DArTseq-Based, High-Throughput Identification of Novel Molecular Markers for the Detection of Blackleg (Leptosphaeria Spp.) Resistance in Rapeseed
Abstract
1. Introduction
2. Results
2.1. Phenotyping
2.2. Genotyping
3. Materials and Methods
3.1. Plant Material
3.2. Field Assessment
- 0
- healthy plant with no visible disease symptoms;
- 1
- slight surface discoloration of the main stem;
- 2
- discoloration covering up to 10% of the main stem;
- 3
- discoloration covering from 11% to 20% of the main stem;
- 4
- discoloration covering from 21% to 30% of the main stem, a few pycnidia;
- 5
- discoloration covering from 31% to 50% of the main stem, several pycnidia;
- 6
- discoloration covering from 51% to 75% of the main stem, numerous pycnidia;
- 7
- discoloration covering over 76% of the main stem and parts of side branches, numerous pycnidia;
- 8
- discoloration covering the whole main stem and extending to side branches, numerous pycnidia, smaller number of siliques as compared to the healthy plants;
- 9
- dead plant with thin stem, very small number or no siliques, numerous pycnidia on the main stem and side branches.
3.3. DNA Extraction
3.4. Genotyping
3.5. Statistical Analysis
3.6. Functional Annotation of Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In Compendium of Plant Genomes; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zheng, X.; Koopmann, B.; Ulber, B.; von Tiedemann, A. A Global Survey on Diseases and Pests in Oilseed Rape—Current Challenges and Innovative Strategies of Control. Front. Agron. 2020, 2, 590908. [Google Scholar] [CrossRef]
- Abbadi, A.; Leckband, G. Rapeseed Breeding for Oil Content, Quality, and Sustainability. Eur. J. Lipid Sci. Technol. 2011, 113, 1198–1206. [Google Scholar] [CrossRef]
- Galanty, A.; Grudzińska, M.; Paździora, W.; Paśko, P. Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef]
- Miklavčič Višnjevec, A.; Tamayo Tenorio, A.; Steenkjær Hastrup, A.C.; Hansen, N.M.L.; Peeters, K.; Schwarzkopf, M. Glucosinolates and Isothiocyantes in Processed Rapeseed Determined by Hplc-dad-qtof. Plants 2021, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic Acid in Feed and Food. EFSA J. 2016, 14, e04593. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Rapeseed Powder from Brassica rapa L. and Brassica napus L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06197. [Google Scholar] [CrossRef]
- Raymer, P.L. Canola: An Emerging Oilseed Crop. In Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Orlovius, K.; Kirkby, E.A. Fertilizing for High Yield and Quality Oilseed Rape. Int. Potash Inst. Bull. 2003, 16, 125. [Google Scholar]
- Van De Wouw, A.P.; Marcroft, S.J.; Howlett, B.J. Blackleg Disease of Canola in Australia. Crop Pasture Sci. 2016, 67, 273–283. [Google Scholar] [CrossRef]
- Hwang, S.F.; Strelkov, S.E.; Peng, G.; Ahmed, H.; Zhou, Q.; Turnbull, G. Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada. Plants 2016, 5, 31. [Google Scholar] [CrossRef]
- Gwiazdowski, R. Hamowanie Wzrostu Leptosphaeria maculans i Leptosphaeria biglobosa Przez Wybrane Fungicydy w Testach Płytkowych. Rośliny Oleiste-Oilseed Crops 2008, 19, 67–73. [Google Scholar]
- Marcroft, S.J.; Sosnowski, M.R.; Scott, E.S.; Ramsey, M.D.; Salisbury, P.A.; Howlett, B.J. Brassica napus Plants Infected by Leptosphaeria maculans after the Third to Fifth Leaf Growth Stage in South-Eastern Australia Do Not Develop Blackleg Stem Canker. Eur. J. Plant Pathol. 2005, 112, 289–292. [Google Scholar] [CrossRef]
- Savage, D.; Barbetti, M.J.; MacLeod, W.J.; Salam, M.U.; Renton, M. Temporal Patterns of Ascospore Release in Leptosphaeria maculans Vary Depending on Geographic Region and Time of Observation. Microb. Ecol. 2013, 65, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Travadon, R.; Sache, I.; Dutech, C.; Stachowiak, A.; Marquer, B.; Bousset, L. Absence of Isolation by Distance Patterns at the Regional Scale in the Fungal Plant Pathogen Leptosphaeria maculans. Fungal Biol. 2011, 115, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Aubertot, J.N.; Pinochet, X.; Doré, T. The Effects of Sowing Date and Nitrogen Availability during Vegetative Stages on Leptosphaeria maculans Development on Winter Oilseed Rape. Crop Prot. 2004, 23, 635–645. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Fernando, W.G.D.; Turkington, T.K.; Mclaren, D.L. Best Management Practices for Blackleg Disease of Canola. Prairie Soils Crops J. 2011, 4, 122–134. [Google Scholar]
- Van de Wouw, A.P.; Marcroft, S.J.; Sprague, S.J.; Scanlan, J.L.; Vesk, P.A.; Idnurm, A. Epidemiology and Management of Blackleg of Canola in Response to Changing Farming Practices in Australia. Australas. Plant Pathol. 2021, 50, 137–149. [Google Scholar] [CrossRef]
- Haddadi, P.; Larkan, N.J.; Van deWouw, A.; Zhang, Y.; Xiang Neik, T.; Beynon, E.; Bayer, P.; Edwards, D.; Batley, J.; Borhan, M.H. Brassica napus Genes Rlm4 and Rlm7, Conferring Resistance to Leptosphaeria maculans, Are Alleles of the Rlm9 Wall-associated Kinase-like Resistance Locus. Plant Biotechnol. J. 2022, 20, 1229. [Google Scholar] [CrossRef] [PubMed]
- Larkan, N.J.; Ma, L.; Haddadi, P.; Buchwaldt, M.; Parkin, I.A.P.; Djavaheri, M.; Borhan, M.H. The Brassica napus Wall-Associated Kinase-like (WAKL) Gene Rlm9 Provides Race-Specific Blackleg Resistance. Plant J. 2020, 104, 892–900. [Google Scholar] [CrossRef]
- Larkan, N.J.; Lydiate, D.J.; Parkin, I.A.P.; Nelson, M.N.; Epp, D.J.; Cowling, W.A.; Rimmer, S.R.; Borhan, M.H. The Brassica Napus Blackleg Resistance Gene LepR3 Encodes a Receptor-like Protein Triggered by the Leptosphaeria maculans Effector AVRLM1. New Phytol. 2013, 197, 595–605. [Google Scholar] [CrossRef]
- Larkan, N.J.; Ma, L.; Borhan, M.H. The Brassica napus Receptor-like Protein RLM2 Is Encoded by a Second Allele of the LepR3/Rlm2 Blackleg Resistance Locus. Plant Biotechnol. J. 2015, 13, 983–992. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Attard, A.; Kühn, M.L.; Rouxel, T. New avirulence genes in the phytopathogenic fungus Leptosphaeria maculans. Phytopathology 2002, 92, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Marcroft, S.J.; Elliott, V.L.; Cozijnsen, A.J.; Salisbury, P.A.; Howlett, B.J.; Van de Wouw, A.P. Identifying resistance genes to Leptosphaeria maculans in Australian Brassica napus cultivars based on reactions to isolates with known avirulence genotypes. Crop Pasture Sci. 2012, 63, 338–350. [Google Scholar] [CrossRef]
- Brun, H.; Chèvre, A.M.; Fitt, B.D.; Powers, S.; Besnard, A.L.; Ermel, M.; Huteau, V.; Marquer, B.; Eber, F.; Renard, M.; et al. Quantitative Resistance Increases the Durability of Qualitative Resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010, 185, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Delourme, R.; Bousset, L.; Ermel, M.; Duffé, P.; Besnard, A.L.; Marquer, B.; Fudal, I.; Linglin, J.; Chadoeuf, J.; Brun, H. Quantitative Resistance Affects the Speed of Frequency Increase but Not the Diversity of the Virulence Alleles Overcoming a Major Resistance Gene to Leptosphaeria Maculans in Oilseed Rape. Infect. Genet. Evol. 2014, 27, 490–499. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Mitrousia, G.K.; Sidique, S.N.M.; Qi, A.; Fitt, B.D.L. Combining R Gene and Quantitative Resistance Increases Effectiveness of Cultivar Resistance against Leptosphaeria maculans in Brassica napus in Different Environments. PLoS ONE 2018, 13, e0197752. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Chawla, H.S.; Obermeier, C.; Dreyer, F.; Abbadi, A.; Snowdon, R. Chromosome-Scale Assembly of Winter Oilseed Rape Brassica napus. Front. Plant Sci. 2020, 11, 514081. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jing, J.; Snowdon, R.J.; Mason, A.S.; Shen, J.; Meng, J.; Zou, J. Exploring the Gene Pool of Brassica napus by Genomics-Based Approaches. Plant Biotechnol. J. 2021, 19, 1693–1712. [Google Scholar] [CrossRef]
- Starosta, E.; Szwarc, J.; Niemann, J.; Szewczyk, K.; Weigt, D. Brassica napus Haploid and Double Haploid Production and Its Latest Applications. Curr. Issues Mol. Biol. 2023, 45, 4431–4450. [Google Scholar] [CrossRef] [PubMed]
- Bocianowski, J. Using NGS Technology and Association Mapping to Identify Candidate Genes Associated with Fusarium Stalk Rot Resistance. Genes 2024, 15, 106. [Google Scholar] [CrossRef]
- Alam, M.; Neal, J.; O’Connor, K.; Kilian, A.; Topp, B. Ultra-High-Throughput DArTseq-Based SilicoDArT and SNP Markers for Genomic Studies in Macadamia. PLoS ONE 2018, 13, e0203465. [Google Scholar] [CrossRef]
- Egea, L.A.; Mérida-García, R.; Kilian, A.; Hernandez, P.; Dorado, G. Assessment of Genetic Diversity and Structure of Large Garlic (Allium aativum) Germplasm Bank, by Diversity Arrays Technology “Genotyping-by-Sequencing” Platform (DArTseq). Front. Genet. 2017, 8, 272084. [Google Scholar] [CrossRef]
- Kilian, A.; Sanewski, G.; Ko, L. The Application of DArTseq Technology to Pineapple. Acta Hortic. 2016, 1111, 181–188. [Google Scholar] [CrossRef]
- Jedryczka, M. Epidemiology and Damage Caused by Stem Canker of Oilseed Rape in Poland. Ph.D. Thesis, Dissertations and Monographs, Abstract of Habilitation Thesis Institute of Plant Genetics, Polish Academy of Sciences, Poznań, Poland, 2006; pp. 42, 150, (In Polish, with English Abstract). [Google Scholar]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C.; et al. Diversity Arrays Technology: A Generic Genome Profiling Technology on Open Platforms. Methods Mol. Biol. 2012, 888, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Wei, L.; Huang, Y.; Liu, D.; Jia, Y.; Luo, C.; Lin, Y.; Liang, C.; Hu, Y.; et al. BnIR: A Multi-Omics Database with Various Tools for Brassica napus Research and Breeding. Mol. Plant 2023, 16, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Malosetti, M.; Ribaut, J.M.; van Eeuwijk, F.A. The Statistical Analysis of Multi-Environment Data: Modeling Genotype-by-Environment Interaction and Its Genetic Basis. Front. Physiol. 2013, 4, 37433. [Google Scholar] [CrossRef] [PubMed]
- van Eeuwijk, F.A.; Bink, M.C.; Chenu, K.; Chapman, S.C. Detection and Use of QTL for Complex Traits in Multiple Environments. Curr. Opin. Plant Biol. 2010, 13, 193–205. [Google Scholar] [CrossRef] [PubMed]
- VSN International. Genstat for Windows, 23rd ed.; VSN International: Hemel Hempstead, UK, 2023. [Google Scholar]
- Patterson, N.; Price, A.L.; Reich, D. Population Structure and Eigenanalysis. PLoS Genet. 2006, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.G.; Haddadi, P.; Wan, J.; Adam, L.; Walker, P.; Larkan, N.J.; Daayf, F.; Hossein Borhan, M.; Belmonte, M.F. Transcriptome Analysis of Rlm2-Mediated Host Immunity in the Brassica napus–Leptosphaeria maculans Pathosystem. Mol. Plant-Microbe Interact. 2019, 32, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Brachaczek, A.; Kaczmarek, J.; Jedryczka, M. Warm and Wet Autumns Favour Yield Losses of Oilseed Rape Caused by Phoma Stem Canker. Agronomy 2021, 11, 1171. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-Wide Importance of Phoma Stem Canker (Leptosphaeria maculans and L. biglobosa) on Oilseed Rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Ha, T.M. A Review on the Development of Integrated Pest Management and Its Integration in Modern Agriculture. Asian J. Agric. Food Sci. 2014, 2, 336–340. [Google Scholar]
- Dhawan, A.K.; Peshin, R. Integrated Pest Management: Concept, Opportunities and Challenges. Integr. Pest Manag. 2009, 1, 51–81. [Google Scholar] [CrossRef]
- Gajardo, H.A.; Wittkop, B.; Soto-Cerda, B.; Higgins, E.E.; Parkin, I.A.P.; Snowdon, R.J.; Federico, M.L.; Iniguez-Luy, F.L. Association Mapping of Seed Quality Traits in Brassica napus L. Using GWAS and Candidate QTL Approaches. Mol. Breed. 2015, 35, 143. [Google Scholar] [CrossRef]
- Helal, M.M.U.; Gill, R.A.; Tang, M.; Yang, L.; Hu, M.; Yang, L.; Xie, M.; Zhao, C.; Cheng, X.; Zhang, Y.; et al. SNP-and Haplotype-Based GWAS of Flowering-Related Traits in Brassica napus. Plants 2021, 10, 2475. [Google Scholar] [CrossRef]
- Dakouri, A.; Lamara, M.; Karim, M.M.; Wang, J.; Chen, Q.; Gossen, B.D.; Strelkov, S.E.; Hwang, S.F.; Peng, G.; Yu, F. Identification of Resistance Loci against New Pathotypes of Plasmodiophora brassicae in Brassica napus Based on Genome-Wide Association Mapping. Sci. Rep. 2021, 11, 6599. [Google Scholar] [CrossRef]
- He, Y.; Wu, D.; Wei, D.; Fu, Y.; Cui, Y.; Dong, H.; Tan, C.; Qian, W. GWAS, QTL Mapping and Gene Expression Analyses in Brassica napus Reveal Genetic Control of Branching Morphogenesis. Sci. Rep. 2017, 7, 15971. [Google Scholar] [CrossRef]
- Yang, H.; Mohd Saad, N.S.; Ibrahim, M.I.; Bayer, P.E.; Neik, T.X.; Severn-Ellis, A.A.; Pradhan, A.; Tirnaz, S.; Edwards, D.; Batley, J. Candidate Rlm6 Resistance Genes against Leptosphaeria maculans Identified through a Genome-Wide Association Study in Brassica juncea (L.) Czern. Theor. Appl. Genet. 2021, 134, 2035–2050. [Google Scholar] [CrossRef]
- Rahman, M.; Mamidi, S.; del Rio, L.; Ross, A.; Kadir, M.M.; Rahaman, M.M.; Arifuzzaman, M. Association Mapping in Brassica napus (L.) Accessions Identifies a Major QTL for Blackleg Disease Resistance on Chromosome A01. Mol. Breed. 2016, 36, 90. [Google Scholar] [CrossRef]
- Raman, H.; McVittie, B.; Pirathiban, R.; Raman, R.; Zhang, Y.; Barbulescu, D.M.; Qiu, Y.; Liu, S.; Cullis, B. Genome-Wide Association Mapping Identifies Novel Loci for Quantitative Resistance to Blackleg Disease in Canola. Front. Plant Sci. 2020, 11, 559815. [Google Scholar] [CrossRef] [PubMed]
- Cantila, A.Y.; Saad, N.S.M.; Amas, J.C.; Edwards, D.; Batley, J. Recent Findings Unravel Genes and Genetic Factors Underlying Leptosphaeria maculans Resistance in Brassica napus and Its Relatives. Int. J. Mol. Sci. 2020, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.H. Current Status of the Gene-For-Gene Concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Liban, S.H.; Cross, D.J.; Kutcher, H.R.; Peng, G.; Fernando, W.G.D. Race Structure and Frequency of Avirulence Genes in the Western Canadian Leptosphaeria maculans Pathogen Population, the Causal Agent of Blackleg in Brassica Species. Plant Pathol. 2016, 65, 1161–1169. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Zhang, Y.; Saad, N.S.M.; Yang, H.; Sheedy, E.; Elliott, C.E.; Batley, J. Molecular Markers for Identifying Resistance Genes in Brassica napus. Agronomy 2022, 12, 985. [Google Scholar] [CrossRef]
- Raman, R.; Diffey, S.; Barbulescu, D.M.; Coombes, N.; Luckett, D.; Salisbury, P.; Cowley, R.; Marcroft, S.; Raman, H. Genetic and Physical Mapping of Loci for Resistance to Blackleg Disease in Canola (Brassica napus L.). Sci. Rep. 2020, 10, 4416. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Jestin, C.; Welham, S.J.; King, G.J.; Manzanares-Dauleux, M.J.; Fitt, B.D.L.; Delourme, R. Identification of Environmentally Stable QTL for Resistance against Leptosphaeria maculans in Oilseed Rape (Brassica Napus). Theor. Appl. Genet. 2016, 129, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Larkan, N.J.; Raman, H.; Lydiate, D.J.; Robinson, S.J.; Yu, F.; Barbulescu, D.M.; Raman, R.; Luckett, D.J.; Burton, W.; Wratten, N.; et al. Multi-Environment QTL Studies Suggest a Role for Cysteine-Rich Protein Kinase Genes in Quantitative Resistance to Blackleg Disease in Brassica napus. BMC Plant Biol. 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Raman, R.; Diffey, S.; Qiu, Y.; McVittie, B.; Barbulescu, D.M.; Salisbury, P.A.; Marcroft, S.; Delourme, R. Stable Quantitative Resistance Loci to Blackleg Disease in Canola (Brassica napus L.) over Continents. Front. Plant Sci. 2018, 871, 412957. [Google Scholar] [CrossRef]
- Amas, J.; Anderson, R.; Edwards, D.; Cowling, W.; Batley, J. Status and Advances in Mining for Blackleg (Leptosphaeria maculans) Quantitative Resistance (QR) in Oilseed Rape (Brassica napus). Theor. Appl. Genet. 2021, 134, 3123–3145. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Bayer, P.E.; Tirnaz, S.; Hurgobin, B.; Edwards, D.; Batley, J. Characterization of Disease Resistance Genes in the Brassica napus Pangenome Reveals Significant Structural Variation. Plant Biotechnol. J. 2020, 18, 969–982. [Google Scholar] [CrossRef]
- Shen, X.; Song, S.; Li, C.; Zhang, J. Synonymous Mutations in Representative Yeast Genes Are Mostly Strongly Non-Neutral. Nature 2022, 606, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-H.; Zhao, Z.-Z.; He, J.-X. Molecular Sciences Brassinosteroid Signaling in Plant-Microbe Interactions. Int. J. Mol. Sci. 2018, 19, 4091. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the Brassinosteroid Biosynthetic Gene DWF4 in Brassica napus Simultaneously Increases Seed Yield and Stress Tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis thaliana and Brassica napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Reignault, P.; Cogan, A.; Muchembled, J.; Lounes-Hadj Sahraoui, A.; Durand, R.; Sancholle, M. Trehalose Induces Resistance to Powdery Mildew in Wheat. New Phytol. 2001, 149, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, C.; Randoux, B.; Vincent, D.; Bourdon, N.; Reignault, P. Exogenous Trehalose Induces Defenses in Wheat Before and During a Biotic Stress Caused by Powdery Mildew. Phytopathol 2014, 104, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Yu, D. Trehalose Phosphate Synthase 5-Dependent Trehalose Metabolism Modulates Basal Defense Responses in Arabidopsis thaliana. J. Integr. Plant Biol. 2019, 61, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Govind, S.R.; Jogaiah, S.; Abdelrahman, M.; Shetty, H.S.; Tran, L.S.P. Exogenous Trehalose Treatment Enhances the Activities of Defense-Related Enzymes and Triggers Resistance against Downy Mildew Disease of Pearl Millet. Front. Plant Sci. 2016, 7, 227073. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, H.; Wang, X.; Jin, W.; Chen, Q.; Yuan, Z.; Yu, H. Physiological and Transcriptomic Analyses Reveal the Molecular Networks of Responses Induced by Exogenous Trehalose in Plant. PLoS ONE 2019, 14, e0217204. [Google Scholar] [CrossRef]
- Sable, A.R.; Danforth, D.; Profile, S.; Agarwal, S.K. Plant Heat Shock Protein Families: Essential Machinery for Development and Defense. J. Biol. Sci. Med. 2018, 4, 51–64. [Google Scholar]
- Shen, L.; Kang, Y.G.G.; Liu, L.; Yu, H. The J-Domain Protein J3 Mediates the Integration of Flowering Signals in Arabidopsis. Plant Cell 2011, 23, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, X.; Li, J.; Su, X.; Liu, J. Overexpression of a Tobacco J-Domain Protein Enhances Drought Tolerance in Transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 83, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Salas-Muñoz, S.; Rodríguez-Hernández, A.A.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Jiménez-Bremont, J.F. Arabidopsis AtDjA3 Null Mutant Shows Increased Sensitivity to Abscisic Acid, Salt, and Osmotic Stress in Germination and Post-Germination Stages. Front. Plant Sci. 2016, 7, 168225. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Xu, M.Z.; Gao, S.Q.; Zhang, Y.; Yang, H.U.; Peng, J.I.N.; Cai, L.N.; Cheng, Y.; Chen, J.P.; Yang, J.; et al. Genome-Wide Identification and Analysis of the Regulation Wheat DnaJ Family Genes Following Wheat Yellow Mosaic Virus Infection. J. Integr. Agric. 2022, 21, 153–169. [Google Scholar] [CrossRef]
- Wang, G.; Cai, G.; Kong, F.; Deng, Y.; Ma, N.; Meng, Q. Overexpression of Tomato Chloroplast-Targeted DnaJ Protein Enhances Tolerance to Drought Stress and Resistance to Pseudomonas solanacearum in Transgenic Tobacco. Plant Physiol. Biochem. 2014, 82, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yang, J.; Shi, Y.; Wang, X.; Wang, G.L. The DnaJ Protein OsDjA6 Negatively Regulates Rice Innate Immunity to the Blast Fungus Magnaporthe oryzae. Mol. Plant Pathol. 2018, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Haliem, A.M.; Joosten, M.H.A.J. Plant Phosphatidylinositol-Specific Phospholipase C at the Center of Plant Innate Immunity. J. Integr. Plant Biol. 2017, 59, 164–179. [Google Scholar] [CrossRef]
- Vossen, J.H.; Abd-El-Haliem, A.; Fradin, E.F.; Van Den Berg, G.C.M.; Ekengren, S.K.; Meijer, H.J.G.; Seifi, A.; Bai, Y.; Ten Have, A.; Munnik, T.; et al. Identification of Tomato Phosphatidylinositol-Specific Phospholipase-C (PI-PLC) Family Members and the Role of PLC4 and PLC6 in HR and Disease Resistance. Plant J. 2010, 62, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Munnik, T.; Testerink, C. Plant Phospholipid Signaling: “In a Nutshell”. J. Lipid Res. 2009, 50, S260–S265. [Google Scholar] [CrossRef]
- Laxalt, A.M.; Munnik, T. Phospholipid Signalling in Plant Defence. Curr. Opin. Plant Biol. 2002, 5, 332–338. [Google Scholar] [CrossRef]
- Mansouripour, S.; Oladzad, A.; Shahoveisi, F.; Rahman, M.M.; del Río Mendoza, L.E.; Mamidi, S.; Moghaddam, S.M. Identification of Genomic Regions Associated with Resistance to Blackleg (Leptosphaeria maculans) in Canola Using Genome Wide Association Study. Eur. J. Plant Pathol. 2021, 161, 693–707. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Coombes, N.; Song, J.; Diffey, S.; Kilian, A.; Lindbeck, K.; Barbulescu, D.M.; Batley, J.; Edwards, D.; et al. Genome-Wide Association Study Identifies New Loci for Resistance to Leptosphaeria maculans in Canola. Front. Plant Sci. 2016, 7, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Paillard, S.; Fopa-Fomeju, B.; Falentin, C.; Deniot, G.; Baron, C.; Vallée, P.; Manzanares-Dauleux, M.J.; Delourme, R. Multi-Year Linkage and Association Mapping Confirm the High Number of Genomic Regions Involved in Oilseed Rape Quantitative Resistance to Blackleg. Theor. Appl. Genet. 2018, 131, 1627–1643. [Google Scholar] [CrossRef]
- Fikere, M.; Barbulescu, D.M.; Malmberg, M.M.; Spangenberg, G.C.; Cogan, N.O.I.; Daetwyler, H.D. Meta-Analysis of GWAS in Canola Blackleg (Leptosphaeria maculans) Disease Traits Demonstrates Increased Power from Imputed Whole-Genome Sequence. Sci. Rep. 2020, 10, 14300. [Google Scholar] [CrossRef] [PubMed]

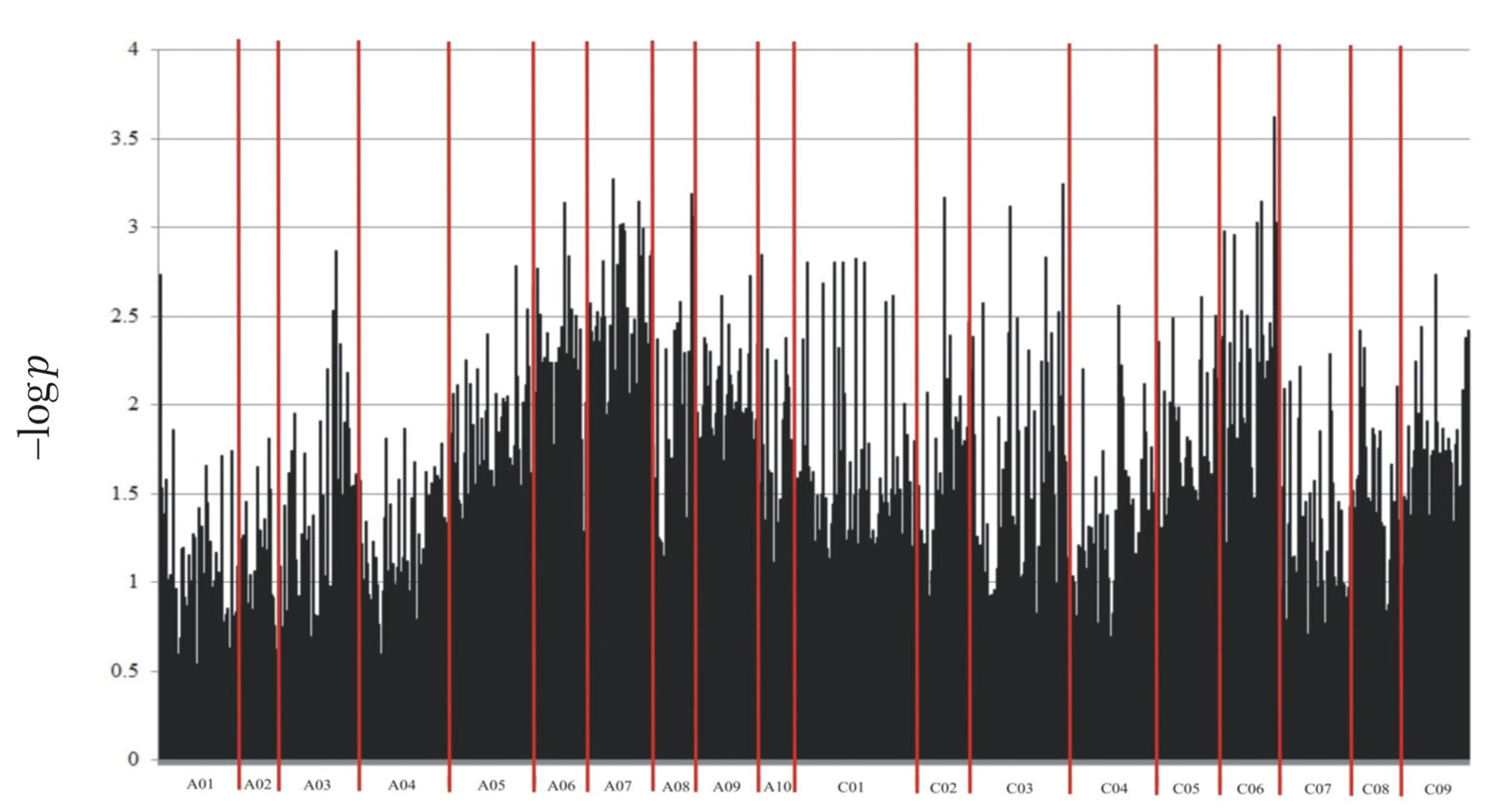
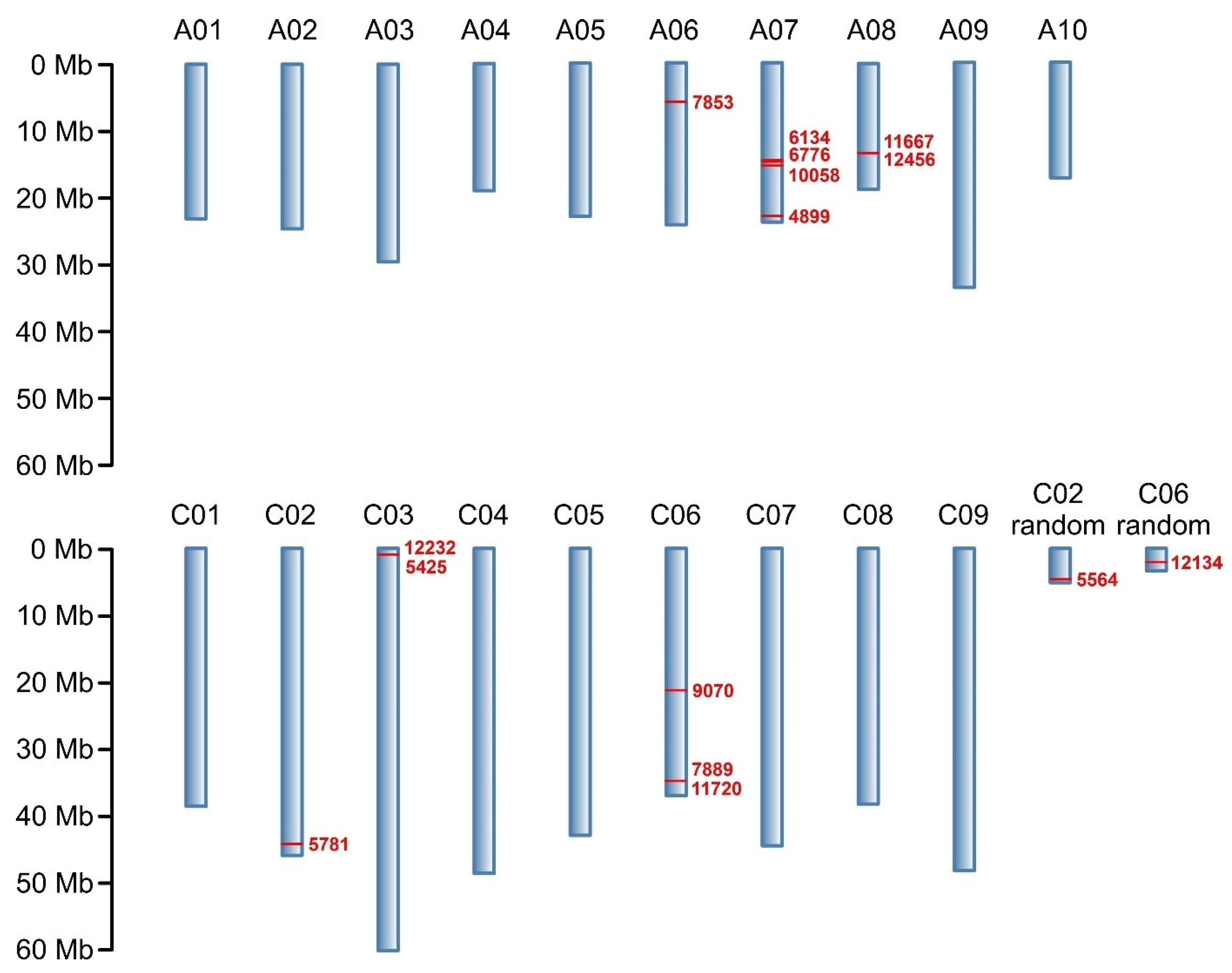
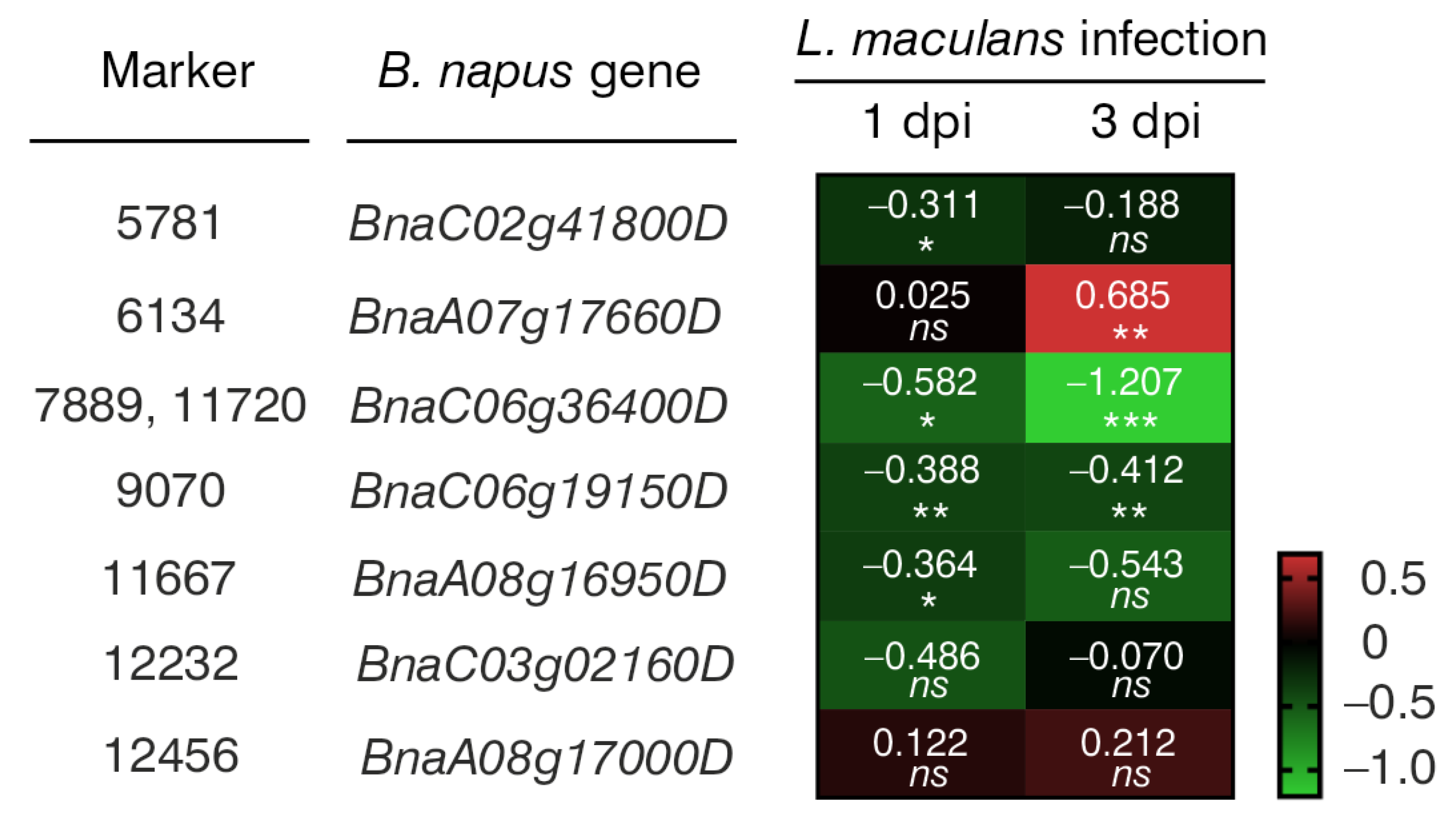
| Marker | Marker Type | Chromosome | DNA Strand | Marker Position on Chromosome (bp) | Marker Sequence * |
|---|---|---|---|---|---|
| 4899 | SilicoDArT | A07 | Minus | 23,194,012–23,193,943 | TGCAGGTTCTAAAAGAGTTTGAGTGGCGATCTGGTTTGGCAATGAGTGTGCAAAAAAGCTCCTTCTATG |
| 5425 | SilicoDArT | C03 | Plus | 1,036,696–1,036,759 | TGCAGTCATGAAGGAACTTATCTTGAACTGCTTCAGTGCTTTGTATTCAATAATGAATTTTAC |
| 5564 | SilicoDArT | C02 random | Minus | 4,895,158–4,895,089 | TGCAGCTCACGTTGAGCTTCAAATTCTACGATCCAGATCCACACCACAATCTAGATCGATCATTTCCAA |
| 5781 | SilicoDArT | C02 | Minus | 44,607,251–44,607,182 | TGCAGTAATTCCAACGTCCATCCTTCAGATCCAATGCCTTCATCTCAAGGTATTTTATTACTAATTTTG |
| 6134 | SilicoDArT | A07 | Minus | 14,719,379–14,719,339 | TGCAGGAGAAGGGAAAATCAAAACTTCAATCCTTACTTAC |
| 6776 | SilicoDArT | A07 | Plus | 14,959,860–14,959,929 | TGCAGCCACGACTGCAAATTTATCTGTTCGGATCAACGAGCCTAAGTCCAAACCTATTTTCTTGATTGG |
| 7853 | SilicoDArT | A06 | Plus | 5,968,268–5,968,337 | TGCAGAAAATGGAATGTTCTTGAGAGATCCTAGTGGAGAATGGGTGACAAATATGCCTCAAGACATGAA |
| 7889 | SilicoDArT | C06 | Plus | 34,959,583–34,959,652 | TGCAGAAGCAGCCATGAGACAGTATTGCTGTTGAGATATATTGTTGCTGTACCTTGGGGAGGAAGCAAC |
| 9070 | SilicoDArT | C06 | Plus | 21,498,041–21,498,110 | TGCAGAATGCCAGGCTAAGTTGAGAGAAGAGAATCCAGGAAATGCACTCCTTGAGGTATATTCATATTC |
| 10058 | SNP (38:G>A) | A07 | Plus | 15,507,858–15,507,927 | TGCAGTTGCACTTTCTCTTTCCAGGAGCTGGTTTTATAGCATTCTTCTCCCTCCATACCTGATAAATCA |
| 11667 | SNP (38:G>A) | A08 | Minus | 13,628,583–13,628,514 | TGCAGCTTGTCAGCACATCCTCTCTTCACACACTCAATGATAAACACCTTGGCGTAACAATGAATCCGG |
| 11720 | SNP (18:A>T) | C06 | Plus | 34,959,583–34,959,652 | TGCAGAAGCAGCCATGAGACAGTATTGCTGTTGAGATATATTGTTGCTGTACCTTGGGGAGGAAGCAAC |
| 12134 | SNP (29:G>A) | C06 random | Minus | 2,170,846–2,170,777 | TGCAGCTTCTACTTTTAGTTGGACAGAGCGCTCAAAGTCAACAATTACAGATCGGAAGAGCGGTTCAGC |
| 12232 | SNP (46:T>A) | C03 | Plus | 1,024,177–1,024,246 | TGCAGAAAAAGATTCAGGTTCCCGGGACCTGAAGATCACTGGATTGTCTGATGCTGTGTTAGGATGCAT |
| 12456 | SNP (45:A>C) | A08 | Plus | 13,650,920–13,650,989 | TGCAGTTTCTACACGTACATATCCAATATTTTAGTTTACTTAGGAAGAAATTTGAAATTTGATTTTATT |
| Marker | Candidate Genes |
|---|---|
| 4899 | Sequence localized between BnaA07g33940D (5704 bp from START codon) and BnaA07g33950D (2160 bp from STOP codon) |
| 5425 | Sequence localized between BnaC03g02170D (2456 bp from STOP codon) and BnaC03g02180D (550 bp from START codon) |
| 5564 | Sequence localized between BnaC02g48660D (6400 bp from START codon) and BnaC02g48670D (3627 bp from START codon) |
| 5781 | Sequence localized within BnaC02g41800D (from 2nd exon to 2nd intron) |
| 6134 | Sequence localized within BnaA07g17660D (1st intron) |
| 6776 | Sequence localized within 16th (last) exon of BnaA07g18190D |
| 7853 | Sequence localized within 13th exon of BnaA06g11460D |
| 7889 | Sequence localized within 12th (last) exon of BnaC06g36400D |
| 9070 | Sequence localized within 4th exon and 4th intron of BnaC06g19150D |
| 10058 | SNP localized within 5th exon of BnaA07g19340D |
| 11667 | SNP localized within the only exon of BnaA08g16950D |
| 11720 | SNP localized within the 12th (last) exon of BnaC06g36400D |
| 12134 | SNP localized between BnaC06g42650D (1255 bp from START codon) and BnaC06g42660D (33,148 bp from STOP codon) |
| 12232 | SNP localized within the 9th exon of BnaC03g02160D |
| 12456 | SNP localized within the 1st intron of BnaA08g17000D |
| B. napus Gene | A. thaliana Orthologue | Protein Encoded by A. thaliana Orthologue | Protein Function |
|---|---|---|---|
| BnaC02g41800D | At5g08130 | BES1-INTERACTING MYC-LIKE1, BIM1 | Transcription factor Brassinosteroid signaling |
| BnaA07g17660 | At3g58180 | ARM repeat superfamily protein | Deoxyhypusine monooxygenase activity |
| BnaA07g18190 | At4g37950 | Rhamnogalacturonate lyase family protein | Enables carbohydrate binding, enables lyase activity, enables rhamnogalacturonan activity |
| BnaA06g11460 | At1g16980 | Trehalose-6-phosphate synthase | Enzyme putatively involved in trehalose biosynthesis |
| BnaC06g36400D | At1g75730 | Hypothetical protein | --- |
| BnaC06g19150D | At1g80380 | p-loop containing nucleoside triphosphate hydrolases superfamily protein | Enables ATP binding, enables glycerate kinase activity, enables kinase activity |
| BnaA07g19340 | At3g62600 | DNAJ heat shock family protein | Enables Hsp70 protein binding, enables unfolded protein binding |
| BnaA08g16950 | At4g38690 | PLC-like phosphodiesterases superfamily protein | Enables phosphoric diester hydrolase activity |
| BnaC03g02160D | At5g05560 | E3 ubiquitin ligase | Encodes a subunit of the A. thaliana E3 ubiquitin ligase complex that plays a synergistic role with APC4 both in female gametogenesis and in embryogenesis |
| BnaA08g17000 | At4g38600 | HECT ubiquitin protein ligase family protein KAK | Enables ubiquitin protein ligase activity, enables ubiquitin-protein transferase activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starosta, E.; Jamruszka, T.; Szwarc, J.; Bocianowski, J.; Jędryczka, M.; Grynia, M.; Niemann, J. DArTseq-Based, High-Throughput Identification of Novel Molecular Markers for the Detection of Blackleg (Leptosphaeria Spp.) Resistance in Rapeseed. Int. J. Mol. Sci. 2024, 25, 8415. https://doi.org/10.3390/ijms25158415
Starosta E, Jamruszka T, Szwarc J, Bocianowski J, Jędryczka M, Grynia M, Niemann J. DArTseq-Based, High-Throughput Identification of Novel Molecular Markers for the Detection of Blackleg (Leptosphaeria Spp.) Resistance in Rapeseed. International Journal of Molecular Sciences. 2024; 25(15):8415. https://doi.org/10.3390/ijms25158415
Chicago/Turabian StyleStarosta, Ewa, Tomasz Jamruszka, Justyna Szwarc, Jan Bocianowski, Małgorzata Jędryczka, Magdalena Grynia, and Janetta Niemann. 2024. "DArTseq-Based, High-Throughput Identification of Novel Molecular Markers for the Detection of Blackleg (Leptosphaeria Spp.) Resistance in Rapeseed" International Journal of Molecular Sciences 25, no. 15: 8415. https://doi.org/10.3390/ijms25158415
APA StyleStarosta, E., Jamruszka, T., Szwarc, J., Bocianowski, J., Jędryczka, M., Grynia, M., & Niemann, J. (2024). DArTseq-Based, High-Throughput Identification of Novel Molecular Markers for the Detection of Blackleg (Leptosphaeria Spp.) Resistance in Rapeseed. International Journal of Molecular Sciences, 25(15), 8415. https://doi.org/10.3390/ijms25158415










