The Vasopressin Receptor Antagonist Tolvaptan Counteracts Tumor Growth in a Murine Xenograft Model of Small Cell Lung Cancer
Abstract
:1. Introduction
2. Results
2.1. Weight and Serum Parameters of Mice
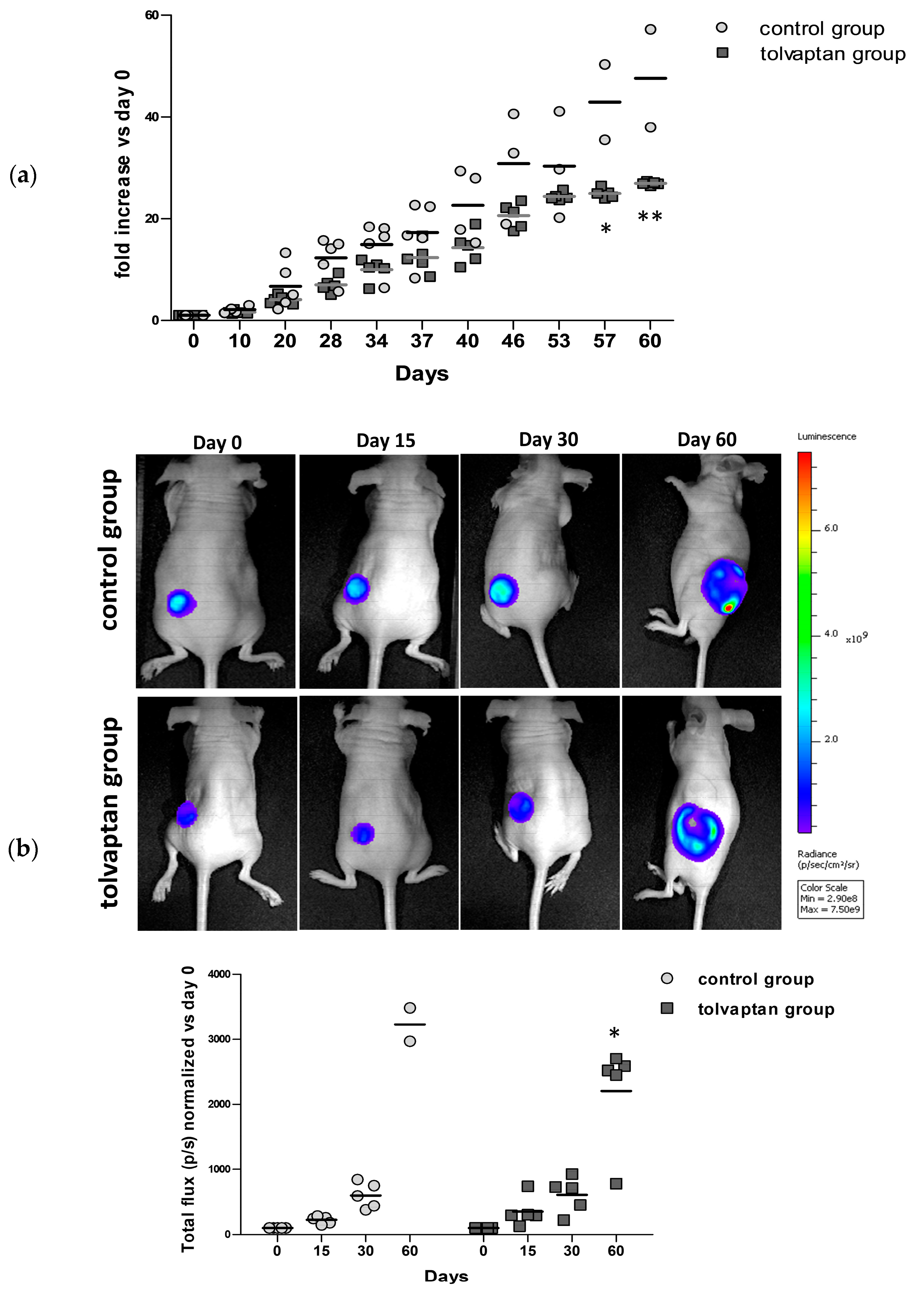
2.2. Tumor Growth
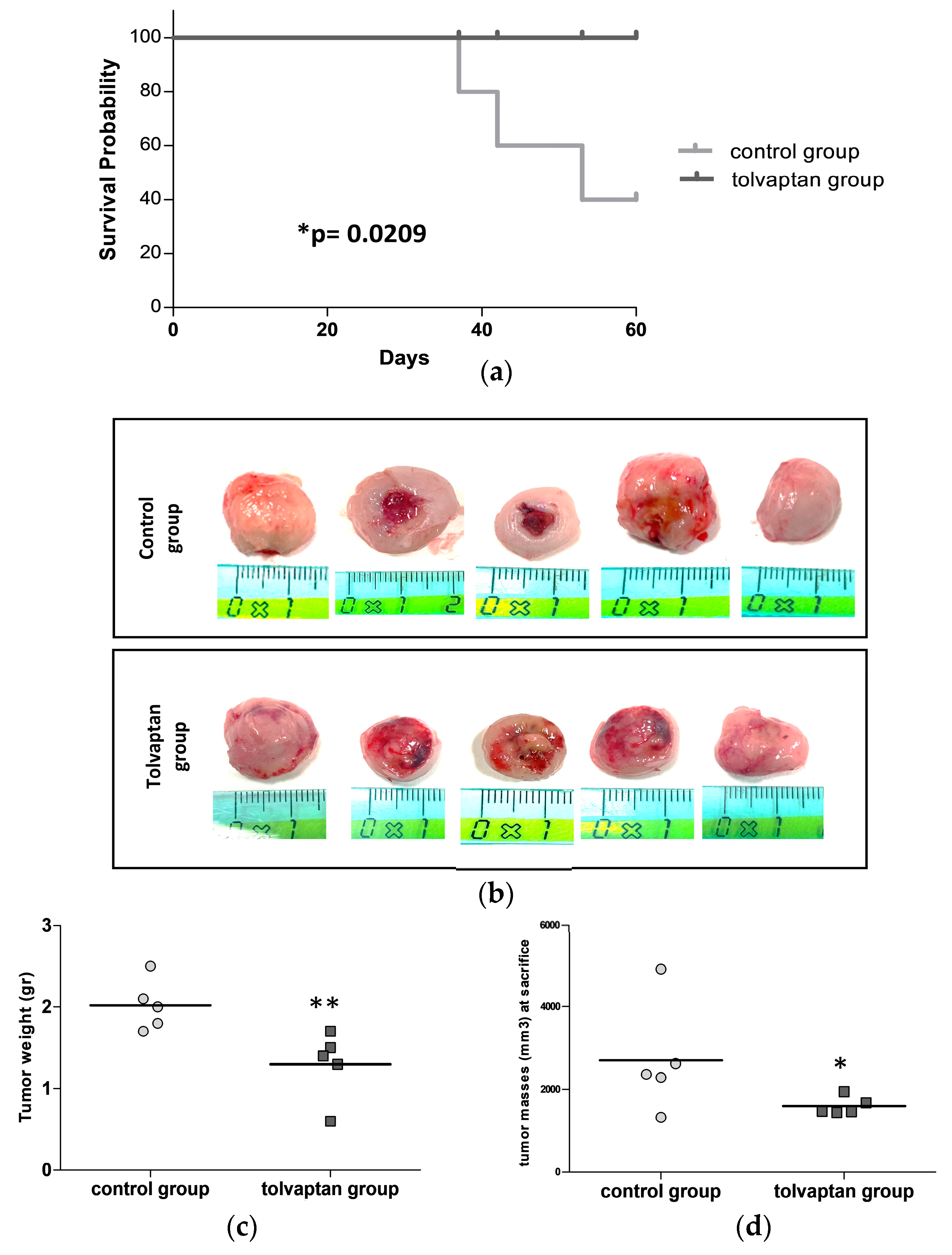
2.3. Mice Survival and Analysis of Excised Tumor Masses
2.4. Cell Signaling Pathways Affected by Tolvaptan
2.5. Proliferative Potential and Apoptosis Analysis
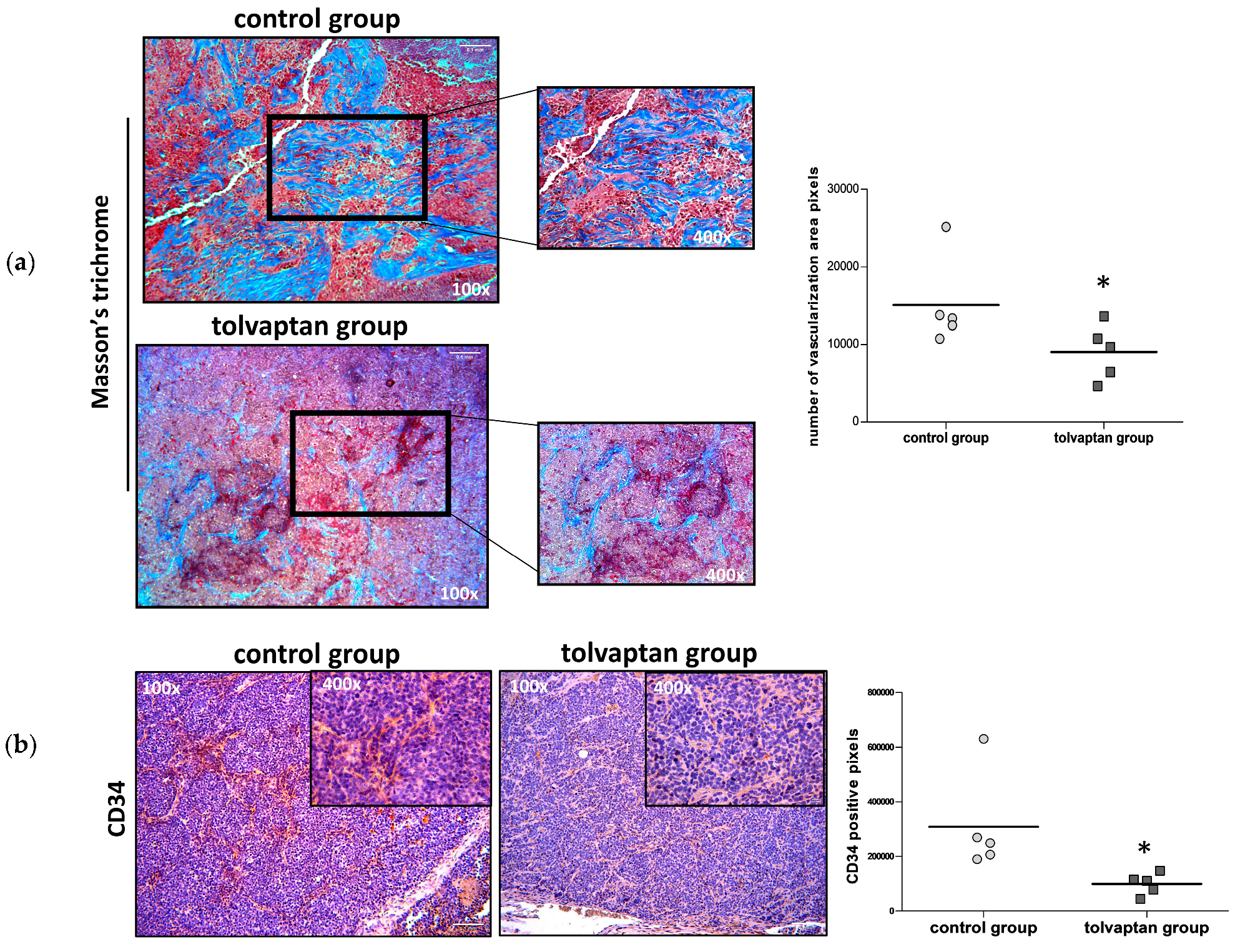
2.6. Tumor Mass Fibrosis and Angiogenesis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Transfection of H69 Cells
4.3. Setup of a Xenograft Mouse Model of SCLC
4.4. In Vivo Imaging
4.5. Serum Analysis
4.6. Tissue Preparation and Masson’s Trichrome Analysis
4.7. Immunohistochemical (IHC) Analysis of Tumor Masses
4.8. Western Blot Analysis of Tumor Masses
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doshi, S.M.; Shah, P.; Lei, X.; Lahoti, A.; Salahudeen, A.K. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am. J. Kidney Dis. 2012, 59, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Rinaldi, S.; Caramanti, M.; Grohè, C.; Santoni, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; Cascinu, S. Hyponatremia in cancer patients: Time for a new approach. Crit. Rev. Oncol. Hematol. 2016, 102, 15–25. [Google Scholar] [CrossRef]
- Sørensen, J.B.; Andersen, M.K.; Hansen, H.H. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J. Intern. Med. 1995, 238, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Caroen, S.; Oronsky, A.; Dobalian, V.E.; Oronsky, N.; Lybeck, M.; Reid, T.R.; Carter, C.A. Electrolyte disorders with platinum-based chemotherapy: Mechanisms, manifestations and management. Cancer Chemother. Pharmacol. 2017, 80, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Santoni, M.; Rinaldi, S.; Nunzi, E.; Smerilli, A.; Caramanti, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; et al. Risk of Hyponatraemia in Cancer Patients Treated with Targeted Therapies: A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2016, 11, e0152079. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, R.; Karam, S.; Uppal, N.N.; Barta, V.S.; Deray, G.; Devoe, C.; Launay-Vacher, V.; Jhaveri, K.D.; on behalf of Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am. J. Nephrol. 2017, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Grohé, C. Hyponatremia in Oncology Patients. Front. Horm. Res. 2019, 52, 161–166. [Google Scholar] [PubMed]
- Raftopoulos, H. Diagnosis and management of hyponatremia in cancer patients. Support. Care Cancer 2007, 15, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, T.; Latta, S.; Jallad, B.; Kheir, F.; Alhosaini, M.N.; Patel, A. Cisplatin-induced renal salt wasting syndrome. South. Med. J. 2010, 103, 793–799. [Google Scholar] [CrossRef]
- Kim, G.H. Pathophysiology of Drug-Induced Hyponatremia. J. Clin. Med. 2022, 11, 5810. [Google Scholar] [CrossRef]
- Fibbi, B.; Marroncini, G.; Naldi, L.; Anceschi, C.; Errico, A.; Norello, D.; Peri, A. Hyponatremia and Cancer: From Bedside to Benchside. Cancers 2023, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Anceschi, C.; Naldi, L.; Fibbi, B.; Baldanzi, F.; Martinelli, S.; Polvani, S.; Maggi, M.; Peri, A. Low sodium and tolvaptan have opposite effects in human small cell lung cancer cells. Mol. Cell Endocrinol. 2021, 537, 111419. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Naldi, L.; Fibbi, B.; Errico, A.; Polvani, S.; Brogi, M.; Fanelli, A.; Maggi, M.; Peri, A. Hyponatremia Promotes Cancer Growth in a Murine Xenograft Model of Neuroblastoma. Int. J. Mol. Sci. 2023, 24, 16680. [Google Scholar] [CrossRef] [PubMed]
- Berl, T.; Quittnat-Pelletier, F.; Verbalis, J.G.; Schrier, R.W.; Bichet, D.G.; Ouyang, J.; Czerwiec, F.S.; Investigators, S. Oral tolvaptan is safe and effective in chronic hyponatremia. J. Am. Soc. Nephrol. 2010, 21, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Peri, A. Clinical review: The use of vaptans in clinical endocrinology. J. Clin. Endocrinol. Metab. 2013, 98, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.; Verbalis, J.G.; Amin, A.N.; Burst, V.R.; Chiodo, J.A.; Chiong, J.R.; Dasta, J.F.; Friend, K.E.; Hauptman, P.J.; Peri, A.; et al. Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int. 2015, 88, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Peri, A.; Cranston, I.; McCool, R.; Shaw, A.; Glanville, J.; Petrakova, L.; O’Reilly, K. A systematic review of known interventions for the treatment of chronic nonhypovolaemic hypotonic hyponatraemia and a meta-analysis of the vaptans. Clin. Endocrinol. 2017, 86, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Vincent, M.; Justice, E. Diagnosis and management of hyponatremia in cancer patients. Oncologist 2012, 17, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Antonuzzo, A.; Blasi, L.; Buosi, R.; Lorusso, V.; Migliorino, M.R.; Montesarchio, V.; Zilembo, N.; Sabbatini, R.; Peri, A. Practical issues for the management of hyponatremia in oncology. Endocrine 2018, 61, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hansen, O.; Sørensen, P.; Hansen, K.H. The occurrence of hyponatremia in SCLC and the influence on prognosis: A retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010, 68, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Okines, A.; Gunapala, R.; Morganstein, D.; Popat, S. Resolution of severe hyponatraemia is associated with improved survival in patients with cancer. BMC Cancer 2015, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Santoni, M.; Newsom-Davis, T.; Caramanti, M.; Rinaldi, S.; Tiberi, M.; Morgese, F.; Torniai, M.; Pistelli, M.; Onofri, A.; et al. Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget 2017, 8, 23871–23879. [Google Scholar] [CrossRef] [PubMed]
- Gattone, V.H.; Wang, X.; Harris, P.C.; Torres, V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 2003, 9, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Beland, F.A.; Chen, S.; Liu, F.; Guo, L.; Fang, J.L. Mechanisms of tolvaptan-induced toxicity in HepG2 cells. Biochem. Pharmacol. 2015, 95, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Dwivedi, N.; Tao, S.; Jamadar, A.; Kakade, V.R.; Neil, M.O.; Weiss, R.H.; Enders, J.; Calvet, J.P.; Thomas, S.M.; et al. Targeting the vasopressin type-2 receptor for renal cell carcinoma therapy. Oncogene 2020, 39, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Anceschi, C.; Naldi, L.; Fibbi, B.; Baldanzi, F.; Maggi, M.; Peri, A. The V2 receptor antagonist tolvaptan counteracts proliferation and invasivity in human cancer cells. J. Endocrinol. Invest. 2022, 45, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Glezerman, I.G.; Boklage, S.H.; Chiodo, J.; Tidwell, B.A.; Lamerato, L.E.; Schulman, K.L. The occurrence of hyponatremia and its importance as a prognostic factor in a cross-section of cancer patients. BMC Cancer 2016, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, T.; Paesmans, M.; Body, J.J. A prospective study on hyponatraemia in medical cancer patients: Epidemiology, aetiology and differential diagnosis. Support. Care Cancer 2000, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I. Safety Profile of Tolvaptan in the Treatment of Autosomal Dominant Polycystic Kidney Disease. Ther. Clin. Risk Manag. 2021, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Coulson, J.M.; Stanley, J.; Woll, P.J. Tumour-specific arginine vasopressin promoter activation in small-cell lung cancer. Br. J. Cancer 1999, 80, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Medici, M.A.; Coppolino, G.; Sciortino, M.T.; Merlo, F.M.; Campo, S.; Donato, V.; Venuti, A.; Sturiale, A.; Zaccaria, D.; et al. Aquaretic inhibits renal cancer proliferation: Role of vasopressin receptor-2 (V2-R). Urol. Oncol. 2010, 28, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Beach, D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell 1993, 4, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kurmi, B.D.; Singh, A.; Singh, D. Potential role of resveratrol and its nano-formulation as anti-cancer agent. Explor. Target. Antitumor Ther. 2022, 3, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Deng, Q.; Chen, Z.; Yan, S.; Dong, Q.; Zhang, Y.; Cui, Y.; Li, L.; He, Y.; Shi, J. Recent development of VEGFR small molecule inhibitors as anticancer agents: A patent review (2021–2023). Bioorg. Chem. 2024, 146, 107278. [Google Scholar] [CrossRef] [PubMed]
- Civin, C.I.; Strauss, L.C.; Brovall, C.; Fackler, M.J.; Schwartz, J.F.; Shaper, J.H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raisedagainst KG-1a cells. J. Immunol. 1984, 133, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Shenoy, S.P.; Bose, B. CD34 cells in somatic, regenerative and cancer stem cells: Developmental biology, cell therapy, and omics big data perspective. J. Cell Biochem. 2020, 121, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Fujiki, H.; Mizuguchi, H.; Hattori, K.; Ohmoto, K.; Ishikawa, M.; Nagano, K.; Yamamura, Y. Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J. Pharmacol. Exp. Ther. 2014, 349, 258–267. [Google Scholar] [CrossRef]
- Garona, J.; Pifano, M.; Ripoll, G.; Alonso, D.F. Development and therapeutic potential of vasopressin synthetic analog [V4Q5]dDAVP as a novel anticancer agent. Vitam. Horm. 2020, 113, 259–289. [Google Scholar] [CrossRef] [PubMed]
- Sobol, N.T.; Solernó, L.M.; Beltrán, B.; Vásquez, L.; Ripoll, G.V.; Garona, J.; Alonso, D.F. Anticancer activity of repurposed hemostatic agent desmopressin on AVPR2-expressing human osteosarcoma. Exp. Ther. Med. 2021, 21, 566. [Google Scholar] [CrossRef] [PubMed]
- Sobol, N.T.; Solerno, L.M.; Llavona, C.; Alonso, D.F.; Garona, J. Vasopressin Analog [V4Q5] dDAVP Exerts Cooperative Anticancer Effects in Combination With Low-Dose 5-Fluorouracil on Aggressive Colorectal Cancer Models. World J. Oncol. 2023, 14, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, A.S.; Fay, M.J.; Memoli, V.A.; North, W.G. Factors regulating the production of vasopressin-associated human neurophysin by small-cell carcinoma of the lung: Evaluation by computer-enhanced quantitative immunocytochemistry. Neuropeptides 1995, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- North, W.G.; Fay, M.J.; Longo, K.A.; Du, J. Expression of all known vasopressin receptor subtypes by small cell tumors implies a multifaceted role for this neuropeptide. Cancer Res. 1998, 58, 1866–1871. [Google Scholar] [PubMed]
- North, W.G. Gene regulation of vasopressin and vasopressin receptors in cancer. Exp. Physiol. 2000, 85, 27S–40S. [Google Scholar] [CrossRef]




| Na+ (mEq/L) | ALT (IU/L) | AST (IU/L) | Urea (mg/dL) | Creatinine (mg/dL) | Urea/Creatinine | |
|---|---|---|---|---|---|---|
| Control group | 152.3 ± 0.3 | 19.3 ± 2.8 | 97.5 ± 23.0 | 62.3 ± 4.8 | 0.09 ± 0.02 | 8.5 ± 1.4 |
| Tolvaptan group | 154.8 ± 2.2 | 20.4 ± 3.1 | 123.8 ± 15.7 | 47.0 ± 3.7 | 0.07 ± 0.01 | 6.9 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naldi, L.; Fibbi, B.; Polvani, S.; Cirillo, C.; Pasella, F.; Bartolini, F.; Romano, F.; Fanelli, A.; Peri, A.; Marroncini, G. The Vasopressin Receptor Antagonist Tolvaptan Counteracts Tumor Growth in a Murine Xenograft Model of Small Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 8402. https://doi.org/10.3390/ijms25158402
Naldi L, Fibbi B, Polvani S, Cirillo C, Pasella F, Bartolini F, Romano F, Fanelli A, Peri A, Marroncini G. The Vasopressin Receptor Antagonist Tolvaptan Counteracts Tumor Growth in a Murine Xenograft Model of Small Cell Lung Cancer. International Journal of Molecular Sciences. 2024; 25(15):8402. https://doi.org/10.3390/ijms25158402
Chicago/Turabian StyleNaldi, Laura, Benedetta Fibbi, Simone Polvani, Chiara Cirillo, Francesca Pasella, Francesca Bartolini, Francesca Romano, Alessandra Fanelli, Alessandro Peri, and Giada Marroncini. 2024. "The Vasopressin Receptor Antagonist Tolvaptan Counteracts Tumor Growth in a Murine Xenograft Model of Small Cell Lung Cancer" International Journal of Molecular Sciences 25, no. 15: 8402. https://doi.org/10.3390/ijms25158402





