rugged, and safe method. J. Sep. Sci. 2021, 44, 1950–1960. [Google Scholar] [CrossRef]
- Hwang, J.I.; Norsworthy, J.K.; Carvalho-Moore, P.; Barber, L.T.; Butts, T.R.; McElroy, J.S. Exploratory Analysis on Herbicide Metabolism and Very-Long-Chain Fatty Acid Production in Metolachlor-Resistant Palmer Amaranth (Amaranthus palmeri S. Wats.). J. Agric. Food Chem. 2023, 71, 6014–6022. [Google Scholar] [CrossRef]
- Jin, H.; Hou, J.; Ruan, Y.; Xu, J.; Yang, K.; Lin, D. Distribution pattern analysis of multiple antibiotics in the soil-rice system using a QuEChERS extraction method. Sci. Total Environ. 2024, 944, 173929. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, R.; Bian, C.; Li, H.; Wang, L.; Fu, J.; Xie, G.; Shi, X.; Li, X.; Li, Z.; et al. Persistence, decontamination and dietary risk assessment of propyrisulfuron residue in natural paddy field environment using QuEChERS@UPLC-Q-TOF-MS/MS. Microchem. J. 2022, 181, 107832. [Google Scholar] [CrossRef]
- Arani, M.H.; Kermani, M.; Kalantary, R.R.; Jaafarzadeh, N.; Arani, S.B. Pesticides residues determination and probabilistic health risk assessment in the soil and cantaloupe by Monte Carlo simulation: A case study in Kashan and Aran-Bidgol, Iran. Ecotox. Environ. Safe. 2023, 263, 115229. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, H.; Lin, W.; Hua, J.; Li, Y.; Li, B. Determination of spinetoram residues in rice field environment by HPLC combined with QuEChERS method, residue elimination and final residues. J. Food Compos. Anal. 2024, 134, 106529. [Google Scholar] [CrossRef]
- NY/T 3150-2017; Guidelines for Evaluating and Calculating Degradation Kinetics in Environmental Media for Pesticide Registration. China Standard Publishing House: Beijing, China, 2017.
- Zhou, R.; Dong, Z.; Wang, L.; Zhou, W.W.; Zhao, W.N.; Wu, T.Q.; Chang, H.L.; Lin, W.; Li, B.T. Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms. Int. J. Mol. Sci. 2023, 24, 10521. [Google Scholar] [CrossRef]
- Nyholm, J.R.; Lundberg, C.; Andersson, P.L. Biodegradation kinetics of selected brominated flame retardants in aerobic and anaerobic soil. Environ. Pollut. 2010, 158, 2235–2240. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Zhou, R.; Xiao, J.; Li, Y.; Ren, Y.; Li, B. Evaluation of Iron Chlorin e6 disappearance and hydrolysis in soil and garlic using salting-out assisted liquid-liquid extraction coupled with high-performance liquid chromatography and ultraviolet-visible detection. Food Chem. 2024, 447, 138960. [Google Scholar] [CrossRef]
- de Oliveira, F.S.; Takeshita, V.; Mendes, K.F.; Tornisielo, V.L.; Alonso, F.G.; Junqueira, L.V.; Neto, M.B.; Lins, H.A.; Silva, D.V. Addition of raw feedstocks and biochars to the soil on the sorption–desorption and biodegradation of 14C-saflufenacil. Int. J. Environ. Sci. Technol. 2023, 20, 1259–1276. [Google Scholar] [CrossRef]
- Ma, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Kinetics, mechanisms and toxicity of the degradation of imidaclothiz in soil and water. J. Hazard. Mater. 2021, 403, 124033. [Google Scholar] [CrossRef]
- Cheng, H.; Xing, D.; Twagirayezu, G.; Lin, S.; Gu, S.; Tu, C.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Effects of field-aging on the impact of biochar on herbicide fate and microbial community structure in the soil environment. Chemosphere 2023, 348, 140682. [Google Scholar] [CrossRef]
- Terol, H.; Thiour-Mauprivez, C.; Devers, M.; Martin-Laurent, F.; Suzuki, M.; Calvayrac, C.; Barthelmebs, L. Structural responses of non-targeted bacterial and hppd communities to the herbicide tembotrione in soil. Sci. Total Environ. 2024, 908, 168198. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, L.; Xu, D.M.; Fu, R.B. Insight into the degradation mechanism of peroxyacetic acid for pentachlorophenol by thermal activation in soil system. Environ. Technol. Innov. 2022, 28, 102882. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, M.; Shi, Y.; Ma, H.; Li, D.; Li, Y.; Lu, L.; Chen, X. High-throughput modeling of atomic diffusion migration energy barrier of fcc metals. Prog. Nat. Sci. 2019, 29, 341–348. [Google Scholar] [CrossRef]
- Dao, D.Q.; Taamalli, S.; Louis, F.; Kdouh, D.; Srour, Z.; Ngo, T.C.; Truong, D.H.; Fèvre-Nollet, V.; Ribaucour, M.; El Bakali, A.; et al. Hydroxyl radical-initiated decomposition of metazachlor herbicide in the gaseous and aqueous phases: Mechanism, kinetics, and toxicity evaluation. Chemosphere 2023, 312, 137234. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Sommers, L. Total Carbon, Organic Carbon and Organic Matter; American Society of Agronomy: Madison, WI, USA, 1985. [Google Scholar]
- L’Huillier, L.; Dupont, S.; Dubus, I.; Becquer, T.; Bourdon, E.; Laubreaux, P.; Bonzon, B. Carence et fixation du phosphore dans les sols ferrallitiques ferritiques de Nouvelle-Caledonie. In Proceedings of the XVIe Congrès Mondial de la Science du Sol, Montpellier, France, 20–26 August 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16 Rev. A.03; Wallingford: Wallingford, CT, USA, 2016. [Google Scholar]
- Andersson, M.P.; Uvdal, P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6-311+G(d,p). J. Phys. Chem. A 2005, 109, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- NY/T 788-2018; Guideline for the Testing of Pesticide Residue in Crops. China Agricultural Press: Beijing, China, 2018.
- Kumar, N.; Jung, U.; Jung, B.; Park, J.; Naushad, M. Zinc hydroxystannate/zinc-tin oxide heterojunctions for the UVC-assisted photocatalytic degradation of methyl orange and tetracycline. Environ. Pollut. 2023, 316, 120353. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, W.; Jia, H.; Huang, H.; Li, B. Adsorption isotherms, degradation kinetics, and leaching behaviors of cyanogen and hydrogen cyanide in eight texturally different agricultural soils from China. Ecotoxicol. Environ. Safe. 2019, 185, 109704. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, H.; Liu, L.; Li, B.; Li, Y.; Gao, M. Environmental behaviors of (E)-Pyriminobac-methyl in agricultural soils. Soil 2022, 8, 237–252. [Google Scholar] [CrossRef]
- Luo, J.; Bian, C.; Rao, L.; Zhou, W.; Li, Y.; Li, B. Determination of the residue behavior of isocycloseram in Brassica oleracea and soil using the QuEChERS method coupled with HPLC. Food Chem. 2022, 367, 130734. [Google Scholar] [CrossRef]
- Woo, S.Y.; Lee, S.Y.; Park, S.B.; Chun, H.S. Simultaneous determination of 17 regulated and non-regulated Fusarium mycotoxins co-occurring in foodstuffs by UPLC-MS/MS with solid-phase extraction. Food Chem. 2024, 438, 137624. [Google Scholar] [CrossRef]
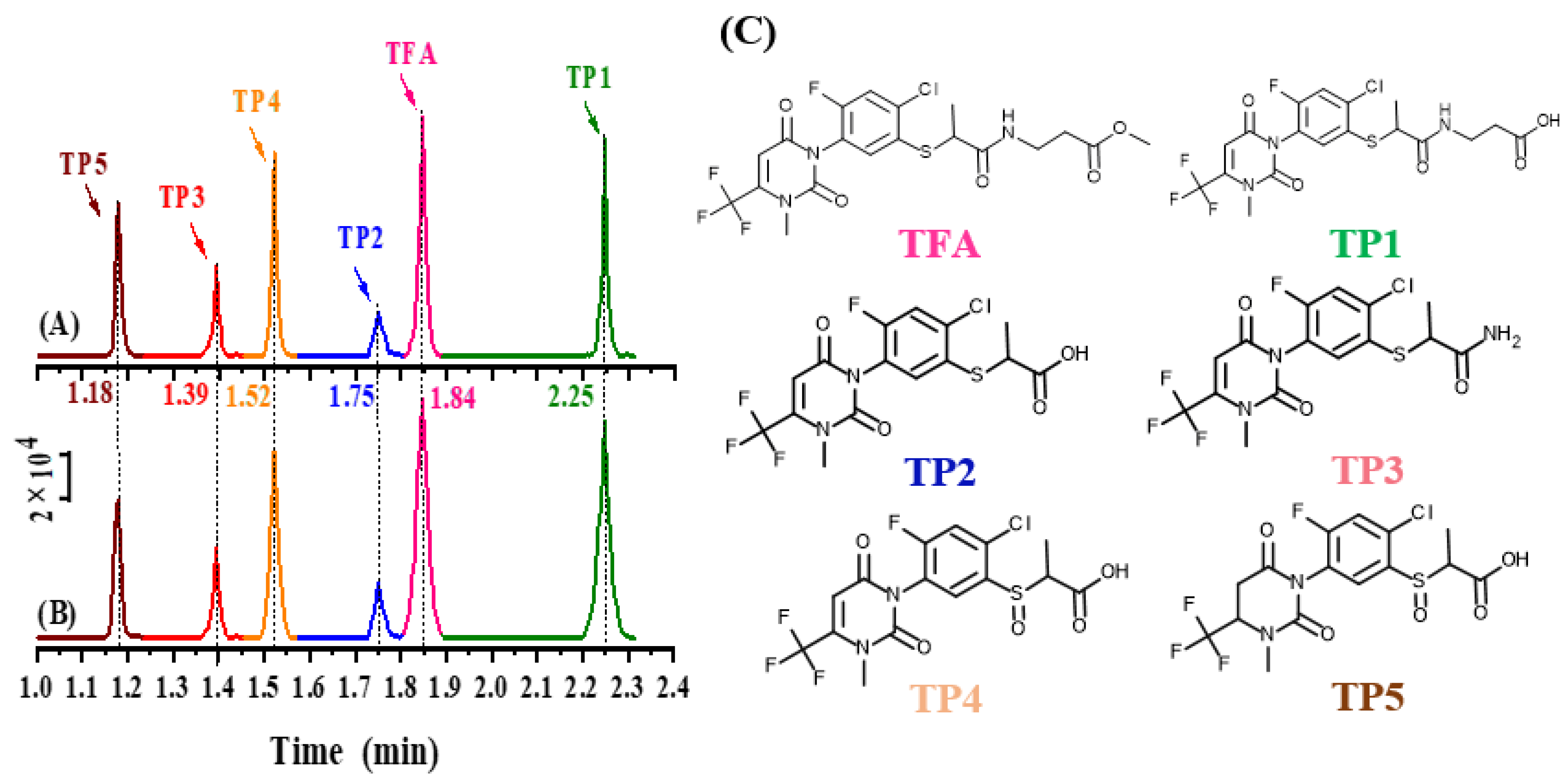



| Compound | Molecular Formula | Retention Time (min) | Fragmentor (V) | Precursor Ion (m/z) | Qualifier Ion (m/z) | Q/q * | Collision Energy (eV) |
|---|---|---|---|---|---|---|---|
| TFA | C19H18ClF4N3O5S | 1.84 | 120 | 512.1 | 480.0 | Q | 37 |
| 426.0 | q | ||||||
| TP1 | C18H16ClF4N3O5S | 2.25 | 120 | 498.1 | 480.0 | Q | 35 |
| 381.0 | q | ||||||
| TP2 | C15H11ClF4N2O4S | 1.75 | 100 | 427.2 | 409.0 | Q | 23 |
| 355.0 | q | ||||||
| TP3 | C15H12ClF4N3O3S | 1.39 | 140 | 426.1 | 409.0 | Q | 27 |
| 381.0 | q | ||||||
| TP4 | C15H11ClF4N2O5S | 1.51 | 100 | 443.1 | 368.8 | Q | 51 |
| 352.9 | q | ||||||
| TP5 | C15H13ClF4N2O5S | 1.18 | 140 | 445.1 | 370.9 | Q | 33 |
| 354.9 | q |
| Compound | Matrix | Regression Equation | R2 | LOQ | Matrix Effect |
|---|---|---|---|---|---|
| (µg/kg) | (%) | ||||
| TFA | Acetonitrile | y = 6269.8x + 102031 | 0.9999 | - | - |
| Anthrosols | y = 7291.8x − 257895 | 0.9954 | 2.00 | 0.82 | |
| Ferralsols | y = 6958.8x + 37158 | 0.9987 | 2.00 | 1.10 | |
| Lixisols | y = 8488.6x − 3944.7 | 0.9988 | 2.00 | 0.84 | |
| Gleysols | y = 7537.7x − 162161 | 0.9986 | 2.00 | 1.05 | |
| TP1 | Aceronitrile | y = 5544x + 105261 | 0.9995 | - | - |
| Anthrosols | y = 6232.2x − 203978 | 0.9973 | 2.00 | 0.97 | |
| Ferralsols | y = 5900.7x + 23821 | 0.9992 | 2.00 | 0.92 | |
| Lixisols | y = 7229.6x + 2410.5 | 0.9983 | 2.00 | 1.08 | |
| Gleysols | y = 7509.2x − 175689 | 0.9975 | 2.00 | 0.90 | |
| TP2 | Aceronitrile | y = 6408.3x + 26722 | 0.9997 | - | - |
| Anthrosols | y = 5325.9x − 158761 | 0.9985 | 2.00 | 1.07 | |
| Ferralsols | y = 4868.1x + 42083 | 0.9987 | 2.00 | 0.84 | |
| Lixisols | y = 6173.6x + 16669 | 0.9968 | 2.00 | 1.12 | |
| Gleysols | y = 6411.3x − 134715 | 0.9988 | 2.00 | 1.16 | |
| TP3 | Aceronitrile | y = 5789.8x − 6729.1 | 0.9974 | - | - |
| Anthrosols | y = 4791.6x − 113832 | 0.9992 | 2.00 | 1.17 | |
| Ferralsols | y = 4365.3x + 60164 | 0.9963 | 2.00 | 0.80 | |
| Lixisols | y = 5527.8x + 1069 | 0.9984 | 2.00 | 1.06 | |
| Gleysols | y = 5741.7x − 135177 | 0.9973 | 2.00 | 0.98 | |
| TP4 | Aceronitrile | y = 6306x − 64930 | 0.9971 | - | - |
| Anthrosols | y = 4548.5x − 117272 | 0.9989 | 2.00 | 1.19 | |
| Ferralsols | y = 4103.4x + 55452 | 0.9963 | 2.00 | 0.75 | |
| Lixisols | y = 5181.8x − 6860.6 | 0.9991 | 2.00 | 1.10 | |
| Gleysols | y = 5367x − 142507 | 0.9949 | 2.00 | 0.94 | |
| TP5 | Aceronitrile | y = 4551.5x + 60842 | 0.9988 | - | - |
| Anthrosols | y = 5048x − 149091 | 0.9978 | 2.00 | 0.69 | |
| Ferralsols | y = 4760.9x + 41728 | 0.9975 | 2.00 | 0.81 | |
| Lixisols | y = 5567.8x + 47773 | 0.9970 | 2.00 | 1.03 | |
| Gleysols | y = 6091.3x − 137091 | 0.9980 | 2.00 | 1.13 |
| Compounds | Instrument Used | LOQ (µg/kg) | LR (µg/kg) | ME% | RSD% | References |
|---|---|---|---|---|---|---|
| TFA | HPLC-MS | (8.80~10.30) × 103 | 0.01~1.0 * | −8.64~962 | 0.25~9.31 | [19] |
| TFA | UHPLC-MS/MS | 10 | 5~1000 | 0.58~0.97 | 1.00~11.40 | [11] |
| TFA | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.82~1.10 | 0.46~12.93 | Present work |
| TP1 | UHPLC-MS/MS | 10 | 5~1000 | 0.61~0.89 | 1.30~7.60 | [11] |
| TP1 | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.92~1.08 | 0.28~8.87 | Present work |
| TP2 | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.84~1.16 | 2.99~10.75 | Present work |
| TP3 | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.80~1.17 | 3.95~9.97 | Present work |
| TP4 | UHPLC-MS/MS | 10 | 5~1000 | 0.65~0.92 | 1.80~9.90 | [11] |
| TP4 | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.75~1.19 | 1.61~11.55 | Present work |
| TP5 | UHPLC-MS/MS | 10 | 5~1000 | 0.64~0.95 | 1.10~9.40 | [11] |
| TP5 | UHPLC-QTOF-MS/MS | 2 | 15~2000 | 0.69~1.13 | 1.49~10.38 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yan, A.; Zhang, S.; Peng, D.; Li, J. Concurrent Analysis of Tiafenacil and Its Transformation Products in Soil by Using Newly Developed UHPLC-QTOF-MS/MS-Based Approaches. Int. J. Mol. Sci. 2024, 25, 8367. https://doi.org/10.3390/ijms25158367
Zhou W, Yan A, Zhang S, Peng D, Li J. Concurrent Analysis of Tiafenacil and Its Transformation Products in Soil by Using Newly Developed UHPLC-QTOF-MS/MS-Based Approaches. International Journal of Molecular Sciences. 2024; 25(15):8367. https://doi.org/10.3390/ijms25158367
Chicago/Turabian StyleZhou, Wenwen, Anqi Yan, Shujie Zhang, Dayong Peng, and Jun Li. 2024. "Concurrent Analysis of Tiafenacil and Its Transformation Products in Soil by Using Newly Developed UHPLC-QTOF-MS/MS-Based Approaches" International Journal of Molecular Sciences 25, no. 15: 8367. https://doi.org/10.3390/ijms25158367






