Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar
Abstract
1. Introduction
2. Results and Discussion
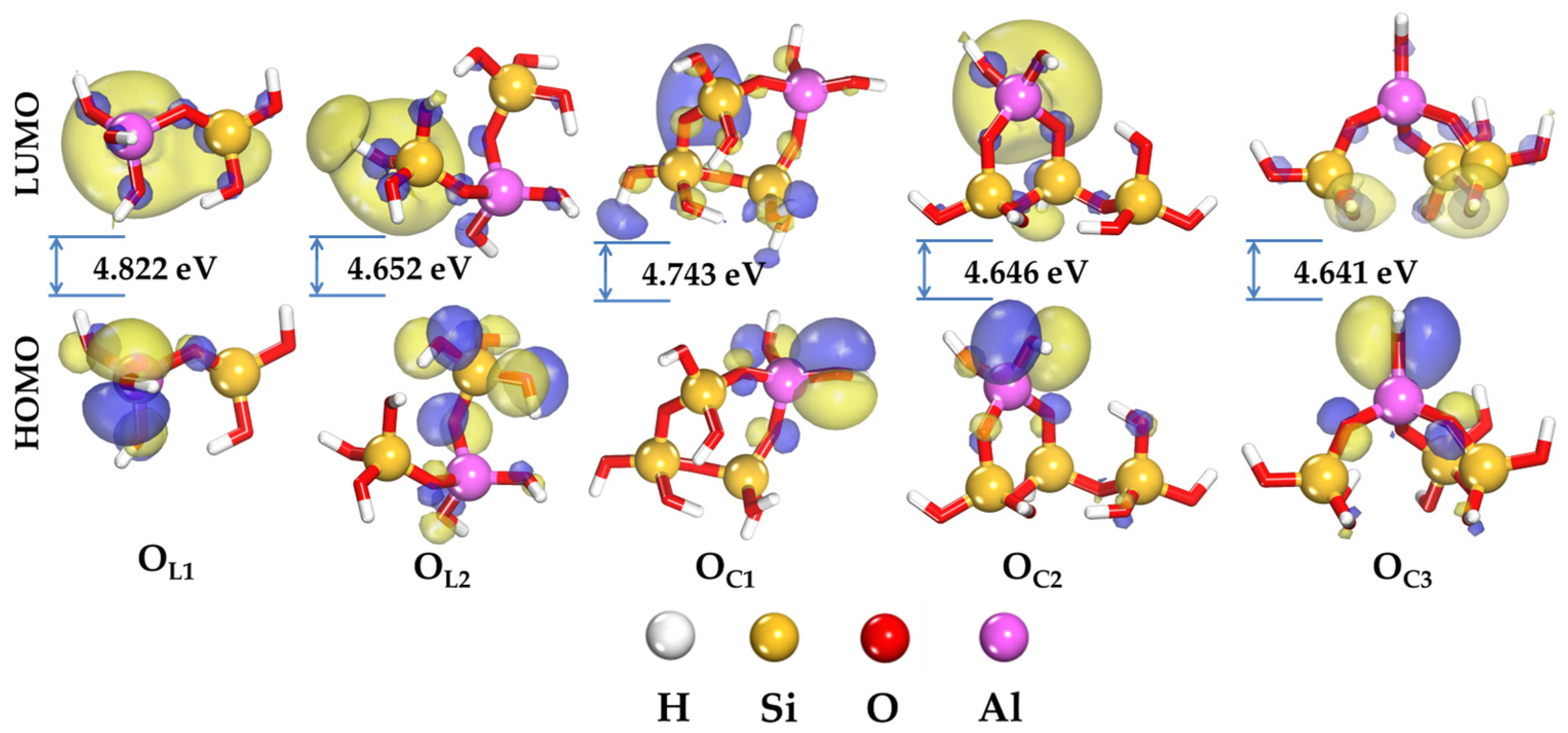
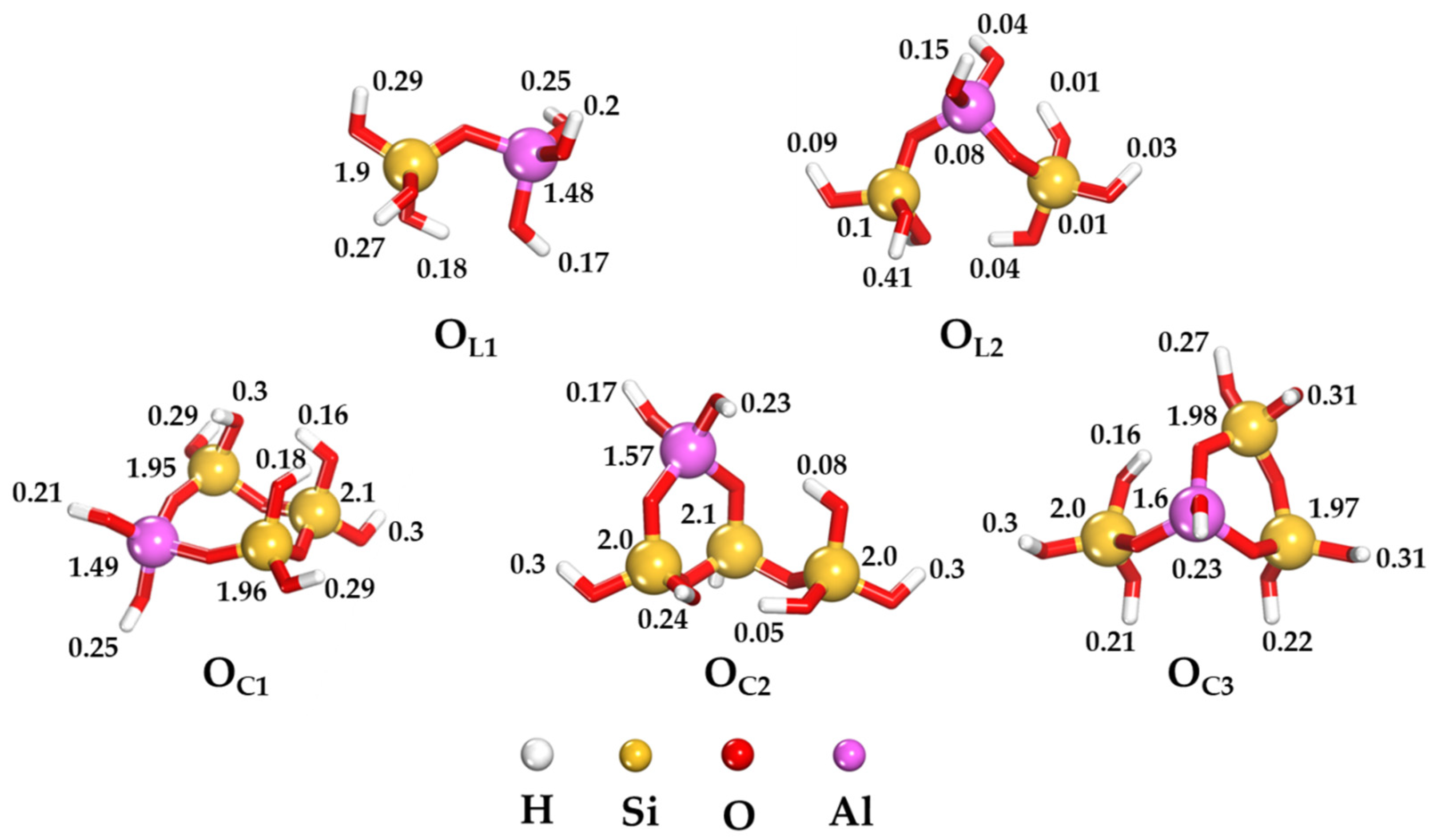
2.1. Local Reactivity of Aluminosilicate Oligomers
2.2. Global Reactivity of Aluminosilicate Oligomers
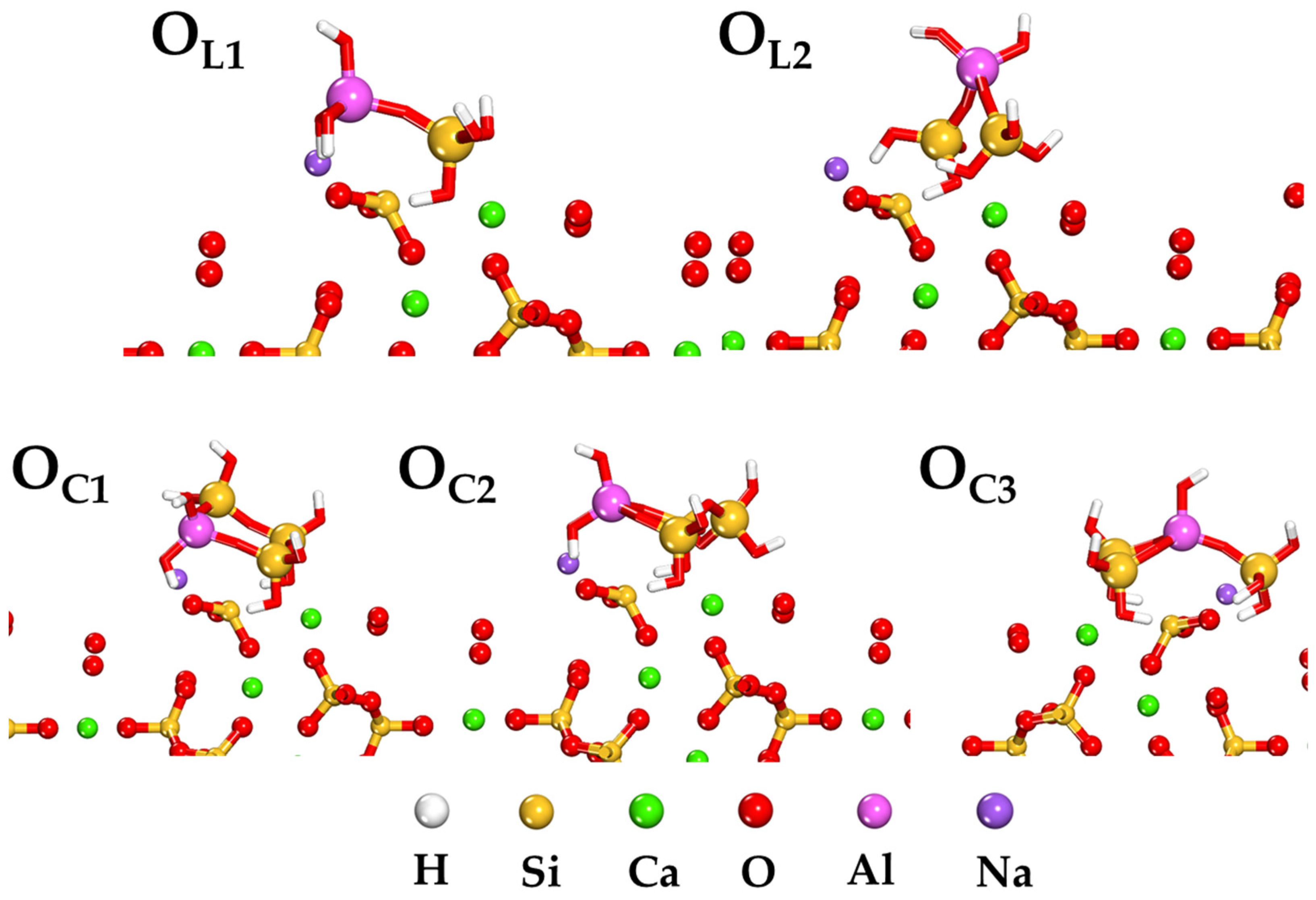
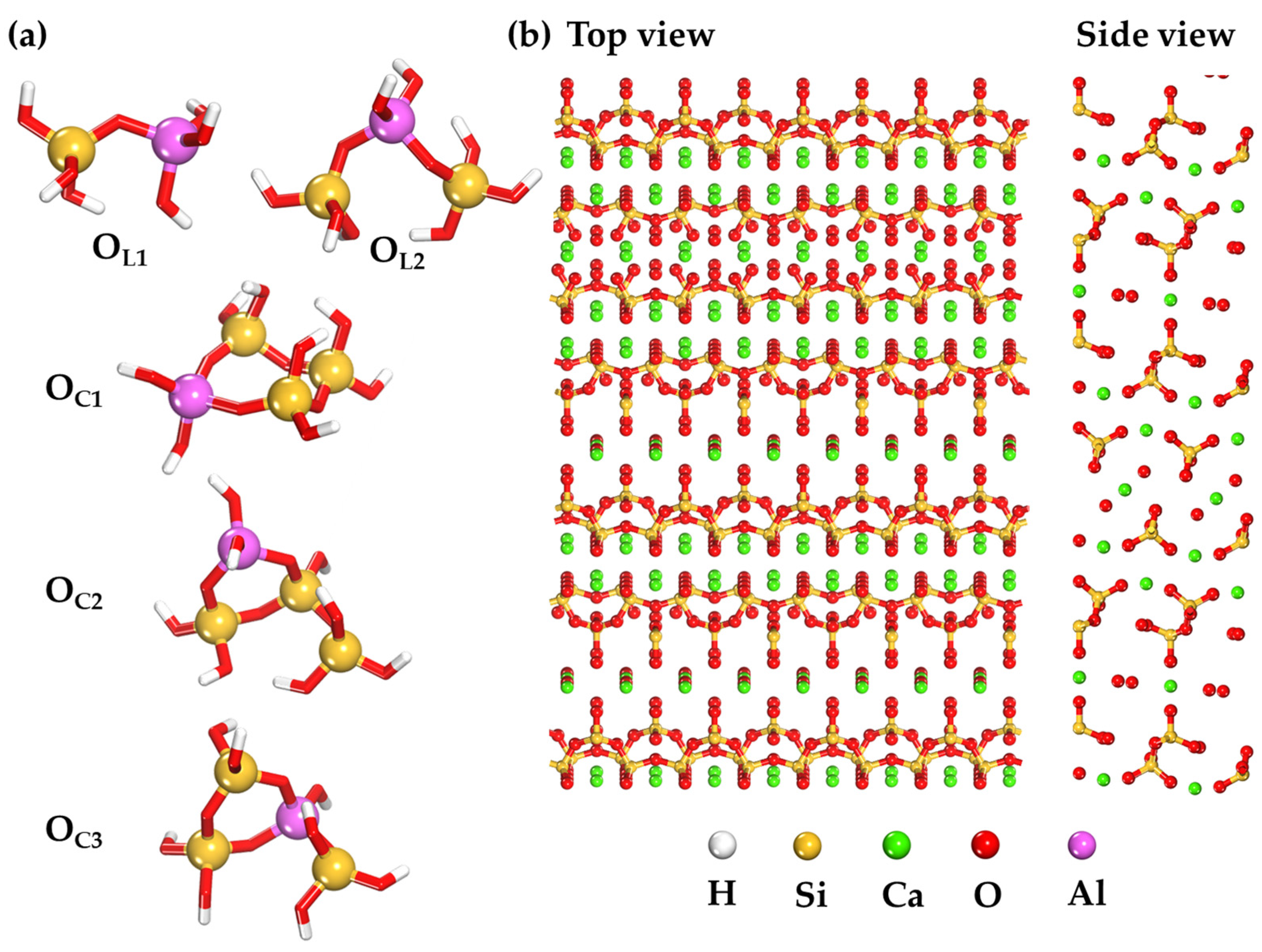
2.3. Adsorption Behavior of Aluminosilicate Oligomers
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhang, Q.; Li, Y. Deterioration of concrete under the coupling effects of freeze–thaw cycles and other actions. Constr. Build. Mater. 2022, 319, 126045. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Dong, W.; Tam, V.W.Y.; Yu, T. Durability deterioration of concrete under marine environment from material to structure. Build. Eng. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Song, X.; Song, X.; Liu, H.; Huang, H.; Anvarovna, K.G.; Ugli, N.A.D.; Huang, Y.; Hu, J.; Wei, J.; Yu, Q. Cement-Based Repair Materials and the Interface with Concrete Substrates: Characterization, Evaluation and Improvement. Polymers 2022, 14, 1485. [Google Scholar] [CrossRef]
- Fang, B.; Hu, Z.; Shi, T.; Liu, Y.; Wang, X.; Yang, D.; Zhu, K.; Zhao, X.; Zhao, Z. Research progress on the properties and applications of magnesium phosphate cement. Ceram. Int. 2023, 49, 4001–4016. [Google Scholar] [CrossRef]
- Mechtcherine, V. Novel cement-based composites for the strengthening and repair of concrete structures. Constr. Build. Mater. 2013, 41, 365–373. [Google Scholar] [CrossRef]
- Hai, R.; Zheng, J.; Li, J.; Hui, C.; Liu, J. Preparation mechanism and properties of thermal activated red mud and its geopolymer repair mortar. Case Stud. Constr. Mater. 2024, 20, e02853. [Google Scholar] [CrossRef]
- Fan, L.F.; Chen, D.K.; Zhong, W.L. Effects of slag and alkaline solution contents on bonding strength of geopolymer-concrete composites. Constr. Build. Mater. 2023, 406, 133391. [Google Scholar] [CrossRef]
- Mohd Tahir, M.F.; Ahmad Zailani, W.W.; Ghazali Abd, A.; Miltiadis, S.K. Morphological analysis of fly ash based geopolymer with different concentration of NaOH as repair material. AIP Conf. Proc. 2021, 2339, 020244. [Google Scholar] [CrossRef]
- Shen, Y.; Kang, S.; Cheng, G.; Wang, J.; Wu, W.; Wang, X.; Zhao, Y.; Li, Q. Effects of silicate modulus and alkali dosage on the performance of one-part electric furnace nickel slag-based geopolymer repair materials. Case Stud. Constr. Mater. 2023, 19, e02224. [Google Scholar] [CrossRef]
- Zhang, B. Repair of ordinary concrete using alkali activated slag/fly ash: High temperature resistance and micro structure evolution of adhesive interface. Constr. Build. Mater. 2023, 386, 131559. [Google Scholar] [CrossRef]
- Wang, B.; Feng, H.; Huang, H.; Guo, A.; Zheng, Y.; Wang, Y. Bonding Properties between Fly Ash/Slag-Based Engineering Geopolymer Composites and Concrete. Materials 2023, 16, 4232. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Z.; Duan, P.; Huang, M.; Hao, X.; Yu, Y. Novel low cost and durable rapid-repair material derived from industrial and agricultural by-products. Minerals 2019, 9, 14516. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Law, D. A state-of-the-art review on the durability of geopolymer concrete for sustainable structures and infrastructure. Constr. Build. Mater. 2021, 291, 123381. [Google Scholar] [CrossRef]
- Frasson, B.J.; Rocha, J.C. Drying shrinkage behavior of geopolymer mortar based on kaolinitic coal gangue. Case Stud. Constr. Mater. 2023, 18, e01957. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Adhesion characterization of tungsten mine waste geopolymeric binder. Influence of OPC concrete substrate surface treatment. Constr. Build. Mater. 2008, 22, 154–161. [Google Scholar] [CrossRef]
- Baloch, W.L.; Siad, H.; Lachemi, M.; Sahmaran, M. A review on the durability of concrete-to-concrete bond in recent rehabilitated structures. Build. Eng. 2021, 44, 103315. [Google Scholar] [CrossRef]
- Daneshvar, D.; Behnood, A.; Robisson, A. Interfacial bond in concrete-to-concrete composite. Constr. Build. Mater. 2022, 359, 129195. [Google Scholar] [CrossRef]
- Feng, S.; Xiao, H.; Liu, R.; Liu, M. The bond properties between ultra-high-performance concrete and normal strength concrete substrate: Bond macro-performance and overlay transition zone microstructure. Cem. Concr. Compos. 2022, 128, 104436. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Peng, K.-D.; Alrefaei, Y.; Dai, J.-G. The bond between geopolymer repair mortars and OPC concrete substrate: Strength and microscopic interactions. Cem. Concr. Compos. 2021, 119, 103991. [Google Scholar] [CrossRef]
- Asayesh, S.; Shirzadi Javid, A.A.; Ziari, H.; Mehri, B. Evaluating fresh state, hardened State, thermal expansion and bond properties of geopolymers for the repairing of concrete pavements under restrained conditions. Constr. Build. Mater. 2021, 292, 123398. [Google Scholar] [CrossRef]
- Fahim Huseien, G.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Abdulameer Hussein, A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Kabanda, M.M.; Murulana, L.C.; Singh, A.K.; Shukla, S.K. Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 12940–12958. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Joseph, A. Electrochemical measurements and theoretical calculations on the inhibitive interaction of Plectranthus amboinicus leaf extract with mild steel in hydrochloric acid. Meas. J. Int. Meas. Confed. 2017, 95, 297–305. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The Use of Global and Local Molecular Parameters for the Analysis of the Gas-Phase Basicity of Amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO-MO molecular wave functions. II. Overlap populations, bond orders, and covalent bond energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- Rong, X.; Wang, Z.; Xing, X.; Zhao, L. Review on the Adhesion of Geopolymer Coatings. ACS Omega 2021, 6, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rong, X.; Zhao, L.; Xing, X.; Ma, H. Effects of Substrate Surface Characteristics on the Adhesion Properties of Geopolymer Coatings. ACS Omega 2022, 7, 11988–11994. [Google Scholar] [CrossRef] [PubMed]
- Słomka-Słupik, B.; Wiśniewska, P.; Bargieł, W. Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research. Materials 2022, 15, 5943. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Azizi, K.; Sufian, S.; Man, Z. Effect of Na/Al and Si/Al Ratios on Adhesion Strength of Geopolymers as Coating Material. Appl. Mech. Mater. 2014, 625, 85–89. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, L.; Fan, D.; Garcia Hernandez, A.; Li, B.; Do, H. Molecular simulations of the structure-property relationships of N-A-S-H gels. Constr. Build. Mater. 2022, 329, 127166. [Google Scholar] [CrossRef]
- Yang, H.; Ma, S.; Zhao, S.; Wang, Q.; Liu, X.; He, P.; Jia, D.; Colombo, P.; Zhou, Y. Mechanistic understanding of geopolymerization at the initial stage: Ab initio molecular dynamics simulations. J. Am. Ceram. Soc. 2023, 106, 4425–4442. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- North, M.R.; Swaddle, T.W. Kinetics of silicate exchange in alkaline aluminosilicate solutions. Inorg. Chem. 2000, 39, 2661–2665. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; Roussel, N.; D’Espinosede Lacaillerie, J.-B. A multinuclear static NMR study of geopolymerisation. Cem. Concr. Res. 2015, 75, 104–109. [Google Scholar] [CrossRef]
- Koleżyński, A.; Król, M.; Żychowicz, M. The structure of geopolymers—Theoretical studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Salha, M.S.; Yada, R.Y.; Farrar, D.H.; Chass, G.A.; Tian, K.V.; Bodo, E. Aluminium catalysed oligomerisation in cement-forming silicate systems. Phys. Chem. Chem. Phys. 2022, 25, 455–461. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Freeman, E.E.; Neeway, J.J.; Motkuri, R.K.; Rimer, J.D.; Mpourmpakis, G. Understanding initial zeolite oligomerization steps with first principles calculations. AIChE J. 2020, 66, e17107. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S.; Kampf, A.R. The Crystal Structure of Tobermorite 14 Å (Plombierite), a C-S-H Phase. Am. Ceram. Soc. 2005, 88, 505–512. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systemes, Materials Studio; Dassault Systemes: San Diego, CA, USA, 2020. [Google Scholar]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Willand, A.; Kvashnin, Y.O.; Genovese, L.; Vázquez-Mayagoitia, Á.; Deb, A.K.; Sadeghi, A.; Deutsch, T.; Goedecker, S. Norm-conserving pseudopotentials with chemical accuracy compared to all-electron calculations. J. Chem. Phys. 2013, 138, 104109. [Google Scholar] [CrossRef]
- Sancho-García, J.; Brédas, J.; Cornil, J. Assessment of the reliability of the Perdew–Burke–Ernzerhof functionals in the determination of torsional potentials in π-conjugated molecules. Chem. Phys. Lett. 2003, 377, 63–68. [Google Scholar] [CrossRef]
- Saßnick, H.-D.; Cocchi, C. Electronic structure of cesium-based photocathode materials from density functional theory: Performance of PBE, SCAN, and HSE06 functionals. Electron. Struct. 2021, 3, 027001. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Inada, Y.; Orita, H. Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: Evidence of small basis set superposition error compared to Gaussian basis sets. J. Comput. Chem. 2008, 29, 225–232. [Google Scholar] [CrossRef]
- Wu, X.; Vargas, M.C.; Nayak, S. Towards extending the applicability of density functional theory to weakly bound systems. Chem. Phys. 2001, 115, 8748–8757. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Sun, H. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Sun, H. Compass: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Yeh, I.-C.; Berkowitz, M.L. Ewald summation for systems with slab geometry. J. Phys. Chem. 1999, 111, 3155–3162. [Google Scholar] [CrossRef]



| Al | Si | Si | Si | |
|---|---|---|---|---|
| OL1 | 2.855 | 1.690 | ||
| OL2 | 4.227 | 1.897 | 2.036 | |
| OC1 | 2.859 | 1.677 | 2.414 | 4.0241 |
| OC2 | 3.123 | 1.941 | 2.353 | 2.5287 |
| OC3 | 3.234 | 2.333 | 1.821 | 1.7913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzhitskii, A.S.; Kruglikov, A.A. Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar. Int. J. Mol. Sci. 2024, 25, 8374. https://doi.org/10.3390/ijms25158374
Kasprzhitskii AS, Kruglikov AA. Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar. International Journal of Molecular Sciences. 2024; 25(15):8374. https://doi.org/10.3390/ijms25158374
Chicago/Turabian StyleKasprzhitskii, Anton S., and Alexander A. Kruglikov. 2024. "Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar" International Journal of Molecular Sciences 25, no. 15: 8374. https://doi.org/10.3390/ijms25158374
APA StyleKasprzhitskii, A. S., & Kruglikov, A. A. (2024). Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar. International Journal of Molecular Sciences, 25(15), 8374. https://doi.org/10.3390/ijms25158374







