Time-Course Analysis and Transcriptomic Identification of a Group III ERF CmTINY2 Involved in Waterlogging Tolerance in Chrysanthemums × morifolium Ramat.
Abstract
1. Introduction
2. Results
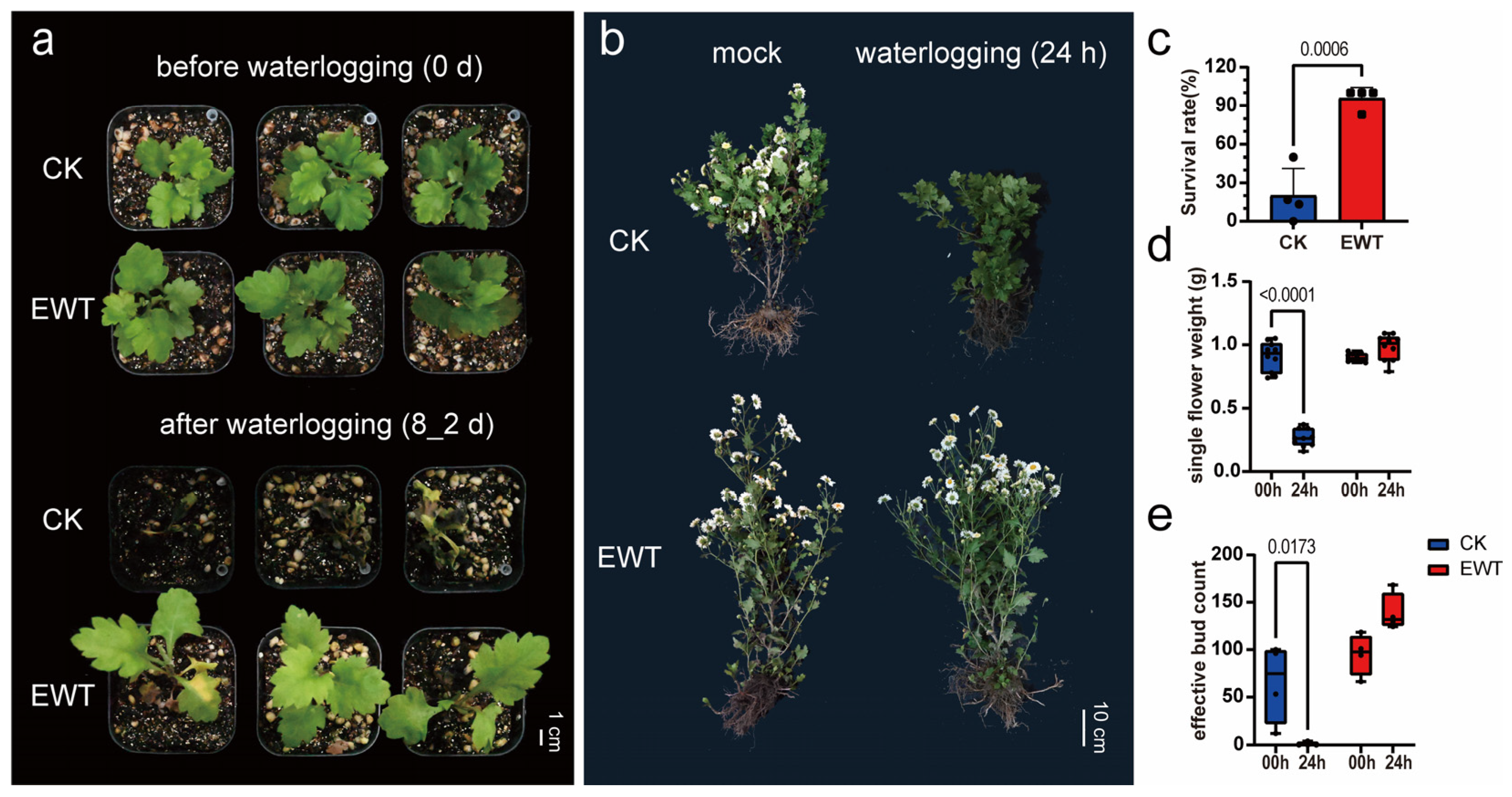
2.1. EWT Showed Greater Tolerance to Waterlogging Than Conventional Variety
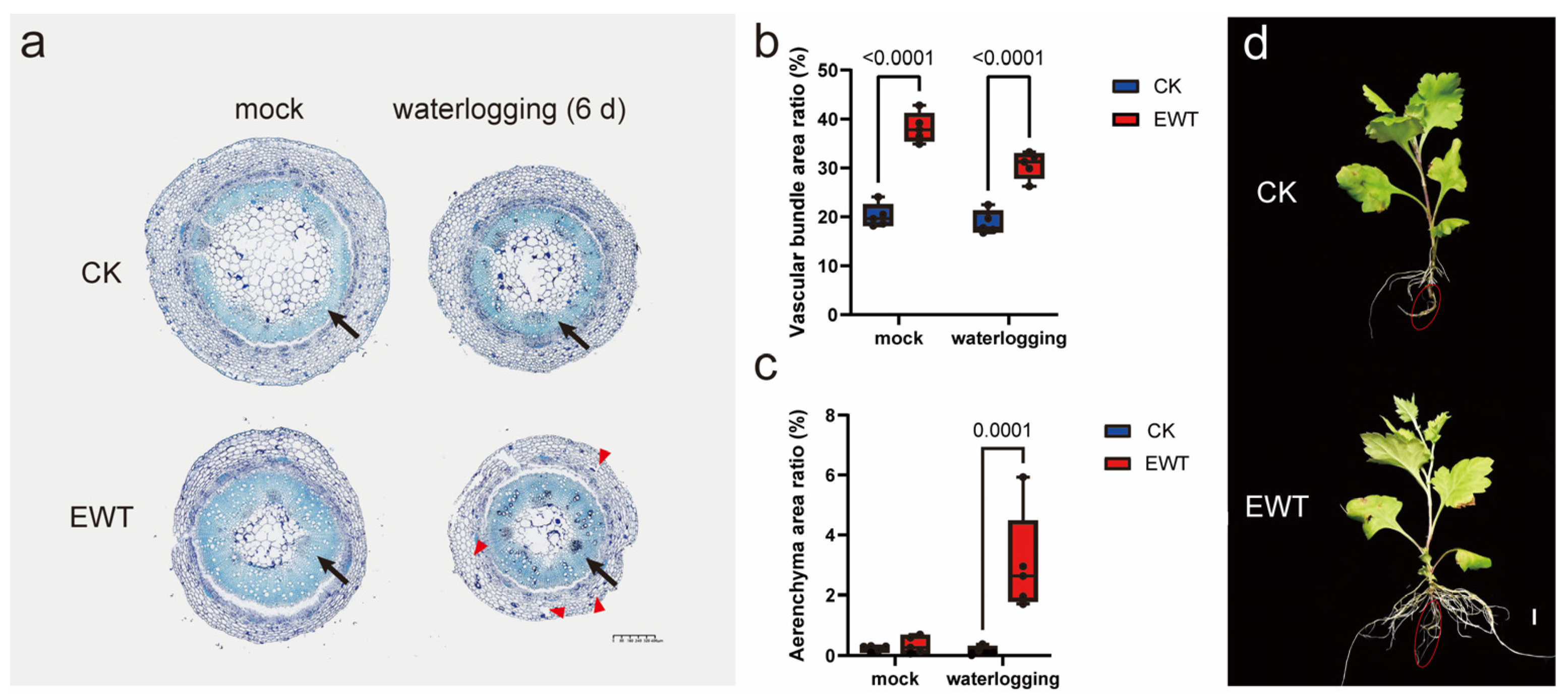
2.2. EWT Exhibited a Well-Developed Aeration Tissue Structure
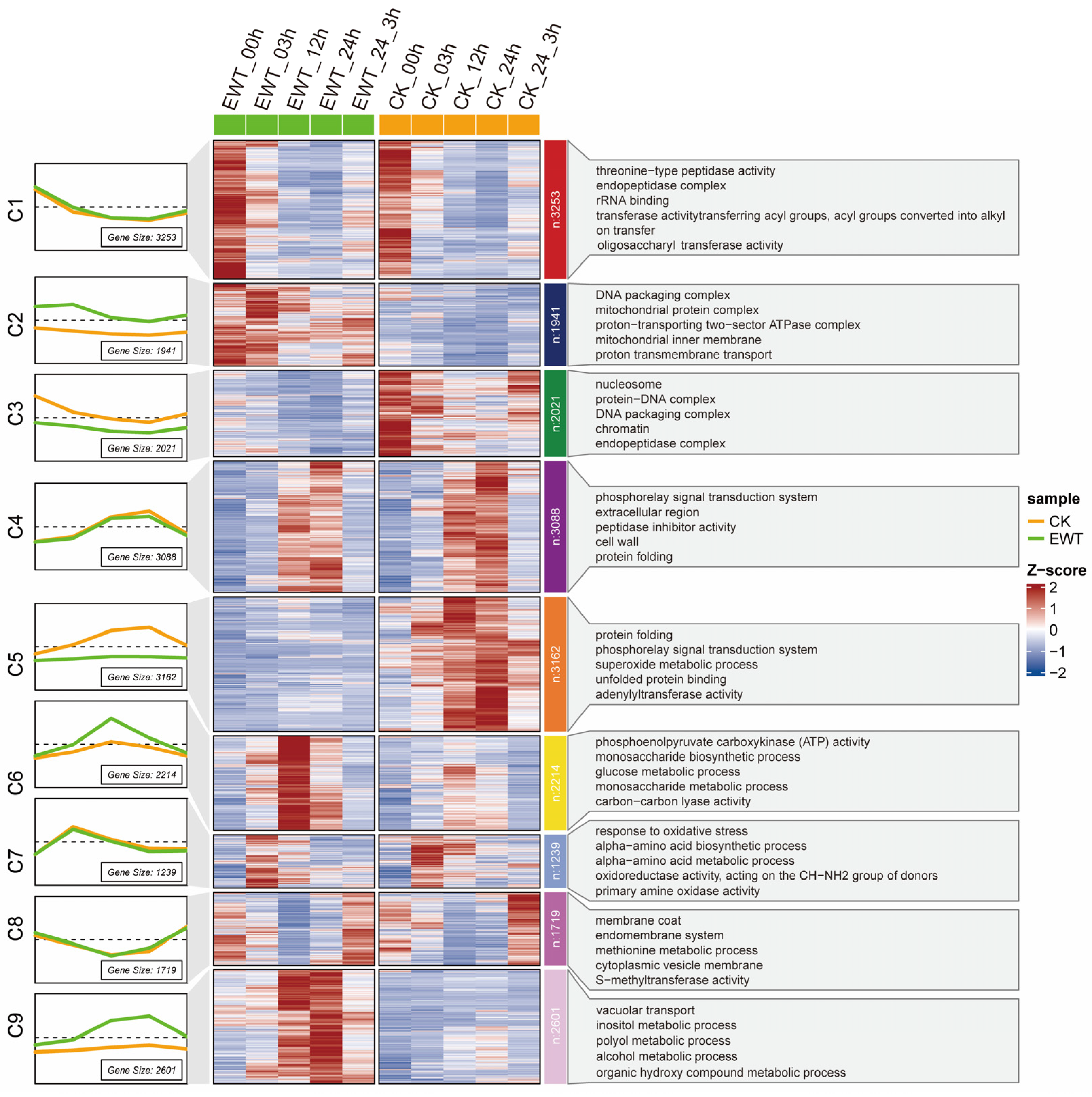
2.3. Transcriptome Profiles of EWT and CK Exposed to Waterlogging Treatment
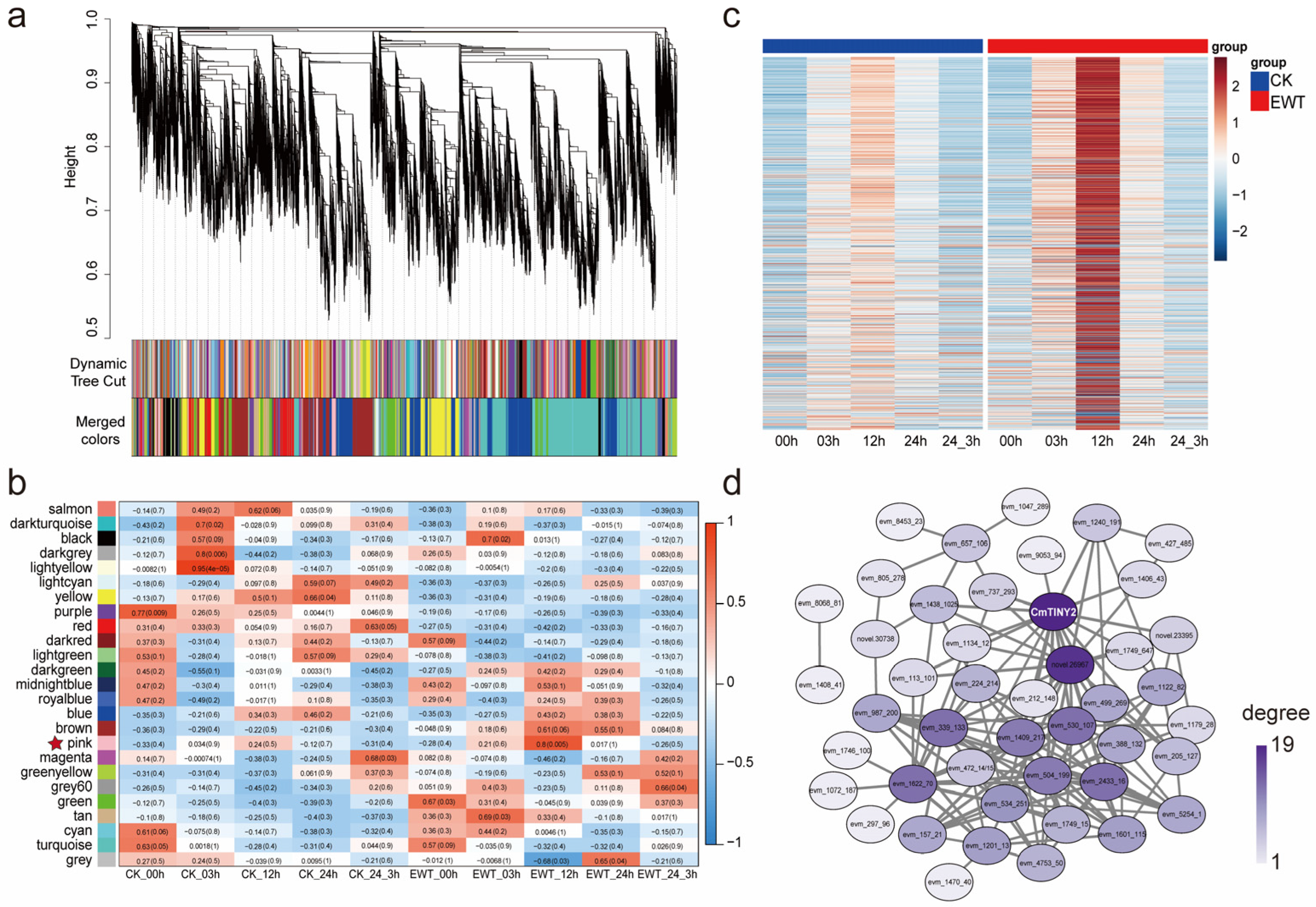
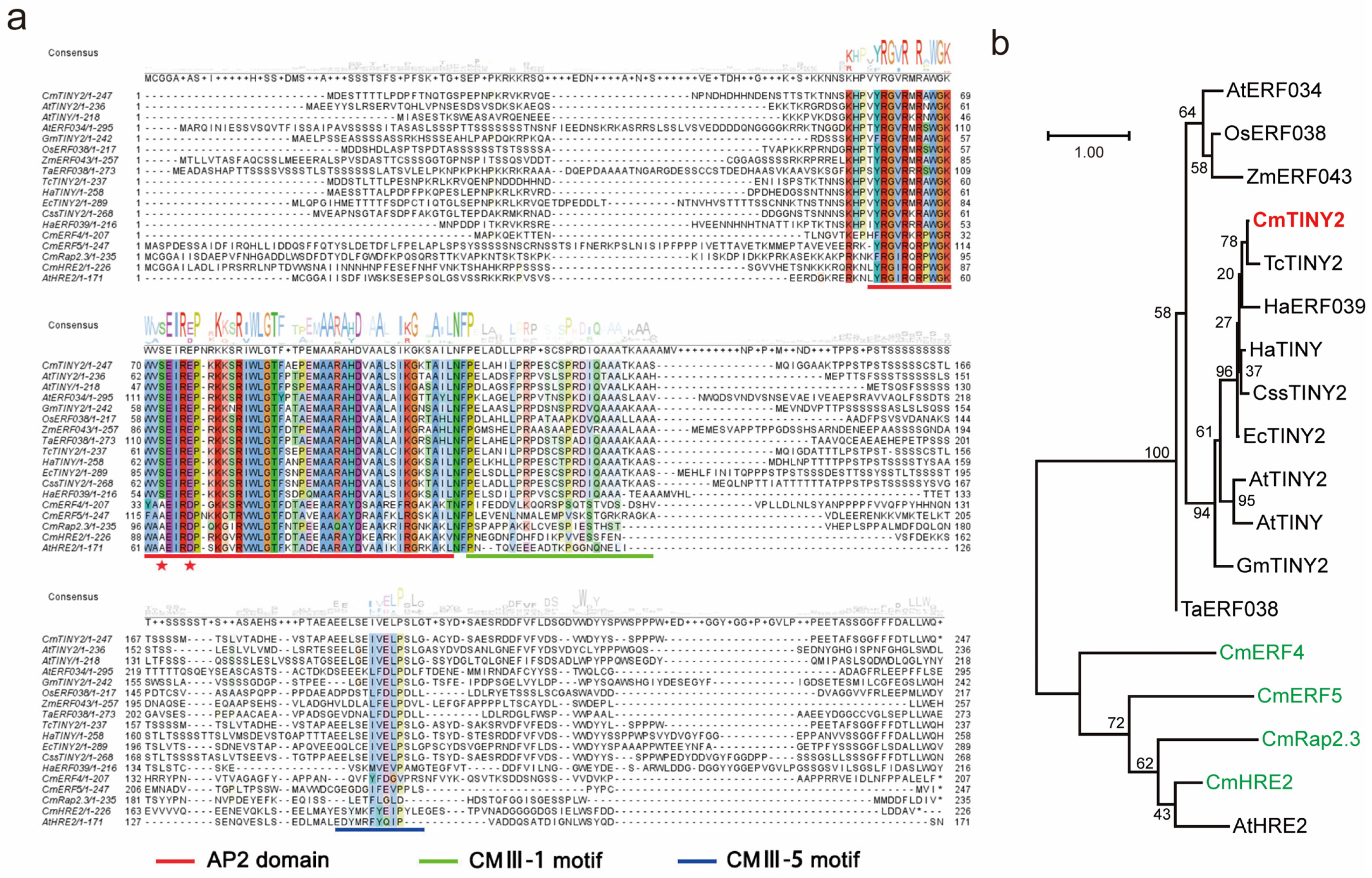
2.4. Mining the Waterlogging Tolerance Regulator Using WGCNA Analysis
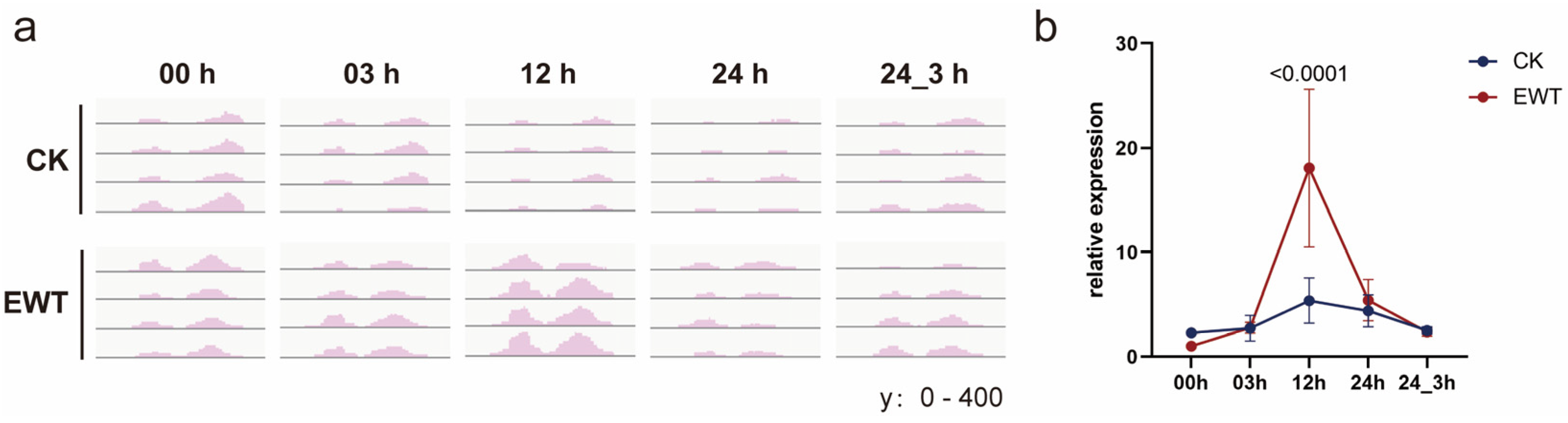
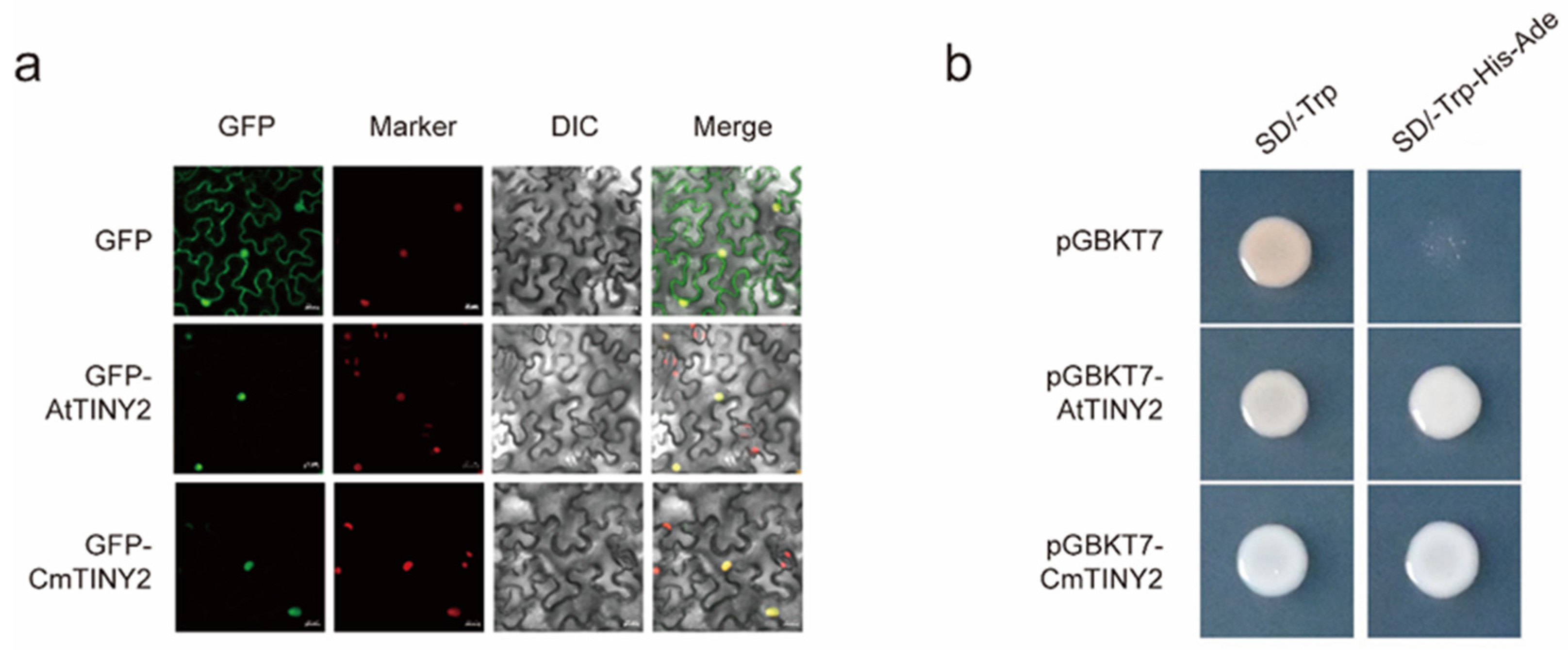
2.5. CmTINY2 Is a Transcriptional Activator of DREB Subfamily
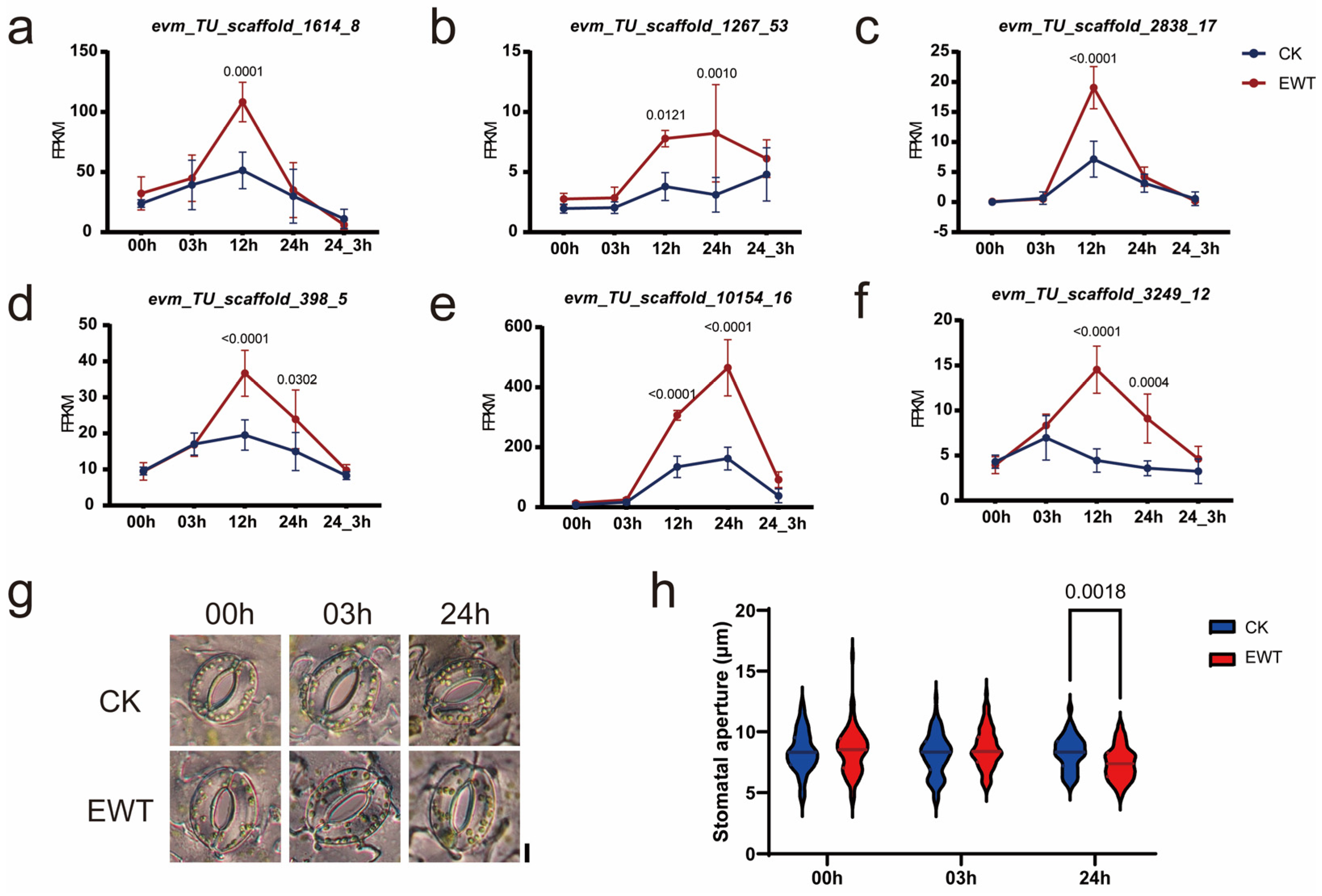
2.6. CmTINY2 Influenced Waterlogging Tolerance Probably through Stomatal Regulation
3. Discussion
3.1. EWT Shows Excellent Waterlogging Tolerance
3.2. CmTINY2 Is an ERF Transcription Factor Induced by Waterlogging Treatment
3.3. CmTINY2 Might Be Involved in the Regulation of Stomatal Movement in EWT
4. Materials and Methods
4.1. Plant Materials
4.2. Waterlogging Treatment of Chrysanthemums
4.3. RNA Extraction, RNA-Seq Library Construction, and Sequencing
4.4. RNA-Seq Data Analysis
4.5. Fluorescence Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.6. Isolation and Sequence Analysis
4.7. Subcellular Localization
4.8. Transcriptional Activity Analysis
4.9. Microscopic Observation
4.10. Statistical Analysis
4.11. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; pp. 323–324. Available online: https://ydz.chp.org.cn/#/main (accessed on 23 July 2024).
- Dai, S.; Wen, X. Efficacy of the medicinal and food chrysanthemum. Chin. Bull. Life Sci. 2015, 27, 1083–1090. (In Chinese) [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.S. Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med. 2005, 71, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lii, C.K.; Lei, Y.P.; Yao, H.T.; Hsieh, Y.S.; Tsai, C.W.; Liu, K.L.; Chen, H.W. Chrysanthemum morifolium Ramat. reduces the oxidized LDL-induced expression of intercellular adhesion molecule-1 and E-selectin in human umbilical vein endothelial cells. J. Ethnopharmacol. 2010, 128, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hao, L.J.; Zhu, J.J.; Zhang, Q.W.; Wang, Z.M.; Zhang, X.; Song, X.M. Study on the effects of sulfur fumigation on chemical constituents and antioxidant activity of Chrysanthemum morifolium cv. Hang-ju. Phytomedicine 2014, 21, 773–779. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Su, J.; Guan, Z.; Fang, W.M.; Chen, F.D.; Zhang, F. Comprehensive evaluation of tea chrysanthemum’s drought and waterlogging tolerance at seedling stage. Acta Hortic. Sin. 2021, 48, 2443–2457. [Google Scholar] [CrossRef]
- Sun, C. Analysis of the impact of flood disasters on agricultural production in the past thirty years. Farm. Econ. Manag. 2024, 1, 33–35. (In Chinese) [Google Scholar]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2020, 11, 627331. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.; Fang, W. Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.; Fang, W. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Su, J.; Li, W.; Tang, Y.; Zhao, N.; Lou, L.; Ou, X.; Jia, D.; Jiang, J.; et al. A group VIIIa ethylene-responsive factor, CmERF4, negatively regulates waterlogging tolerance in chrysanthemum. J. Exp. Bot. 2023, 75, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Su, J.S.; Zhao, N.; Lou, L.; Ou, X.; Yan, Y.; Wang, L.; Jiang, J.; Chen, S.; Chen, F. CmERF5-CmRAP2.3 transcriptional cascade positively regulates waterlogging tolerance in Chrysanthemum morifolium. Plant Biotechnol. J. 2023, 21, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Zhang, Z.; Liu, W.; Cheng, P.; Mu, W.; Su, T.; Chen, S.; Chen, F.; Jiang, J. Overexpression of CmSOS1 confers waterlogging tolerance in Chrysanthemum. J. Integr. Plant Biol. 2020, 62, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, C.; Yan, Y.; Wang, H.; Wang, L.; Jiang, J.; Chen, S.; Chen, F. The transcriptional coactivator CmMBF1c is required for waterlogging tolerance in Chrysanthemum morifolium. Hortic. Res. 2022, 9, uhac215. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. MaRAP2-4, a waterlogging-responsive ERF from Mentha, regulates bidirectional sugar transporter AtSWEET10 to modulate stress response in Arabidopsis. Plant Biotechnol. J. 2018, 16, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dossa, K.; You, J.; Zhang, Y.; Li, D.; Zhou, R.; Yu, J.; Wei, X.; Zhu, X.; Jiang, S.; et al. High-resolution temporal transcriptome sequencing unravels ERF and WRKY as the master players in the regulatory networks underlying sesame responses to waterlogging and recovery. Genomics 2021, 113, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, C.; Yan, Y.; Cao, W.; Song, A.; Wang, H.; Chen, S.; Jiang, J.; Chen, F. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Waterlogging-Tolerant Chrysanthemum morifolium Cultivars under Waterlogging Stress and Reoxygenation Conditions. Int. J. Mol. Sci. 2018, 19, 1455. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Guo, Q.; Zhang, X.M.; Song, L.S. Effects of flood stress on anthocyanins synthesis with relative enzymes and genes of Chrysanthemum morifolium cv. ‘Hangju’. China J. Chin. Mater. Medica 2016, 42, 1847–1852. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, T.; Guo, Q.S.; Zou, Q.; Yang, F.; Lu, D.; Liu, J. Effect of soil moisture regimes in the early flowering stage on inflorescence morphology and medicinal ingredients of Chrysanthemum morifolium Ramat. Cv. ‘Hangju’. Sci. Hortic. 2020, 260, 108849. [Google Scholar] [CrossRef]
- Song, A.; Su, J.; Wang, H.; Zhang, Z.; Zhang, X.; Van de Peer, Y.; Chen, F.; Fang, W.; Guan, Z.; Zhang, F.; et al. Analyses of a chromosome-scale genome assembly reveal the origin and evolution of cultivated chrysanthemum. Nat. Commun. 2023, 14, 2021. [Google Scholar] [CrossRef]
- Sun, S.; Yu, J.P.; Chen, F.; Zhao, T.-J.; Fang, X.-H.; Li, Y.-Q.; Sui, S.-F. TINY, a dehydration-responsive element (DRE)-binding protein-like transcription factor connecting the DRE- and ethylene-responsive element-mediated signaling pathways in Arabidopsis. J. Biol. Chem. 2008, 283, 6261–6271. [Google Scholar] [CrossRef]
- Wei, G.; Pan, Y.; Lei, J.; Zhu, Y.-X. Molecular cloning, phylogenetic analysis, expressional profiling and in vitro studies of TINY2 from Arabidopsis thaliana. J. Biochem. Mol. Biol. 2005, 38, 440–446. [Google Scholar] [CrossRef]
- Nakata, M.T.; Sakamoto, S.; Nuoendagula; Kajita, S.; Mitsuda, N. Fiber cell-specific expression of the VP16-fused ethylene response factor 41 protein increases biomass yield and alters lignin composition. Front. Plant Sci. 2021, 12, 654–655. [Google Scholar] [CrossRef]
- Hsu, P.K.; Takahashi, Y.; Merilo, E.; Costa, A.; Zhang, L.; Kernig, K.; Lee, K.H.; Schroeder, J.I. Raf-like kinases and receptor-like (pseudo)kinase GHR1 are required for stomatal vapor pressure difference response. Proc. Natl. Acad. Sci. USA 2021, 118, e2107280118. [Google Scholar] [CrossRef]
- van Veen, H.; Akman, M.; Jamar, D.C.; Vreugdenhil, D.; Kooiker, M.; van Tienderen, P.; Voesenek, L.A.C.J.; Schranz, M.E.; Sasidharan, R. Group VII ethylene response factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ. 2014, 37, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Waters, I.; Kuiper, P.J.C.; Watkin, E.; Greenway, H. Effects of Anoxia on Wheat Seedlings. J. Exp. Bot. 1991, 42, 1427–1435. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.G.; Jia, L.T.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2021, 229, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.L.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef]
- Xie, Z.L.; Nolan, R.O.R.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; Yin, Y. The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef]
- Pan, Y.; Seymour, G.B.; Lu, C.G.; Hu, Z.; Chen, X.; Chen, G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012, 31, 349–360. [Google Scholar] [CrossRef]
- Jin, X.Y.; Yin, X.F.; Ndayambaza, B.; Zhang, Z.; Min, X.; Lin, X.; Wang, Y.; Liu, W. Genome-wide identification and expression profiling of the ERF gene family in Medicago sativa L. under various abiotic stresses. DNA Cell Biol. 2019, 38, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Zhang, Z.G.; Luo, H.L. Anatomical responses to waterlogging in Chrysanthemum zawadskii. Sci. Hortic. 2012, 146, 86–91. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Yuan, P.; Song, C.; Song, S.; Jiao, J.; Wang, M.; Hao, P.; Zheng, X.; Bai, T. Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks. Int. J. Mol. Sci. 2023, 24, 9298. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Du, X.; Mao, B. Production of virus-free chrysanthemum (Chrysanthemum morifolium Ramat) by tissue culture techniques. Methods Mol. Biol. 2022, 2400, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-tasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue o. Stain. Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Liu, X.; Sha, H.; Du, X.; Zhang, H.; Miao, Y.; Chen, W.; Mao, B. Time-Course Analysis and Transcriptomic Identification of a Group III ERF CmTINY2 Involved in Waterlogging Tolerance in Chrysanthemums × morifolium Ramat. Int. J. Mol. Sci. 2024, 25, 8417. https://doi.org/10.3390/ijms25158417
Gu X, Liu X, Sha H, Du X, Zhang H, Miao Y, Chen W, Mao B. Time-Course Analysis and Transcriptomic Identification of a Group III ERF CmTINY2 Involved in Waterlogging Tolerance in Chrysanthemums × morifolium Ramat. International Journal of Molecular Sciences. 2024; 25(15):8417. https://doi.org/10.3390/ijms25158417
Chicago/Turabian StyleGu, Xueting, Xinyi Liu, Haodong Sha, Xuejie Du, Han Zhang, Yuexiao Miao, Weiliang Chen, and Bizeng Mao. 2024. "Time-Course Analysis and Transcriptomic Identification of a Group III ERF CmTINY2 Involved in Waterlogging Tolerance in Chrysanthemums × morifolium Ramat." International Journal of Molecular Sciences 25, no. 15: 8417. https://doi.org/10.3390/ijms25158417
APA StyleGu, X., Liu, X., Sha, H., Du, X., Zhang, H., Miao, Y., Chen, W., & Mao, B. (2024). Time-Course Analysis and Transcriptomic Identification of a Group III ERF CmTINY2 Involved in Waterlogging Tolerance in Chrysanthemums × morifolium Ramat. International Journal of Molecular Sciences, 25(15), 8417. https://doi.org/10.3390/ijms25158417





