The “Forgotten” Subtypes of Breast Carcinoma: A Systematic Review of Selected Histological Variants Not Included or Not Recognized as Distinct Entities in the Current World Health Organization Classification of Breast Tumors
Abstract
:1. Introduction
2. Methods
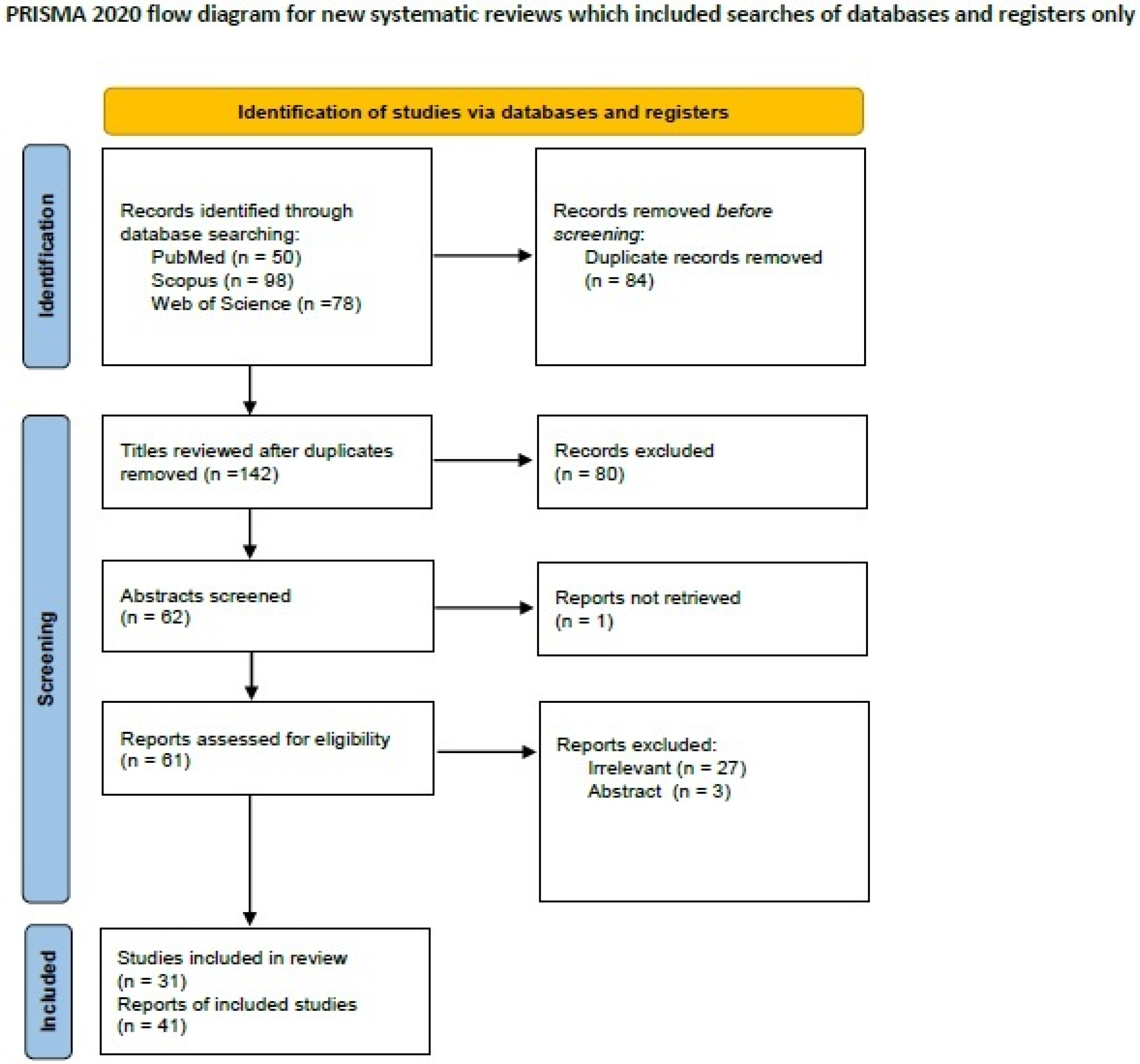
2.1. Systematic Review of the Literature of Lymphoepithelioma-like Breast Carcinoma
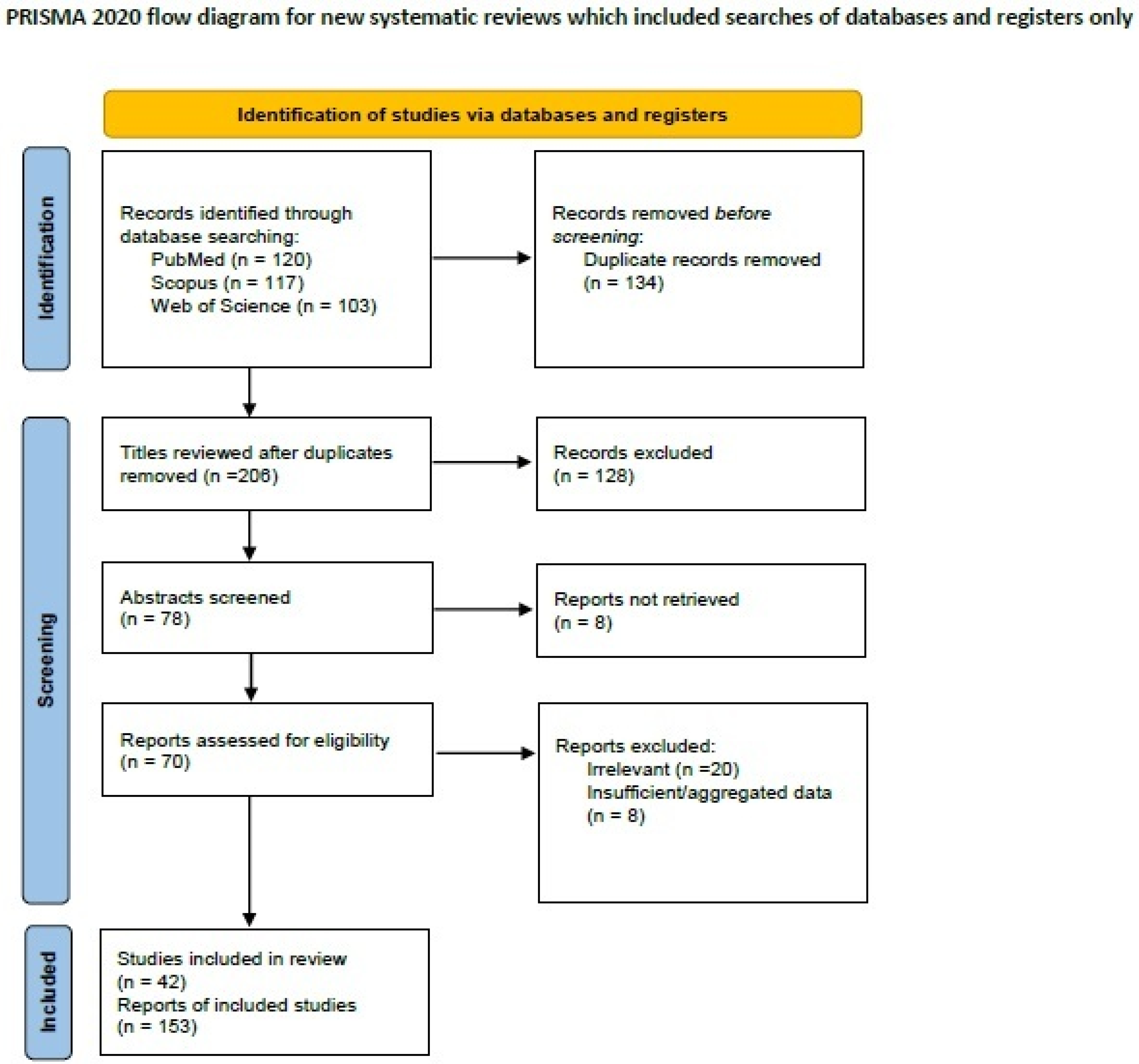
2.2. Systematic Review of the Literature on Breast Carcinoma with Osteoclast-like Giant Cells
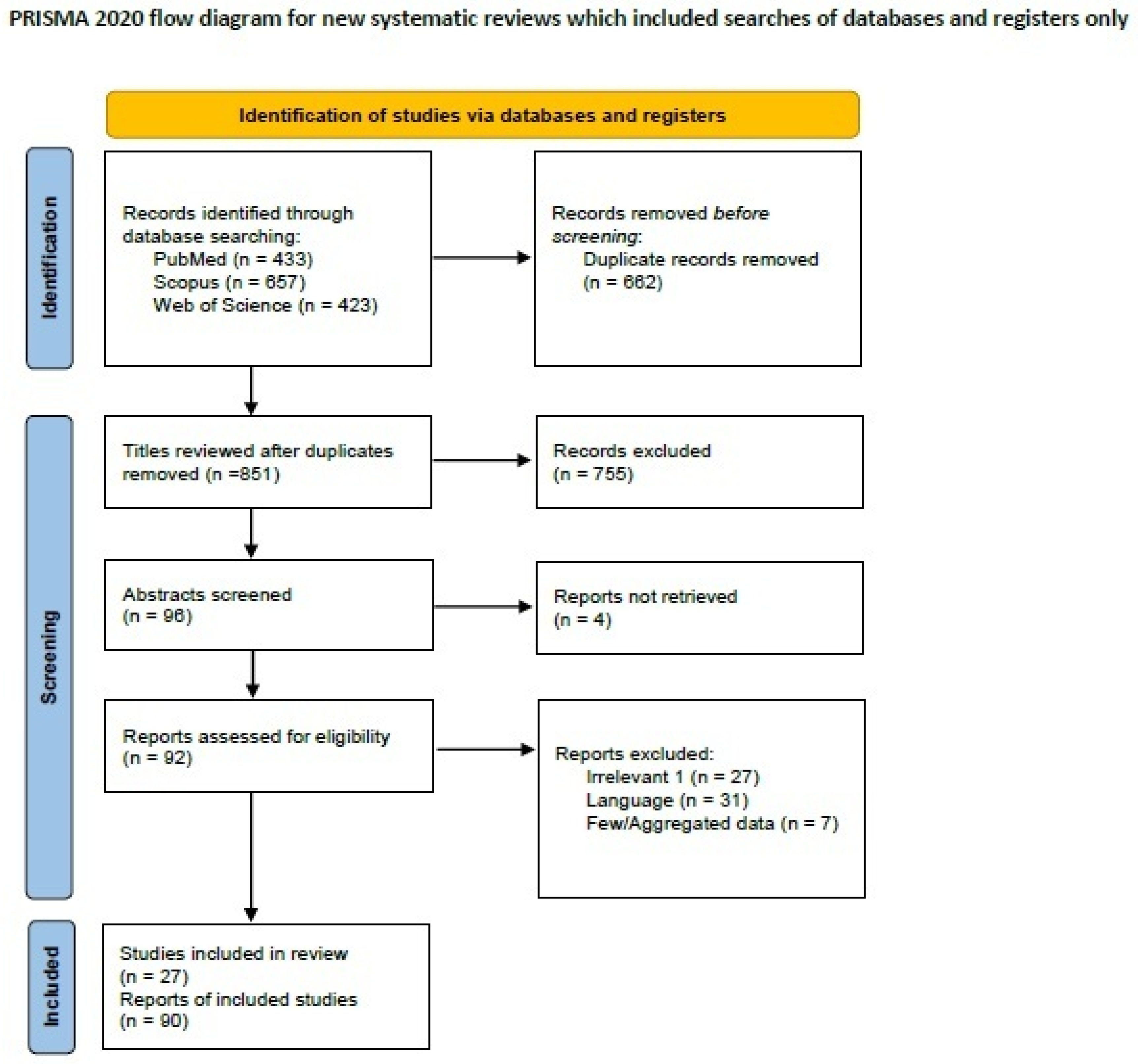
2.3. Systematic Review of the Literature on Signet-Ring Cell Breast Carcinoma
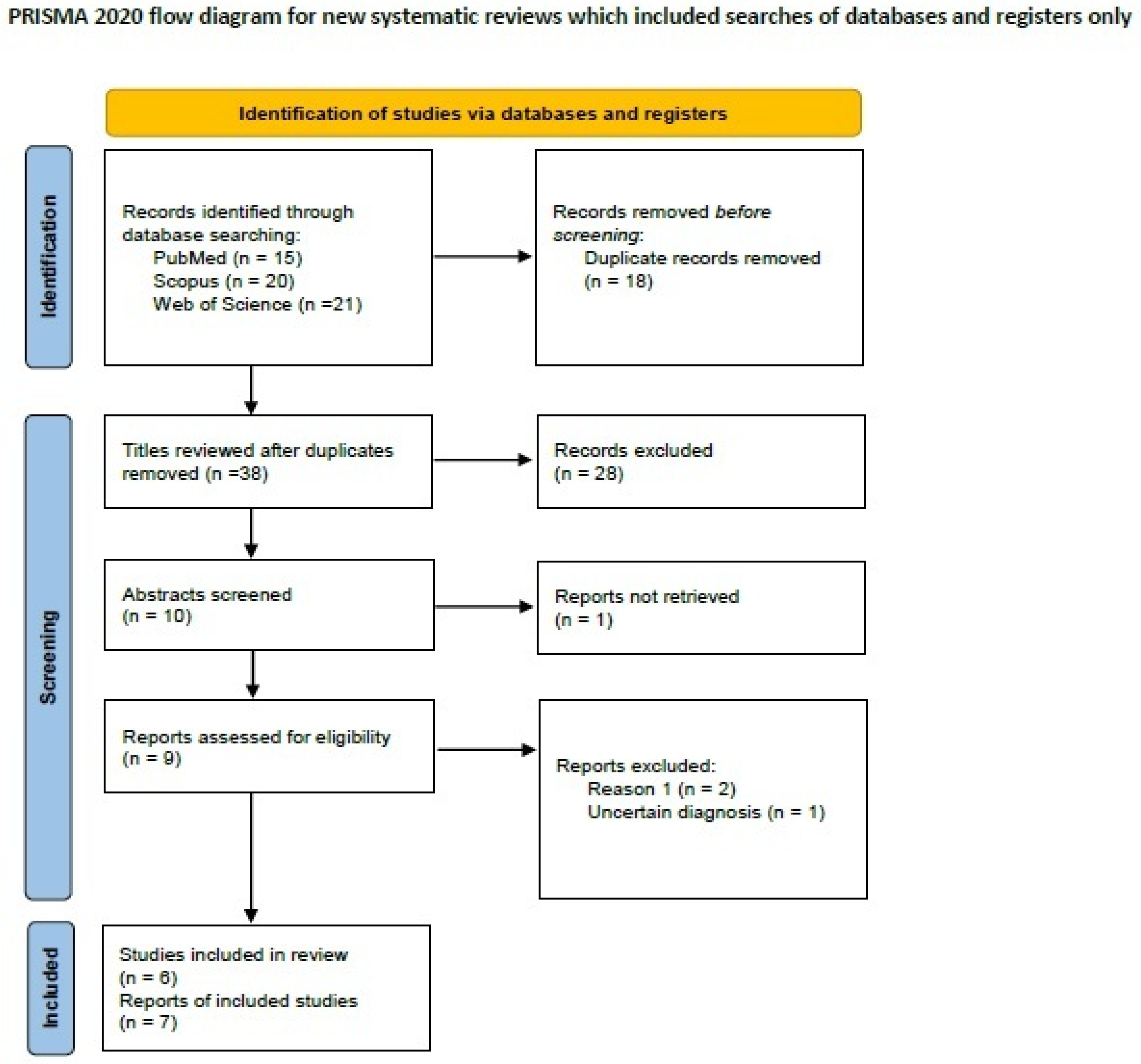
2.4. Systematic Review of the Literature on Metaplastic Breast Carcinoma with Melanocytic Differentiation
3. Results and Discussion
3.1. Lymphoepithelioma-like Breast Carcinoma
3.1.1. Demographic and Clinicopathological Features
3.1.2. Imaging Findings
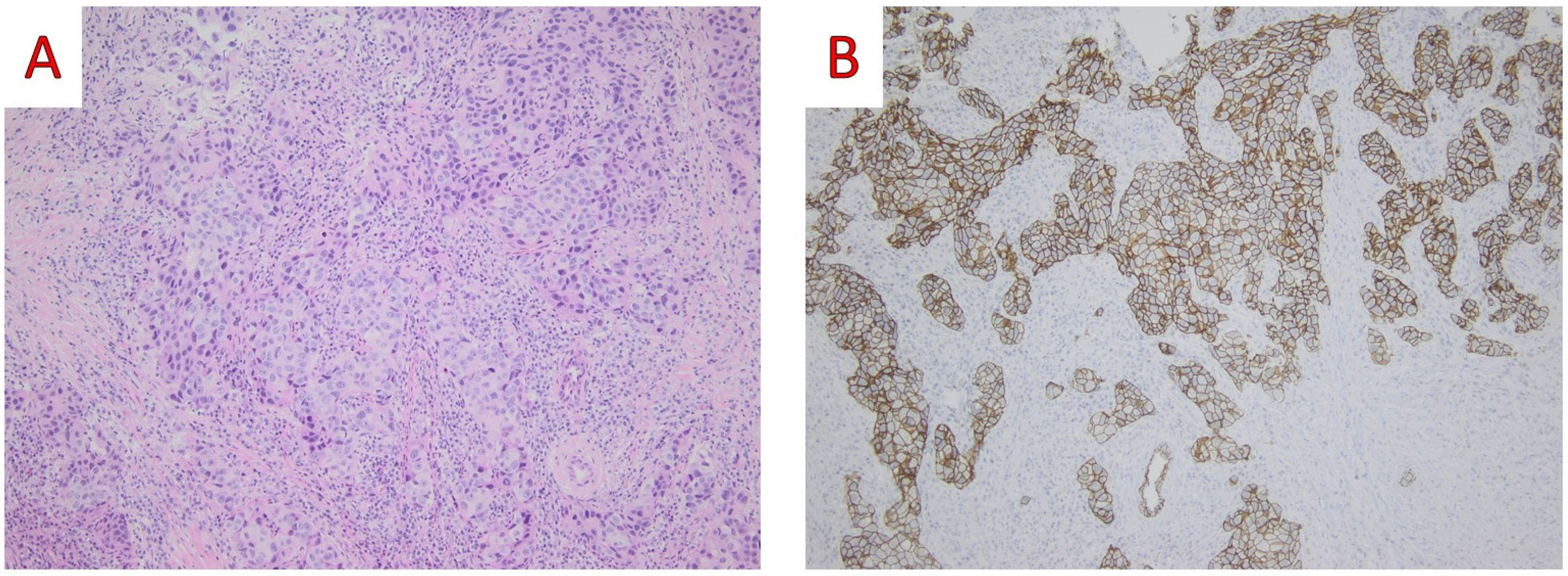
3.1.3. Histological Findings and Differential Diagnosis
3.1.4. Molecular Studies
3.1.5. Treatment
3.1.6. Outcome
3.2. Breast Carcinoma with Osteoclast-like Giant Cells
3.2.1. Demographic and Clinicopathological Features
3.2.2. Imaging Findings
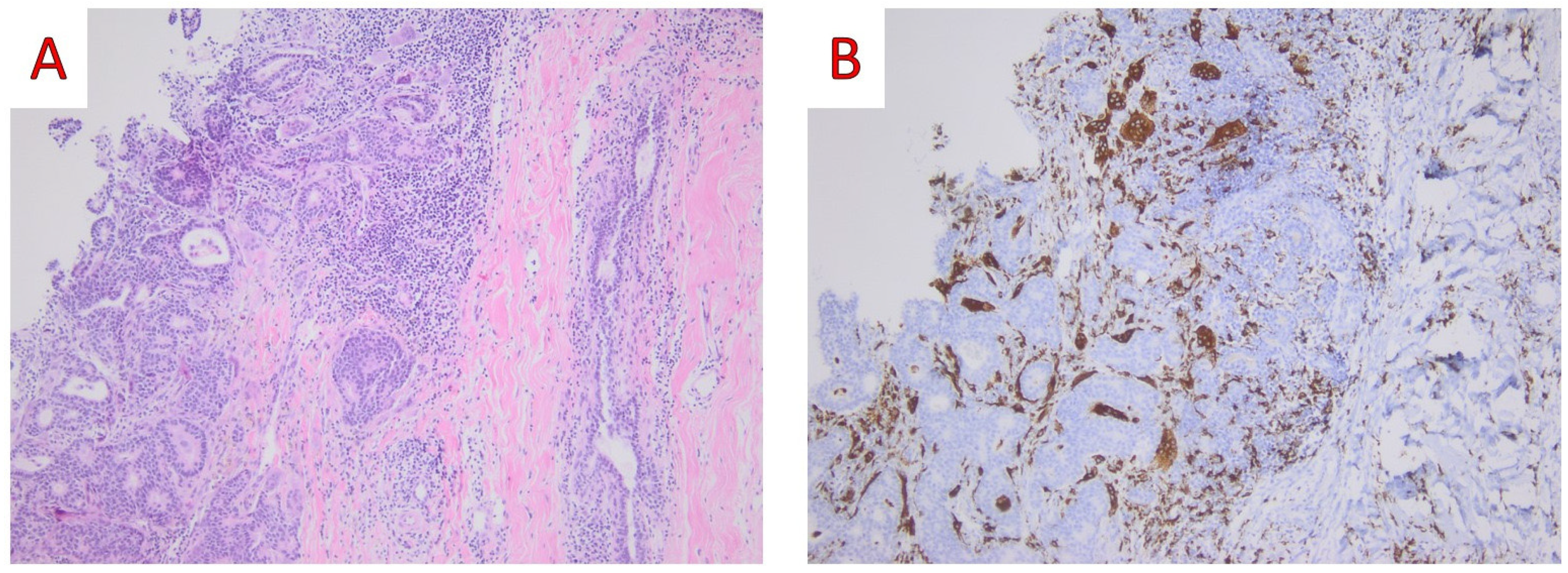
3.2.3. Histological Findings and Differential Diagnosis
3.2.4. Molecular Studies
3.2.5. Treatment
3.2.6. Outcome
3.3. Signet-Ring Cell Carcinoma
3.3.1. Demographic, Clinical and Pathological Features
3.3.2. Imaging Findings
3.3.3. Histological Findings and Differential Diagnosis
3.3.4. Molecular Studies
3.3.5. Treatment
3.3.6. Outcome
3.4. Metaplastic Breast Carcinoma with Melanocytic Differentiation
3.4.1. Demographic, Clinical and Pathological Features
3.4.2. Imaging Findings
3.4.3. Histological Findings and Differential Diagnosis
3.4.4. Molecular Studies
3.4.5. Treatment
3.4.6. Outcome
3.5. Cumulative Results
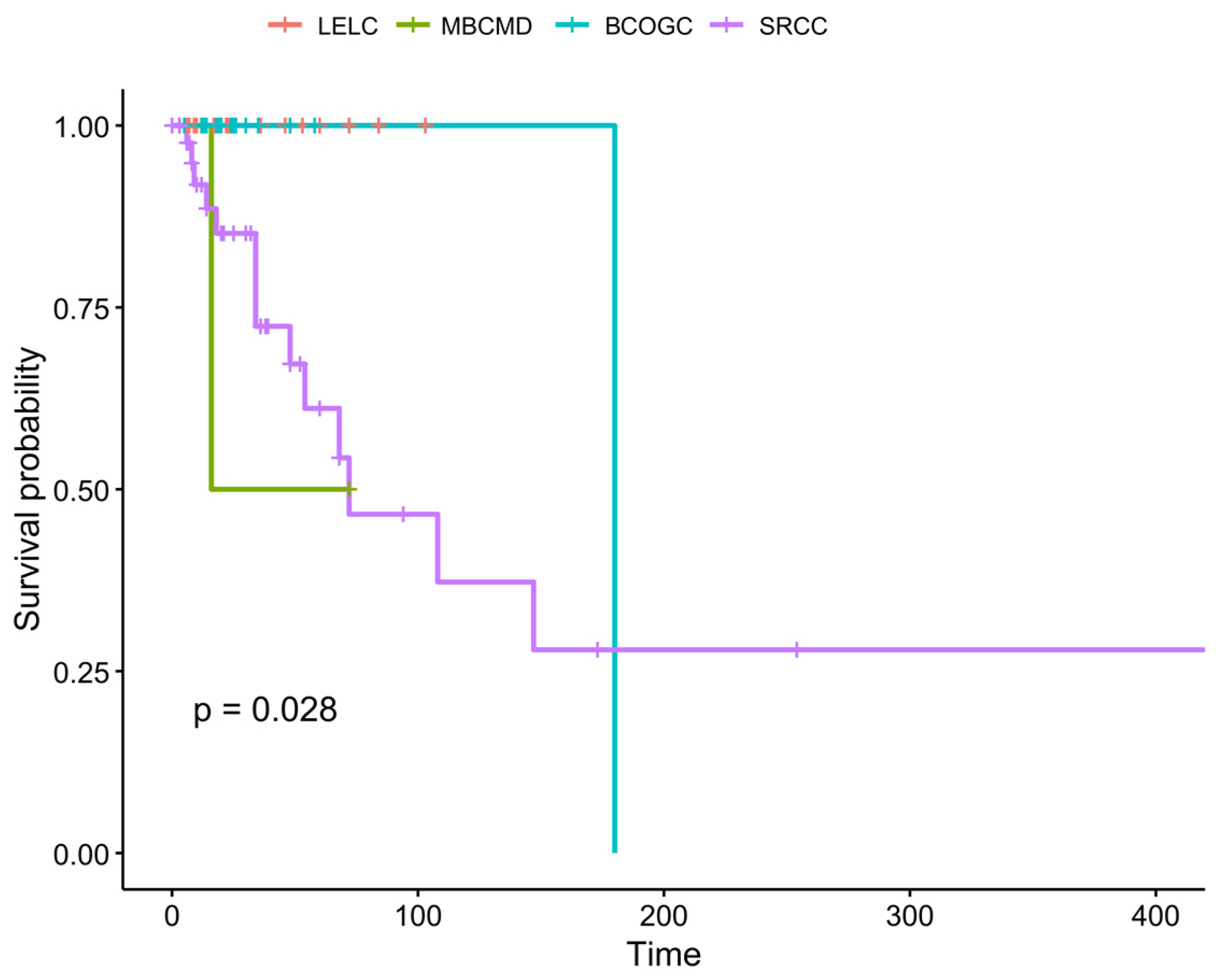
3.5.1. Survival
3.5.2. Clinicopathological Features of the Four Entities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E.; on behalf of the ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- National Breast Cancer Foundation Inc. Available online: https://www.nationalbreastcancer.org/breast-cancer-facts/ (accessed on 24 June 2024).
- Lokuhetty, D.; White, V.A.; Watanave, R.; Cree, I.A. (Eds.) Breast Tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Cserni, G.; Floris, G.; Koufopoulos, N.; Kovács, A.; Nonni, A.; Regitnig, P.; Stahls, A.; Varga, Z. Invasive lobular carcinoma with extracellular mucin production-a novel pattern of lobular carcinomas of the breast. Clinico-pathological description of eight cases. Virchows Arch. 2017, 471, 3–12. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Antoniadou, F.; Kokkali, S.; Pigadioti, E.; Khaldi, L. Invasive Lobular Carcinoma with Extracellular Mucin Production: Description of a Case and Review of the Literature. Cureus 2019, 11, e5550. [Google Scholar] [CrossRef]
- Hayes, M.M. Adenomyoepithelioma of the breast: A review stressing its propensity for malignant transformation. J. Clin. Pathol. 2011, 64, 477–484. [Google Scholar] [CrossRef]
- Wei, S. Papillary lesions of the breast: An update. Arch. Pathol. Lab. Med. 2016, 140, 628–643. [Google Scholar] [CrossRef] [PubMed]
- McBoyle, M.F.; Razek, H.A.; Carter, J.L.; Helmer, S.D. Tubular carcinoma of the breast: An institutional review. Am. Surg. 1997, 63, 639–644. [Google Scholar] [PubMed]
- Diab, S.G.; Clark, G.M.; Osborne, C.K.; Libby, A.; Allred, D.C.; Elledge, R.M. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J. Clin. Oncol. 1999, 17, 1442–1448. [Google Scholar] [CrossRef]
- Liu, X.Y.; Jiang, Y.Z.; Liu, Y.R.; Zuo, W.J.; Shao, Z.M. Clinicopathological characteristics and survival outcomes of invasive cribriform carcinoma of breast: A SEER population-based study. Medicine 2015, 94, e1309. [Google Scholar] [CrossRef] [PubMed]
- Di Saverio, S.; Gutierrez, J.; Avisar, E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res. Treat. 2008, 111, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Koufopoulos, N.; Goudeli, C.; Syrios, J.; Filopoulos, E.; Khaldi, L. Mucinous cystadenocarcinoma of the breast: The challenge of diagnosing a rare entity. Rare Tumors 2017, 9, 98–100. [Google Scholar] [CrossRef]
- Chen, H.; Wu, K.; Wang, M.; Wang, F.; Zhang, M.; Zhang, P. Invasive micropapillary carcinoma of the breast has a better long-term survival than invasive ductal carcinoma of the breast in spite of its aggressive clinical presentations: A comparison based on large population database and case–control analysis. Cancer Med. 2017, 6, 2775–2786. [Google Scholar] [CrossRef]
- Marchiò, C.; Iravani, M.; Natrajan, R.; Lambros, M.; Savage, K.; Tamber, N.; Fenwick, K.; Mackay, A.; Senetta, R.; Di Palma, S.; et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J. Pathol. 2008, 215, 398–410. [Google Scholar] [CrossRef]
- Mills, A.M.; Gottlieb, C.E.; Wendroth, S.M.; Brenin, C.M.; Atkins, K.A. Pure apocrine carcinomas represent a clinicopathologically distinct androgen receptor–positive subset of triple-negative breast cancers. Am. J. Surg. Pathol. 2016, 40, 1109–1116. [Google Scholar] [CrossRef]
- Mills, M.N.; Yang, G.Q.; Oliver, D.E.; Liveringhouse, C.L.; Ahmed, K.A.; Orman, A.G.; Laronga, C.; Hoover, S.J.; Khakpour, N.; Costa, R.L.B.; et al. Histologic heterogeneity of triple negative breast cancer: A National Cancer Centre Database analysis. Eur. J. Cancer 2018, 98, 48–58. [Google Scholar] [CrossRef]
- Schroeder, M.C.; Rastogi, P.; Geyer, C.E., Jr.; Miller, L.D.; Thomas, A. Early and locally advanced metaplastic breast cancer: Presentation and survival by receptor status in surveillance, epidemiology, and end results (SEER) 2010–2014. Oncologist 2018, 23, 481–488. [Google Scholar] [CrossRef]
- Boujelbene, N.; Khabir, A.; Sozzi, W.J.; Mirimanoff, R.; Khanfir, K. Clinical review–breast adenoid cystic carcinoma. Breast 2012, 21, 124–127. [Google Scholar] [CrossRef]
- Damiani, S.; Pasquinelli, G.; Lamovec, J.; Peterse, J.; Eusebi, V. Acinic cell carcinoma of the breast: An immunohistochemical and ultrastructural study. Virchows Arch. 2000, 437, 74–81. [Google Scholar] [CrossRef]
- Tavassoli, F.; Norris, H. Secretory carcinoma of the breast. Cancer 1980, 45, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Sheikh, F.S.; Allenby, P.A.; Rosen, P.P. Invasive secretory (juvenile) carcinoma arising in ectopic breast tissue of the axilla. Arch. Pathol. Lab. Med. 2001, 125, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, L.; Foschini, M.P.; Ragazzini, T.; Magrini, E.; Fornelli, A.; Ellis, I.O.; Eusebi, V. Mucoepidermoid carcinoma of the breast. Virchows Arch. 2004, 444, 13–19. [Google Scholar] [CrossRef]
- Basbug, M.; Akbulut, S.; Arikanoglu, Z.; Sogutcu, N.; First, U.; Kucukoner, M. Mucoepidermoid carcinoma in a breast affected by burn scars: Comprehensive literature review and case report. Breast Care 2011, 6, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Asioli, S.; Marucci, G.; Ficarra, G.; Stephens, M.; Foschini, M.P.; Ellis, I.O.; Eusebi, V. Polymorphous adenocarcinoma of the breast. Report of three cases. Virchows Arch. 2006, 448, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Trihia, H.J.; Valavanis, C.; Novkovic, N.; Koutsodontis, G.; Petraki, M.; Efstathiou, E. Polymorphous adenocarcinoma of the breast—An exceptionally rare entity: Clinicopathological description of a case and brief review. Breast J. 2020, 26, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Trihia, H.J.; Lampropoulos, P.; Karelis, L.; Souka, E.; Galanopoulos, G.; Provatas, I. Tall cell carcinoma with reversed polarity: A case report of a very rare breast tumor entity and mini-review. Breast J. 2021, 27, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sapino, A.; Papotti, M.; Righi, L.; Cassoni, P.; Chiusa, L.; Bussolati, G. Clinical significance of neuroendocrine carcinoma of the breast. Ann. Oncol. 2001, 12, S115–S117. [Google Scholar] [CrossRef]
- Lopez-Bonet, E.; Alonso-Ruano, M.; Barraza, G.; Vazquez-Martin, A.; Bernado, L.; Menendez, J.A. Solid neuroendocrine breast carcinomas: Incidence, clinico-pathological features and immunohistochemical profiling. Oncol. Rep. 2008, 20, 1369–1374. [Google Scholar]
- Shin, S.J.; DeLellis, R.A.; Ying, L.; Rosen, P.P. Small cell carcinoma of the breast: A clinicopathologic and immunohistochemical study of nine patients. Am. J. Surg. Pathol. 2000, 24, 1231–1238. [Google Scholar] [CrossRef]
- Hare, F.; Giri, S.; Patel, J.K.; Hahn, A.; Martin, M.G. A population-based analysis of outcomes for small cell carcinoma of the breast by tumor stage and the use of radiation therapy. Springerplus 2015, 4, 138. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Pateras, I.S.; Gouloumis, A.R.; Ieronimaki, A.I.; Zacharatou, A.; Spathis, A.; Leventakou, D.; Economopoulou, P.; Psyrri, A.; Arkadopoulos, N.; et al. Diagnostically Challenging Subtypes of Invasive Lobular Carcinomas: How to Avoid Potential Diagnostic Pitfalls. Diagnostics 2022, 12, 2658. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Goudeli, C.; Pigadioti, E.; Balalis, D.; Manatakis, D.K.; Antoniadou, F.; Korkolis, D.P. Synchronous colonic adenocarcinoma and metastatic lobular carcinoma in a colectomy specimen: A rare finding. Cureus 2018, 10, e3207. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D. Lymphoepithelioma-like carcinoma of the breast. Mod. Pathol. 1994, 7, 129–131. [Google Scholar]
- Cristina, S.; Boldorini, R.; Brustia, F.; Monga, G. Lymphoepithelioma-like carcinoma of the breast. An unusual pattern of infiltrating lobular carcinoma. Virchows Arch. 2000, 437, 198–202. [Google Scholar] [CrossRef]
- Dadmanesh, F.; Peterse, J.; Sapino, A.; Fonelli, A.; Eusebi, V. Lymphoepithelioma-like carcinoma of the breast: Lack of evidence of Epstein–Barr virus infection. Histopathology 2001, 38, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, P.; Chetty, R. Lymphoepithelioma-like carcinoma of the breast with associated sclerosing lymphocytic lobulitis. Arch. Pathol. Lab. Med. 2001, 125, 669–672. [Google Scholar] [CrossRef]
- Peştereli, H.E.; Erdoğan, O.; Kaya, R.; Karaveli, F.Ş. Lymphoepithelioma-like carcinoma of the breast: A newly recognized subtype of lobular carcinoma. APMIS 2002, 110, 447–450. [Google Scholar] [CrossRef]
- Ilvan, S.; Celik, V.; Akyildiz, E.U.; Bese, N.S.; Ramazanoglu, R.; Calay, Z. Lymphoepithelioma-like carcinoma of the breast: Is it a distinct entity?: Clinicopathological evaluation of two cases and review of the literature. Breast 2004, 13, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Ayala, A.G.; Middleton, L.P. Lymphoepithelioma-like carcinoma of the breast: Report of a case mimicking lymphoma. Ann. Diagn. Pathol. 2004, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kurose, A.; Ichinohasama, R.; Kanno, H.; Kobayashi, T.; Ishida, M.; Nishinari, N.; Sawai, T. Lymphoepithelioma-like carcinoma of the breast. Report of a case with the first electron microscopic study and review of the literature. Virchows Arch. 2005, 447, 653–659. [Google Scholar] [CrossRef]
- Saleh, R.A.; DaCamara, P.; Radhi, J.; Boutross-Tadross, O. Lymphoepithelioma-like Carcinoma of the Breast Mimicking Nodular Sclerosing Hodgkin’s Lymphoma. Breast J. 2005, 11, 353–354. [Google Scholar] [CrossRef]
- Kulka, J.; Kovalszky, I.; Svastics, E.; Berta, M.; Füle, T. Lymphoepithelioma-like carcinoma of the breast: Not Epstein-Barr virus–, but human papilloma virus–positive. Hum. Pathol. 2008, 39, 298–301. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan-Mejia, E.; Idowu, M.O.; Davis Masssey, H.; Cardenosa, G.; Grimes, M.M. Lymphoepithelioma-like Carcinoma of the Breast: Diagnosis by Core Needle Biopsy. Breast J. 2009, 15, 658–660. [Google Scholar] [CrossRef]
- Jeong, A.K.; Park, S.B.; Kim, Y.M.; Ko, B.K.; Yang, M.J.; Kwon, W.J.; Lee, H.J.; Weon, Y.C. Lymphoepithelioma-like Carcinoma of the Breast. J. Ultrasound. Med. 2010, 29, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Kucukzeybek, B.B.; Postaci, H.; Ozguzer, A.; Yakan, S.; Denecli, A.G. Lymphoepithelioma-like Carcinoma of the Breast: A Case Report and Review of the Literature. Inter. J. Hematol. Oncol. 2011, 33, 115–119. [Google Scholar] [CrossRef]
- Trihia, H.; Siatra, H.; Gklisty, H.; Diamantopoulos, P.; Arapantoni-Dadiotis, P.; Kalogerakos, K. Lymphoepithelioma-like carcinoma of the breast: Cytological and histological features and review of the literature. Acta Cytol. 2012, 56, 85–91. [Google Scholar] [CrossRef]
- Nio, Y.; Tsuboi, K.; Tamaoki, M.; Tamaoki, M.; Maruyama, R. Lymphoepithelioma-like carcinoma of the breast: A case report with a special analysis of an association with human papilloma virus. Anticancer Res. 2012, 32, 1435–1441. [Google Scholar]
- Dinniwell, R.; Hanna, W.; Mashhour, M.; Saad, R.; Czarnota, G. Lymphoepithelioma-like carcinoma of the breast: A diagnostic and therapeutic challenge. Curr. Oncol. 2012, 19, 177–183. [Google Scholar] [CrossRef]
- Top, Ö.E.; Vardar, E.; Yağcı, A.; Deniz, S.; Öztürk, R.; Zengel, B. Lymphoepithelioma-like carcinoma of the breast: A case report. J. Breast Health 2014, 10, 177. [Google Scholar] [CrossRef]
- Abdou, A.G.; Asaad, N.Y. Lymphoepithelioma-like carcinoma of the breast: Cytological, histological, and immunohistochemical characteristics. Diagn. Cytopathol. 2015, 43, 210–213. [Google Scholar] [CrossRef]
- Suzuki, I.; Chakkabat, P.; Goicochea, L.; Campassi, C.; Chumsri, S. Lymphoepithelioma-like carcinoma of the breast presenting as breast abscess. World J. Clin. Oncol. 2014, 5, 1107. [Google Scholar] [CrossRef]
- Jansari, T.R.; Gupta, T.; Trivedi, P.P.; Shah, M.J. Lymphoepithelioma-like carcinoma of the breast: A case report. Ann. Pathol. Lab. Med. 2015, 2, C235–C238. [Google Scholar]
- Nankin, N.L.; Gondusky, C.J.; Abasolo, P.A.; Kalantari, B.N. Lymphoepithelioma-like carcinoma of the breast. Radiol. Case Rep. 2015, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Goepfert, R.; Caro-Sánchez, C.; Maafs-Molina, E. Lymphoepithelioma-like carcinoma of the breast: A singular morphological pattern with an expected outcome. Austin. J. Clin. Case Rep. 2016, 3, 1102. [Google Scholar]
- Shet, T.; Pai, T.; Shetty, O.; Desai, S. Lymphoepithelioma-like carcinoma of breast—Evaluation for Epstein-Barr virus–encoded RNA, human papillomavirus, and markers of basal cell differentiation. Ann. Diagn. Pathol. 2016, 25, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kawasaki, T.; Abo-Yashima, A.; Yoshida, T.; Kobayashi, S.; Kashiwaba, M.; Sugai, T.; Ichihara, S. Cytological features of lymphoepithelioma-like carcinoma of the breast. Cytopathology 2017, 28, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Abouelfad, D.M.; Yassen, N.N.; Amin, H.A.A.; Shabana, M.E. Lymphoepithelioma-like carcinoma of the breast mimicking granulomatous mastitis-case report and review of the literature. Asian Pac. J. Cancer Prev. 2017, 18, 1737. [Google Scholar] [CrossRef] [PubMed]
- Koufopoulos, N.; Syrios, J.; Papanikolaou, A.; Misitzis, I.; Kapatou, K.A.; Dimas, D.; Khaldi, L. Lymphoepithelioma-like breast carcinoma. Pol. J. Pathol. 2018, 69, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Aridi, T.; Fawwaz, M.; Kassab, A.; Bahmad, M.; Houcheimi, F.; Mshiek, M.; Boulos, F.; Kanj, A.; Ramadan, G.; Bahmad, H.F.; et al. Lymphoepithelioma-like carcinoma of the breast: A case report unveiling several clinical and histopathological challenges. Case Rep. Surg. 2018, 2018, 8240534. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Coronel, M.T.; Perez-Sanchez, V.M.; Salazar-Campos, J.E.; Diaz-Molina, R.; Arce-Salinas, C.H. Lymphoepithelioma-like carcinoma of breast: A case report and review of the literature. Indian J. Pathol. Microbiol. 2019, 62, 125. [Google Scholar] [CrossRef]
- Fadila, K.; Faycal, A.; Lamiaa, J.; Mohammed, B.; Nabil, I. Lymphoepithelioma-like carcinoma of the breast: A case report and review of the literature. Pan. Afr. Med. J. 2019, 32, e29231. [Google Scholar] [CrossRef]
- Salehiazar, S.; Huang, H.; Aghighi, M.; Venegas, R. Lymphoepithelioma-like Carcinoma of the Breast: A Case Report of a Rare Type of Invasive Carcinoma. Cureus 2022, 14, e29231. [Google Scholar] [CrossRef] [PubMed]
- Nanev, V.; Naneva, S.; Yordanov, A.; Strashilov, S.; Konsoulova, A.; Vasileva-Slaveva, M.; Betova, T.; Ivanov, I. Lymphoepithelioma-like carcinoma of the breast synchronous with a high-grade invasive ductal carcinoma and ductal carcinoma in situ in a different quadrant of the same breast: A case report. Medicina 2022, 58, 1146. [Google Scholar] [CrossRef] [PubMed]
- Agnantis, N.T.; Rosen, P.P. Mammary carcinoma with osteoclast-like giant cells: A study of eight cases with follow-up data. Am. J. Clin. Pathol. 1979, 72, 383–389. [Google Scholar] [CrossRef]
- Levin, A.; Rywlin, A.; Tachmes, P. Carcinoma of the breast with stromal epulis-like giant cells. South Med. J. 1981, 74, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Sugano, I.; Nagao, K.; Kondo, Y.; Nabeshima, S.; Murakami, S. Cytologic and ultrastructural studies of a rare breast carcinoma with osteoclast-like giant cells. Cancer 1983, 52, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; van Haelst, U.J. Mammary carcinoma with osteoclast-like giant cells: Additional observations on six cases. Cancer 1984, 53, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Tobioka, N.; Samoto, T.; Kobayashi, M.; Iwase, H.; Masaoka, A.; Nakamura, T.; Shibata, H.; Amoh, H.; Matsuyama, M. Breast cancer with osteoclast-like multinucleated giant cells. Acta Pathol. Jpn. 1984, 34, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.; Kiaer, H. Carcinoma of the breast with stromal multinucleated giant cells. Histopathology 1985, 9, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Manivel, J.; Picone, A.; Petrella, G.; Insabato, L. Alveolar variant of infiltrating lobular carcinoma of the breast with stromal osteoclast-like giant cells. Pathol. Res. Pract. 1989, 185, 388–394. [Google Scholar] [CrossRef]
- Athanasou, N.; Wells, C.; Quinn, J.; Ferguson, D.; Heryet, A.; McGee, J.D. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: Implications for tumour osteolysis and macrophage biology. Br. J. Cancer 1989, 59, 491–498. [Google Scholar] [CrossRef]
- Stewart, C.; Mutch, A. Breast carcinoma with osteoclast-like giant cells. Cytopathology 1991, 2, 215–219. [Google Scholar] [CrossRef]
- Herrington, C.; Tarin, D.; Buley, I.; Athanasou, N. Osteosarcomatous differentiation in carcinoma of the breast: A case of ‘metaplastic’carcinoma with osteoclasts and osteoclast-like giant cells. Histopathology 1994, 24, 282–285. [Google Scholar] [CrossRef]
- Viacava, P.; Naccarato, A.G.; Nardini, V.; Bevilacqua, G. Breast carcinoma with osteoclast-like giant cells: Immunohistochemical and ultrastructural study of a case and review of the literature. Tumori 1995, 81, 135–141. [Google Scholar] [CrossRef]
- Takahashi, T.; Moriki, T.; Hiroi, M.; Nakayama, H. Invasive Lobular Carcinoma of the Breast with Osteoclastlike Giant Cells. A Case Report. Acta Cytol. 1998, 42, 734–741. [Google Scholar] [CrossRef]
- Saimura, M.; Fukutomi, T.; Tsuda, H.; Akashi-Tanaka, S.; Nanasawa, T. Breast carcinoma with osteoclast-like giant cells: A case report and review of the Japanese literature. Breast Cancer 1999, 6, 121–126. [Google Scholar] [CrossRef]
- Iacocca, M.V.; Maia, D.M. Bilateral infiltrating lobular carcinoma of the breast with osteoclast-like giant cells. Breast J. 2001, 7, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, L.; Lauridsen, M.; Sørensen, F.B. Breast carcinoma with osteoclast-like giant cells: Morphological and ultrastructural studies of a case with review of the literature. Breast 2001, 10, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, R.; Watanabe, Y.; Shimizu, M. Best cases from the AFIP: Invasive ductal carcinoma with osteoclast-like giant cells. Radiographics 2002, 22, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Simsir, A.; Cangiarella, J. Invasive mammary carcinoma with osteoclast-like giant cells diagnosed by fine-needle aspiration biopsy: Review of the cytologic literature and distinction from other mammary lesions containing giant cells. Diagn. Cytopathol. 2004, 30, 396–400. [Google Scholar] [CrossRef]
- Vicandi, B.; Jimenez-Heffernan, J.A.; Lopez-Ferrer, P.; Hardisson, D.; Perez-Campos, A.; Gonzalez-Peramato, P.; Viguer, J.M. Fine needle aspiration cytology of mammary carcinoma with osteoclast-like giant cells. Cytopathology 2004, 15, 321–325. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.B.; Min, K.W. Metaplastic mammary carcinoma with osteoclast-like giant cells: Identical point mutation of p53 gene only identified in both the intraductal and sarcomatous components. Virchows Arch. 2004, 444, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Kikuchi, K.; Zhao, C.; Kobayashi, M.; Nakanishi, Y.; Nemoto, N. Osteoclastogenesis in human breast carcinoma. Virchows Arch. 2004, 444, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Koizumi, J.; Vazquez, M. Mammary carcinoma with osteoclast-like giant cells: A study of four cases and a review of literature. Diagn. Cytopathol. 2005, 33, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Mouri, Y.; Asano, A.; Kamei, K.; Iwata, Y.; Isogai, M.; Saga, S.; Ichihara, S. Pleomorphic carcinoma with osteoclastic giant cells of the breast: Immunohistochemical differentiation between coexisting neoplastic and reactive giant cells. Pathol. Int. 2009, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.; Gill, S.A. Solid neuroendocrine carcinoma of the breast with osteoclast-like giant cells. Breast J. 2009, 15, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Shishido-Hara, Y.; Kurata, A.; Fujiwara, M.; Itoh, H.; Imoto, S.; Kamma, H. Two cases of breast carcinoma with osteoclastic giant cells: Are the osteoclastic giant cells pro-tumoural differentiation of macrophages? Diagn. Pathol. 2010, 5, 55. [Google Scholar] [CrossRef]
- Jacquet, S.F.; Balleyguier, C.; Garbay, J.R.; Bourgier, C.; Mathieu, M.C.; Delaloge, S.; Viehl, P. Fine-needle aspiration cytopathology—An accurate diagnostic modality in mammary carcinoma with osteoclast-like giant cells: A study of 8 consecutive cases. Cancer Cytopathol. 2010, 118, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Stratton, A.; Plackett, T.P.; Belnap, C.M.; Lin-Hurtubise, K.M. Infiltrating mammary carcinoma with osteoclast-like giant cells. Hawaii Med. J. 2010, 69, 284. [Google Scholar] [PubMed]
- Richter, G.; Uleer, C.; Noesselt, T. Multifocal invasive ductal breast cancer with osteoclast-like giant cells: A case report. J. Med. Case Rep. 2011, 5, 85. [Google Scholar] [CrossRef]
- Jovičić-Milentijević, M.; Bašić, M.; Petrović, A. Multinucleated stromal giant cells in adenoid cystic carcinoma of the breast: A case report and literature review. Vojnosanit. Pregl. 2011, 68, 178–180. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, L.; Zhou, R.; Li, X.; Yang, W. Invasive breast carcinomas of no special type with osteoclast-like giant cells frequently have a luminal phenotype. Virchows Arch. 2014, 464, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, I.; Ciancia, G.; Limite, G.; Di Micco, R.; Varone, V.; Cortese, A.; Vatrella, A.; Di Crescenzo, V.; Zeppa, P. Neuroendocrine differentiation in breast carcinoma with osteoclast-like giant cells. Report of a case and review of the literature. Int. J. Surg. 2014, 12, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Albawardi, A.S.; Awwad, A.A.; Almarzooqi, S.S. Mammary carcinoma with osteoclast-like giant cells: A case report. Int. J. Clin. Exp. Pathol. 2014, 7, 9038. [Google Scholar] [PubMed]
- Zagelbaum, N.K.; Ward, M.F.; Okby, N.; Karpoff, H. Invasive ductal carcinoma of the breast with osteoclast-like giant cells and clear cell features: A case report of a novel finding and review of the literature. World J. Surg. Oncol. 2016, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Hayama, A.; Matsubara, M.; Watarai, Y.; Sakatani, T.; Naito, Z.; Shimizu, A. Breast carcinoma with osteoclast-like giant cells: A cytological-pathological correlation with a literature review. Ann. Diagn. Pathol. 2018, 33, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Peña-Jaimes, L.; González-García, I.; Reguero-Callejas, M.E.; Pinilla-Pagnon, I.; Pérez-Mies, B.; Albarrán-Artahona, V.; Martínez-Jañez, N.; Rosa-Rosa, J.M.; Palacios, J. Pleomorphic lobular carcinoma of the breast with osteoclast-like giant cells: A case report and review of the literature. Diagn. Pathol. 2018, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, I.; Flechter, E.; Vlodavsky, E.; Militianu, D.; Keidar, Z.; Haddad, E.; Bar-Sela, G. Fortuitous administration of denosumab in breast carcinoma with osteoclastic giant cells. Anticancer Drugs 2018, 29, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Güth, U.; Borovecki, A.; Amann, E.; Rechsteiner, M.; Tinguely, M. Pleomorphic lobular breast carcinoma with osteoclast like giant cells in the era of genomic testing. Curr. Probl. Cancer Case Rep. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Irelli, A.; Sirufo, M.M.; Quaglione, G.R.; De Pietro, F.; Bassino, E.M.; D’Ugo, C.; Ginaldi, L.; De Martinis, M. Invasive ductal breast cancer with osteoclast-like giant cells: A case report based on the gene expression profile for changes in management. J. Pers. Med. 2021, 11, 156. [Google Scholar] [CrossRef]
- Cyrta, J.; Benoist, C.; Masliah-Planchon, J.; Vieira, A.F.; Pierron, G.; Fuhrmann, L.; Richardot, C.; Caly, M.; Leclere, R.; Mariani, O.; et al. Breast carcinomas with osteoclast-like giant cells: A comprehensive clinico-pathological and molecular portrait and evidence of RANK-L expression. Mod. Pathol. 2022, 35, 1624–1635. [Google Scholar] [CrossRef]
- Sajjadi, E.; Gaudioso, G.; Terrasi, A.; Boggio, F.; Venetis, K.; Ivanova, M.; Bertolasi, L.; Lopez, G.; Runza, L.; Premoli, A.; et al. Osteoclast-like stromal giant cells in breast cancer likely belong to the spectrum of immunosuppressive tumor-associated macrophages. Front. Mol. Biosci. 2022, 9, 894247. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Tomasicchio, G.; Montanaro, A.E.; Telgrafo, M.; Mastropasqua, M.G.; Punzo, C. Osteoclast-like stromal giant cells in invasive ductal breast cancer: A case series. Int. J. Surg. Case Rep. 2022, 97, 107421. [Google Scholar] [CrossRef] [PubMed]
- d’Amati, A.; Mariano, M.; Addante, F.; Giliberti, G.; Tomasicchio, G.; Mastropasqua, M.G. When Histological Tumor Type Diagnosed on Core Biopsy Changes Its Face after Surgery: Report of a Deceptive Case of Breast Carcinoma. Reports 2022, 5, 38. [Google Scholar] [CrossRef]
- Wang, Y.J.; Huang, C.P.; Hong, Z.J.; Liao, G.S.; Yu, J.C. Invasive breast carcinoma with osteoclast-like stromal giant cells: A case report. World J. Clin. Cases 2023, 11, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, J.S.; Silverberg, S.G. Signet-ring cell carcinoma of the breast. The mucinous variant of infiltrating lobular carcinoma? Cancer 1976, 37, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Wells, S.; Vasudev, K. Primary signet ring cell carcinoma of the breast. Histopathology 1978, 2, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Hariri, J.A. Primary signet ring cell carcinoma of the breast. Virchows Arch. 1980, 388, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.T.; Seo, I.S.; Battersby, J.; Csicsko, J.F. Signet-ring cell carcinoma of the breast: A clinicopathologic study of 24 cases. Am. J. Clin. Pathol. 1980, 73, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Hatanaka, S.; Oneda, S.; Yoshida, H. Signet ring cells in breast carcinoma: An immunohistochemical and ultrastructural study. Acta Pathol. Jpn. 1992, 42, 523–528. [Google Scholar] [CrossRef]
- Ruiz, V.; Henríquez, I.; Reig, A.; Conill, C.; Verger, E. Has radiation therapy any role in signet-ring cell breast adenocarcinoma? Eur. J. Cancer 1997, 33, 977–978. [Google Scholar] [CrossRef]
- Yim, H.; Jin, Y.M.; Shim, C.; Park, H.B. Gastric metastasis of mammary signet ring cell carcinoma—A differential diagnosis with primary gastric signet ring cell carcinoma. J. Korean Med. Sci. 1997, 12, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Mizuguchi, K.; Yoshimoto, M.; Tanaka, M.; Motegi, S.; Matushima, H.; Ishizawa, M.; Nakamura, K. Cytologic diagnosis of signet-ring cell carcinoma of the breast. Acta Cytol. 1998, 42, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kennebeck, C.H.; Alagoz, T. Signet ring breast carcinoma metastases limited to the endometrium and cervix. Gynecol. Oncol. 1998, 71, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Izuishi, K.; Imoto, S.; Hasebe, T. Signet ring cell carcinoma associated with invasive ductal carcinoma of the breast: A case report. Breast Cancer 1999, 6, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Chen, D.R. Signet-ring cell carcinoma of the breast. Pathol. Int. 2000, 50, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Colak, T.; Akca, T.; Dirlik, M.; Caglikulekci, M.; Seyrek, E.; Cinel, L.; Bozdogan, R.; Aydin, S. Signet ring cell carcinoma of the breast as a source of pelvic floor metastatic mass. A case report. Acta Chir. Belg. 2005, 105, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Subramanya, H.; Rajaram, T.; Vincent, P.; Rai, R. Signet ring carcinoma of the breast: An uncommon type of breast carcinoma. Med. J. Armed. Forces India 2005, 61, 84–85. [Google Scholar] [CrossRef]
- Kuroda, N.; Fujishima, N.; Ohara, M.; Hirouchi, T.; Mizuno, K.; Lee, G.H. Invasive ductal carcinoma of the breast with signet-ring cell and mucinous carcinoma components: Diagnostic utility of immunocytochemistry of signet-ring cells in aspiration cytology materials. Diagn. Cytopathol. 2007, 35, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, F.; Ereño, C.; Martínez, A.; Sánchez del Rey, A.; Zabala, A. Mandibular metastasis of a signet ring cell carcinoma of the breast in a patient who underwent bilateral mastectomy more than 25 years earlier. Breast Care 2009, 4, 192–194. [Google Scholar] [CrossRef]
- Hara, F.; Kiyoto, S.; Takabatake, D.; Takashima, S.; Aogi, K.; Ohsumi, S.; Teramoto, N.; Nishimura, R.; Takashima, S. Metastatic breast cancer to the stomach resembling early gastric cancer. Case Rep. Oncol. 2010, 3, 142–147. [Google Scholar] [CrossRef]
- Leung, K.M.; Yeoh, G.P.; Chan, J.K.; Cheung, P.S.; Chan, K.W. Ductal type signet ring cell carcinoma of breast with growth pattern of pure mucinous carcinoma. Pathology 2011, 43, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Ertas, I.E.; Sayhan, S.; Karagoz, G.; Yildirim, Y. Signet-ring cell carcinoma of the breast with uterine metastasis treated with extensive cytoreductive surgery: A case report and brief review of the literature. J. Obstet. Gynaecol. Res. 2012, 38, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Karabagli, P.; Kilic, H. Primary pure signet cell carcinoma of the breast: A case report and review of the literature. Breast Cancer 2013, 20, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Y.F.; Wei, W.D.; Liu, P.; Xie, Z.M.; Wang, J.; Xie, X.M. Signet-ring cell carcinoma of the breast: A case report. World J. Surg. Oncol. 2013, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Dubey, V.; Makkar, M.; Suri, V. Pure primary signet ring cell carcinoma breast: A rare cytological diagnosis. J. Cytol. 2013, 30, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bal, A.; Das, A.; Kohli, P.S.; Singh, G.; Mittal, B.R. Invasive duct carcinoma of the breast with dominant signet-ring cell differentiation: A microsatellite stable tumor with aggressive behavior. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 720–724. [Google Scholar] [CrossRef]
- Acharfi, N.; Boujarnija, R.; Messoudi, K.; Darif, K.; Ouahbi, H.; Ouafki, I.; Amaadour, L.; Oualla, K.; Benbrahim, Z.; Arifi, S.; et al. Signet Ring Cell Carcinoma of the Breast: About A Case and Literature Review. Int. J. Innov. Res. Med. Sci. 2020, 5, 114–116. [Google Scholar] [CrossRef]
- Wang, T.; Shen, B.; Wang, L.; Liu, F. Primary signet ring cell carcinoma of the breast: A rare entity with unique biological behavior—A clinical study based on pure signet ring cell carcinoma cohort. Pathol. Res. Pract. 2020, 216, 152948. [Google Scholar] [CrossRef]
- Principe, D.R.; Raicu, A.; Cataneo, J.; Beverley, H.R.; Hyser, M. Perforating duodenal ulcer with umbilical herniation as a metastatic complication of primary signet ring cell carcinoma of the breast. J. Surg. Case Rep. 2021, 2021, rjab034. [Google Scholar] [CrossRef]
- Yang, W.; Ding, S.; Wang, L.; Ren, F.; Lai, Y.; Wang, H.; Hong, G.; Gao, W. Carcinoma with signet ring cell differentiation associated with invasive breast cancer: A case report. Oncol. Lett. 2023, 25, 212. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.; Yang, W.; Yao, J.; Guo, J.; Liu, C. The clinicopathological and immunohistochemical features of breast carcinomas with signet-ring-cell differentiation. World J. Surg. Oncol. 2023, 21, 181. [Google Scholar] [CrossRef]
- Padmore, R.F.; Lara, J.F.; Ackerman, D.J.; Gales, T.; Sigurdson, E.R.; Ehya, H.; Cooper, H.S.; Patchefsky, A.S. Primary combined malignant melanoma and ductal carcinoma of the breast: A report of two cases. Cancer 1996, 78, 2515–2525. [Google Scholar] [CrossRef]
- Nobukawa, B.; Fujii, H.; Hirai, S.; Kumasaka, T.; Shimizu, H.; Matsumoto, T.; Suda, K.; Futagawa, S. Breast carcinoma diverging to aberrant melanocytic differentiation: A case report with histopathologic and loss of heterozygosity analyses. Am. J. Surg. Pathol. 1999, 23, 1280. [Google Scholar] [CrossRef]
- Yen, H.; Florentine, B.; Kelly, L.K.; Bu, X.; Crawford, J.; Martin, S. Fine-needle aspiration of a metaplastic breast carcinoma with extensive melanocytic differentiation: A case report. Diagn. Cytopathol. 2000, 23, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Noske, A.; Schwabe, M.; Pahl, S.; Fallenberg, E.; Richter-Ehrenstein, C.; Dietel, M.; Kristiansen, G. Report of a metaplastic carcinoma of the breast with multi-directional differentiation: An adenoid cystic carcinoma, a spindle cell carcinoma and melanoma. Virchows Arch. 2008, 452, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Bendic, A.; Bozic, M.; Durdov, M.G. Metaplastic breast carcinoma with melanocytic differentiation. Pathol. Int. 2009, 59, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Nzegwu, M.A.; Sule, E.; Uzoigwe, J.; Achi, F. Metaplastic breast carcinoma; melanocytic variant, a very rare tumour. J. Surg. Case Rep. 2015, 2015, rju158. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, R.L.; Rosen, P.P.; Port, A.; Kinne, D.; Miké, V. Medullary carcinoma of the breast. A clinicopathologic study with 10 year follow-up. Cancer 1977, 40, 1365–1385. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, H.; Mori-Shiraishi, K.; Nakajima, M.; Ueki, H. Defining lymphocyte-predominant breast cancer by the proportion of lymphocyte-rich stroma and its significance in routine histopathological diagnosis. Pathol. Int. 2015, 65, 644–651. [Google Scholar] [CrossRef]
- Molberg, K.H.; Heffess, C.; Delgado, R.; Albores-Saavedra, J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer 1998, 82, 1279–1287. [Google Scholar] [CrossRef]
- Bégin, P.; Sahai, S.; Wang, N.S. Giant cell formation in small cell carcinoma of the lung. Cancer 1983, 52, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Stracca-Pansa, V.; Menegon, A.; Donisi, P.M.; Bozzola, L.; Fedeli, F.; Quarto, F.; Nappi, O.; Pettinato, G. Gastric carcinoma with osteoclast-like giant cells: Report of four cases. Am. J. Clin. Pathol. 1995, 103, 453–459. [Google Scholar] [CrossRef]
- Hood, D.L.; Bauer, T.W.; Leibel, S.A.; McMahon, J.T. Hepatic giant cell carcinoma: An ultrastructural and immunohistochemical study. Am. J. Clin. Pathol. 1990, 93, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M.; Prat, J.; Tabernero, M. Giant-cell tumor-like proliferation associated with a papillary transitional cell carcinoma of the renal pelvis. Am. J. Surg. Pathol. 1984, 8, 139–144. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Gaber, K.; Ordonez, N.G. Renal cell carcinoma with osteoclast-like giant cells. Virchows Arch. 1993, 422, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, M.J.; Lack, E.E.; Christ, M.L.; Weiss, L.M. Anaplastic thyroid carcinoma with osteoclast-like giant cells: A clinicopathologic, immunohistochemical, and ultrastructural study. Am. J. Surg. Pathol. 1991, 15, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Alpers, C.E.; Beckstead, J.H. Malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells: Enzyme histochemistry distinguishes tumor cells from giant cells. Am. J. Surg. Pathol. 1985, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Batsakis, J.G.; Ordonez, N.G.; Sevidal, P.A.; Baker, J.R. Osteoclast-type giant cell neoplasms of the parotid gland. J. Laryngol. Otol. 1988, 102, 901–904. [Google Scholar] [CrossRef]
- Mentzel, T.; Calonje, E.; Fletcher, C. Leiomyosarcoma with prominent osteoclast-like giant cells. Analysis of eight cases closely mimicking the so-called giant cell variant of malignant fibrous histiocytoma. Am. J. Surg. Pathol. 1994, 18, 258–265. [Google Scholar] [CrossRef]
- Yu, G.; Lin, C.; Wang, W.; Han, Y.; Qu, G.; Zhang, T. Squamous cell carcinoma of the uterine cervix associated with osteoclast-like giant cells: A case report and review of the literature. Oncol. Lett. 2014, 8, 1595–1598. [Google Scholar] [CrossRef]
- Lamovec, J.; Sobel, H.J.; Zidar, A.; Jerman, J. Epithelioid hemangioendothelioma of the anterior mediastinum with osteoclast-like giant cells: Light microscopic, immunohistochemical, and electron microscopic study. Am. J. Clin. Pathol. 1990, 93, 813–817. [Google Scholar] [CrossRef]
- D’Antonio, A.; Caleo, A.; Marsilia, G.; Boscaino, A. An unusual appearance of a common skin tumour. Cytopathology 2005, 16, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.P. Multinucleated mammary stromal giant cells. A benign lesion that simulates invasive carcinoma. Cancer 1979, 44, 1305–1308. [Google Scholar] [CrossRef]
- Berean, K.; Tron, V.; Churg, A.; Clement, P. Mammary fibroadenoma with multinucleated stromal giant cells. Am. J. Surg. Pathol. 1986, 10, 823–827. [Google Scholar] [CrossRef]
- Tse, G.M.; Law, B.K.; Chan, K.F.; Mas, T.K. Multinucleated stromal giant cells in mammary phyllodes tumours. Pathology 2001, 33, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Longacre, T.A. Ductal carcinoma in situ of the breast with osteoclast-like giant cells. Hum. Pathol. 2006, 37, 369–372. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Ieronimaki, A.I.; Zacharatou, A.; Gouloumis, A.R.; Leventakou, D.; Boutas, I.; Dimas, D.T.; Kontogeorgi, A.; Sitara, K.; Khaldi, L.; et al. A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review. J. Pers. Med. 2023, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Shi, S.; Zhang, Z.; Liu, H. Primary signet-ring cell melanoma of the anorectum: A case report. Oncol. Lett. 2023, 25, 220. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.P.; Ernsberger, D. Low-grade adenosquamous carcinoma: A variant of metaplastic mammary carcinoma. Am. J. Surg. Pathol. 1987, 11, 351–358. [Google Scholar] [CrossRef]
- Van Hoeven, K.; Drudis, T.; Cranor, M.L.; Erlandson, R.A.; Rosen, P.P. Low-grade adenosquamous carcinoma of the breast: A clinocopathologic study of 32 cases with ultrastructural analysis. Am. J. Surg. Pathol. 1993, 17, 248–258. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Clark, B.Z. Low-grade fibromatosis-like spindle cell carcinoma of the breast. Arch. Pathol. Lab. Med. 2015, 139, 552–557. [Google Scholar] [CrossRef]
- Sneige, N.; Yaziji, H.; Mandavilli, S.R.; Perez, E.R.; Ordonez, N.G.; Gown, A.M.; Ayalla, A. Low-grade (fibromatosis-like) spindle cell carcinoma of the breast. Am. J. Surg. Pathol. 2001, 25, 1009–1016. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Krishnamurthy, S.; Giordano, S.; Buchholz, T.A.; Kau, S.W.; Duan, Z.; Valero, V.; Hortobagyi, G.N. Squamous cell carcinoma of the breast. J. Clin. Oncol. 2005, 23, 7827–7835. [Google Scholar] [CrossRef]
- Grabowski, J.; Saltzstein, S.L.; Sadler, G.; Blair, S. Squamous cell carcinoma of the breast: A review of 177 cases. Am. Surg. 2009, 75, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.; Martinez, A.; Hernández, G.; Hardisson, D.; De Santiago, J. Squamous cell carcinoma of the breast. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 222–226. [Google Scholar] [CrossRef]
- Carter, M.R.; Hornick, J.L.; Lester, S.; Fletcher, C.D. Spindle cell (sarcomatoid) carcinoma of the breast: A clinicopathologic and immunohistochemical analysis of 29 cases. Am. J. Surg. Pathol. 2006, 30, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.; Giordano, S.; Broglio, K.; Duan, Z.; Trent, J.; Buchholz, T.; Babiera, G.; Hortobagyi, G.N.; Valero, V. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann. Oncol. 2006, 17, 605–613. [Google Scholar] [CrossRef]
- Kiakou, M.; Tolia, M.; Koufopoulos, N.; Tsapakidis, K.; Arvanitou, E.; Konstantinos, G.; Charalambakis, N.; Nikolaou, M.; Matthaios, D.; Tsoukalas, N. A Rare Case of Primary Carcinosarcoma of the Breast. Forum. Clin. Oncol. 2022, 13, 48–52. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Kokkali, S.; Antoniadou, F.; Dimas, D.T.; Missitzis, I.L. Matrix-producing Breast Carcinoma: A Rare Subtype of Metaplastic Breast Carcinoma. Cureus 2019, 11, e5188. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Dimas, D.; Antoniadou, F.; Sitara, K.; Balalis, D.; Boutas, I.; Gouloumis, A.R.; Kontogeorgi, A.; Khaldi, L. Metaplastic matrix-producing carcinoma and apocrine lobular carcinoma in situ associated with microglandular adenosis: A unique case report. Diagnostics 2022, 12, 1458. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Rosen, L.; Williams, W.; Benson, J.; Rywlin, A. A malignant neoplasm with features of both squamous cell carcinoma and malignant melanoma. Am. J. Dermatopathol. 1984, 6, 213–219. [Google Scholar] [PubMed]
- Charlton, R. A melanomatous carcinoma. A case report and commentary. Am. J. Dermatopathol. 1984, 6, 221–229. [Google Scholar] [PubMed]
- Sirsat, M.; Shrikhande, S.S. Collision tumour in the oral cavity. Indian J. Pathol. Microbiol. 1966, 9, 340–343. [Google Scholar]
- Davis, J.; Maclennan, K.; Schofield, J.; Watkinson, J.; Gluckman, P. Synchronous primary mucosal melanoma and mucoepidermoid carcinoma of the maxillary antrum. J. Laryngol. Otol. 1991, 105, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, T.; Tsuru, T.; Tsuneyoshi, M.; Sueishi, K.; Sibuya, T.; Fukuda, T. Primary collision neoplasm of malignant melanoma and adenocarcinoma in the lung: A case report. Pathol. Res. Pract. 1993, 189, 178–183. [Google Scholar] [CrossRef]
- Kajo, K.; Zubor, P.; Spacek, J.; Ryska, A. Carcinosarcoma of the uterus with melanocytic differentiation. Pathol. Res. Pract. 2007, 203, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Davel, G.H.; De Vos, R.; Vergote, I.; Lindeque, B.; de Jonge, E. Uterine carcinosarcoma with melanocytic differentiation. Int. J. Gynecol. Pathol. 2001, 20, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Genega, E.M.; Zhuang, L.; Zhou, M. High-grade urothelial carcinoma with malignant melanocytic differentiation. Int. J. Surg. Pathol. 2021, 29, 794–797. [Google Scholar] [CrossRef]

| LELC (N = 41) | MBCMD (N = 7) | BCOGC (N = 83) | SRCC (N = 67) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 53.0 (8.79) | 49.7 (11.7) | 50.2 (12.8) | 58.3 (11.4) | <0.001 |
| Median [Min, Max] | 53.0 [37.0, 69.0] | 45.0 [38.0, 72.0] | 47.0 [27.0, 84.0] | 58.0 [32.0, 86.0] | |
| Tumor grade | |||||
| I | 0 (0%) | 0 (0%) | 20 (24.1%) | 3 (4.5%) | 0.779 † |
| II | 0 (0%) | 0 (0%) | 18 (21.7%) | 5 (7.5%) | |
| III | 0 (0%) | 0 (0%) | 14 (16.9%) | 4 (6.0%) | |
| Not reported | 41 (100%) | 7 (100%) | 31 (37.3%) | 55 (82.1%) | |
| Tumor size (mm) | |||||
| Mean (SD) | 24.8 (8.42) | 40.0 (23.0) | 26.9 (14.5) | 47.6 (37.8) | <0.001 |
| Median [Min, Max] | 22.0 [10.0, 45.0] | 30.0 [20.0, 80.0] | 25.0 [4.00, 87.0] | 37.5 [10.0, 200] | |
| Not reported | 0 (0%) | 1 (14.3%) | 7 (8.4%) | 11 (16.4%) | |
| Lymph nodes (positive) | |||||
| Mean (SD) | 0.629 (1.50) | 0.833 (0.983) | 0.984 (2.70) | 6.03 (10.5) | <0.001 |
| Median [Min, Max] | 0 [0, 8.00] | 0.500 [0, 2.00] | 0 [0, 14.0] | 1.50 [0, 46.0] | |
| Not reported | 6 (14.6%) | 1 (14.3%) | 20 (24.1%) | 33 (49.3%) | |
| Lymph nodes (total) | |||||
| Mean (SD) | 15.9 (9.12) | 12.0 (5.96) | 15.4 (10.0) | 17.5 (9.33) | 0.515 |
| Median [Min, Max] | 18.0 [1.00, 33.0] | 13.0 [2.00, 17.0] | 15.0 [1.00, 42.0] | 19.0 [2.00, 46.0] | |
| Not reported | 8 (19.5%) | 2 (28.6%) | 64 (77.1%) | 42 (62.7%) | |
| Lymph nodes positivity | |||||
| Yes | 10 (24.4%) | 4 (57.1%) | 23 (27.7%) | 30 (44.8%) | |
| No | 25 (61.0%) | 3 (42.9%) | 50 (60.2%) | 15 (22.4%) | <0.001 † |
| Not reported | 6 (14.6%) | 0 (0%) | 10 (12.0%) | 22 (32.8%) | |
| Type of lymph node dissection | |||||
| ALND | 31 (75.6%) | 6 (85.7%) | 21 (25.3%) | 14 (20.9%) | <0.001 † |
| SLNB | 6 (14.6%) | 0 (0%) | 13 (15.7%) | 2 (3.0%) | |
| None | 0 (0%) | 0 (0%) | 2 (2.4%) | 6 (9.0%) | |
| Not reported | 4 (9.8%) | 1 (14.3%) | 47 (56.6%) | 45 (67.2%) | |
| pTNM (tumor component) | |||||
| pT1 | 0 (0%) | 1 (14.3%) | 26 (31.3%) | 0 (0%) | 0.295 † |
| pT2 | 0 (0%) | 4 (57.1%) | 31 (37.3%) | 0 (0%) | |
| pT3 | 0 (0%) | 2 (28.6%) | 7 (8.4%) | 0 (0%) | |
| pT4 | 0 (0%) | 0 (0%) | 1 (1.2%) | 0 (0%) | |
| Not reported | 41 (100%) | 0 (0%) | 18 (21.7%) | 67 (100%) | |
| Breast preserving surgery | |||||
| No | 16 (39.0%) | 5 (71.4%) | 24 (28.9%) | 15 (22.4%) | 0.078 † |
| Yes | 23 (56.1%) | 1 (14.3%) | 16 (19.3%) | 7 (10.4%) | |
| Not reported | 2 (4.9%) | 1 (14.3%) | 43 (51.8%) | 45 (67.2%) | |
| Radiotherapy | |||||
| No | 16 (39.0%) | 1 (14.3%) | 7 (8.4%) | 13 (19.4%) | 0.392 † |
| Yes | 17 (41.5%) | 3 (42.9%) | 11 (13.3%) | 8 (11.9%) | |
| Not reported | 8 (19.5%) | 3 (42.9%) | 65 (78.3%) | 46 (68.7%) | |
| Chemotherapy | |||||
| Chemotherapy | 18 (43.9%) | 0 (0%) | 4 (4.8%) | 12 (17.9%) | <0.001 † |
| Hormonal therapy | 2 (4.9%) | 0 (0%) | 6 (7.2%) | 1 (1.5%) | |
| Chemotherapy and hormonal therapy | 1 (2.4%) | 0 (0%) | 3 (3.6%) | 4 (6.0%) | |
| Nothing | 12 (29.3%) | 0 (0%) | 4 (4.8%) | 3 (4.5%) | |
| Not reported | 8 (19.5%) | 7 (100%) | 65 (78.3%) | 47 (70.1%) | |
| Chemotherapy and hormonal and immunotherapy | 0 (0%) | 0 (0%) | 1 (1.2%) | 0 (0%) | |
| Monitoring time (months) | |||||
| Mean (SD) | 31.4 (25.8) | 28.0 (29.4) | 28.4 (34.5) | 47.3 (75.1) | 0.972 |
| Median [Min, Max] | 24.0 [3.00, 103] | 14.0 [12.0, 72.0] | 19.5 [3.00, 180] | 23.0 [0, 423] | |
| Not reported | 13 (31.7%) | 3 (42.9%) | 59 (71.1%) | 21 (31.3%) | |
| Life status | |||||
| ANED | 29 (70.7%) | 2 (28.6%) | 36 (43.4%) | 27 (40.3%) | <0.001 † |
| AWD | 0 (0%) | 1 (14.3%) | 3 (3.6%) | 6 (9.0%) | |
| DOD | 0 (0%) | 1 (14.3%) | 4 (4.8%) | 16 (23.9%) | |
| DUC | 0 (0%) | 0 (0%) | 1 (1.2%) | 0 (0%) | |
| DOC | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7.5%) | |
| Not reported | 12 (29.3%) | 3 (42.9%) | 39 (47.0%) | 13 (19.4%) | |
| Chemotherapy Type | |||||
| Adjuvant | 19 (46.3%) | 0 (0%) | 9 (10.8%) | 14 (20.9%) | 0.729 † |
| Neoadjuvant | 1 (2.4%) | 0 (0%) | 1 (1.2%) | 2 (3.0%) | |
| Neoadjuvant and Adjuvant | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | |
| Not reported | 21 (51.2%) | 7 (100%) | 73 (88.0%) | 50 (74.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufopoulos, N.I.; Boutas, I.; Pouliakis, A.; Samaras, M.G.; Kotanidis, C.; Kontogeorgi, A.; Dimas, D.T.; Ieronimaki, A.-I.; Leventakou, D.; Spathis, A.; et al. The “Forgotten” Subtypes of Breast Carcinoma: A Systematic Review of Selected Histological Variants Not Included or Not Recognized as Distinct Entities in the Current World Health Organization Classification of Breast Tumors. Int. J. Mol. Sci. 2024, 25, 8382. https://doi.org/10.3390/ijms25158382
Koufopoulos NI, Boutas I, Pouliakis A, Samaras MG, Kotanidis C, Kontogeorgi A, Dimas DT, Ieronimaki A-I, Leventakou D, Spathis A, et al. The “Forgotten” Subtypes of Breast Carcinoma: A Systematic Review of Selected Histological Variants Not Included or Not Recognized as Distinct Entities in the Current World Health Organization Classification of Breast Tumors. International Journal of Molecular Sciences. 2024; 25(15):8382. https://doi.org/10.3390/ijms25158382
Chicago/Turabian StyleKoufopoulos, Nektarios I., Ioannis Boutas, Abraham Pouliakis, Menelaos G. Samaras, Christakis Kotanidis, Adamantia Kontogeorgi, Dionysios T. Dimas, Argyro-Ioanna Ieronimaki, Danai Leventakou, Aris Spathis, and et al. 2024. "The “Forgotten” Subtypes of Breast Carcinoma: A Systematic Review of Selected Histological Variants Not Included or Not Recognized as Distinct Entities in the Current World Health Organization Classification of Breast Tumors" International Journal of Molecular Sciences 25, no. 15: 8382. https://doi.org/10.3390/ijms25158382







