α-IRAK-4 Suppresses the Activation of RANK/RANKL Pathway on Macrophages Exposed to Endodontic Microorganisms
Abstract
:1. Introduction
2. Results
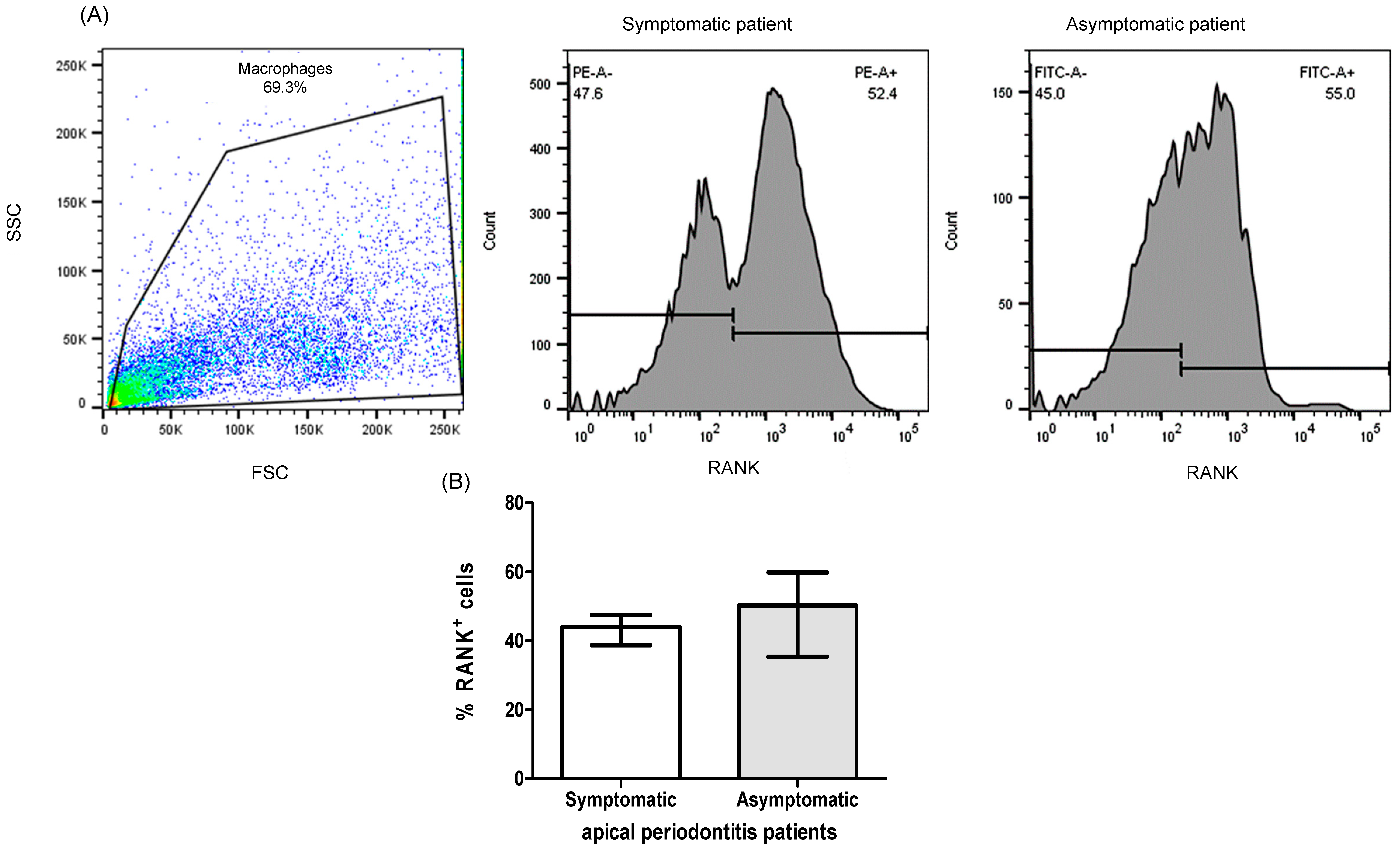
2.1. Patients
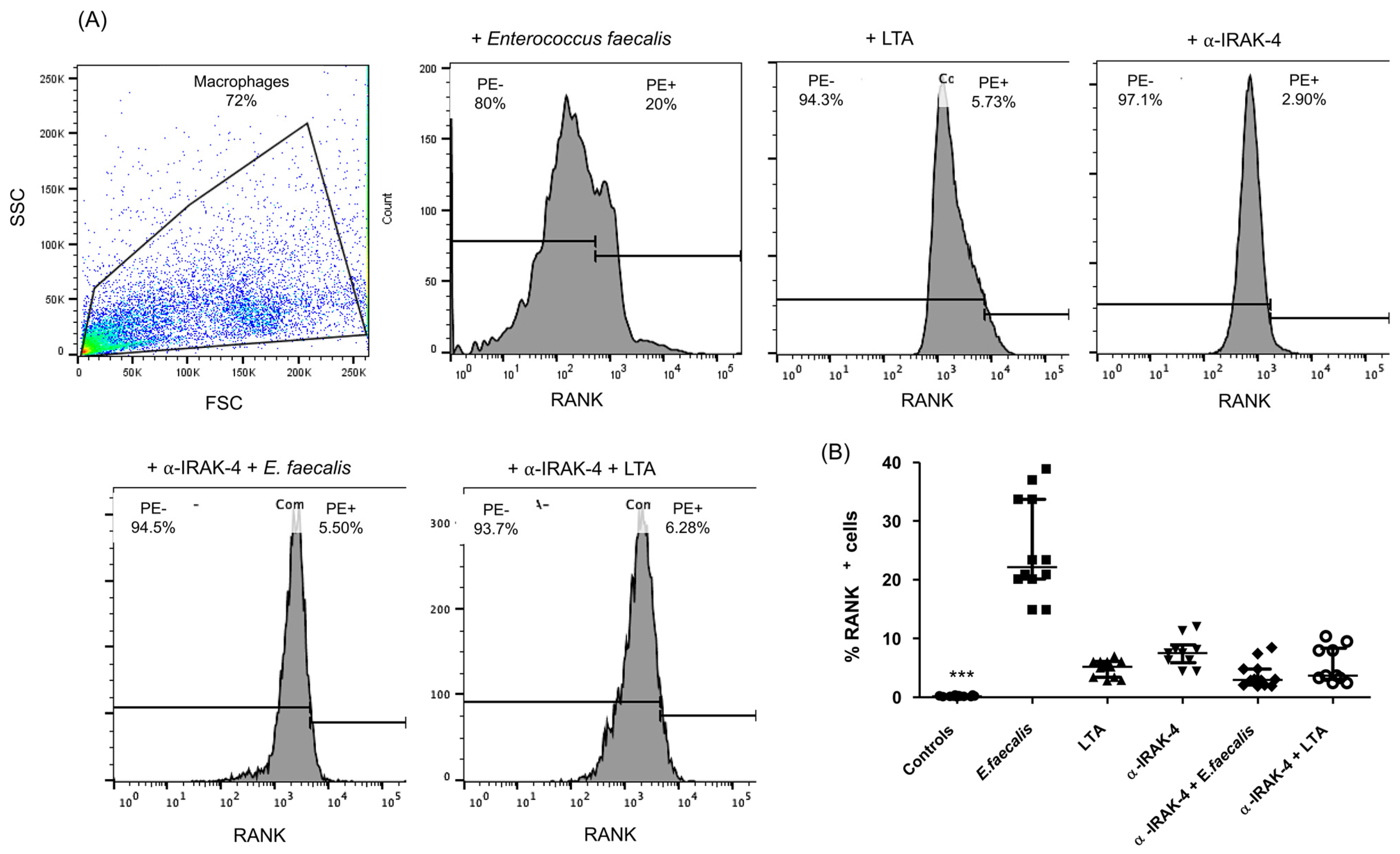
2.2. Expression of RANK+ Cells of THP-1 Macrophages
2.3. Expression of RANK+, RANKL+, OPG+ Cells of PBMC from Patients with Apical Periodontitis
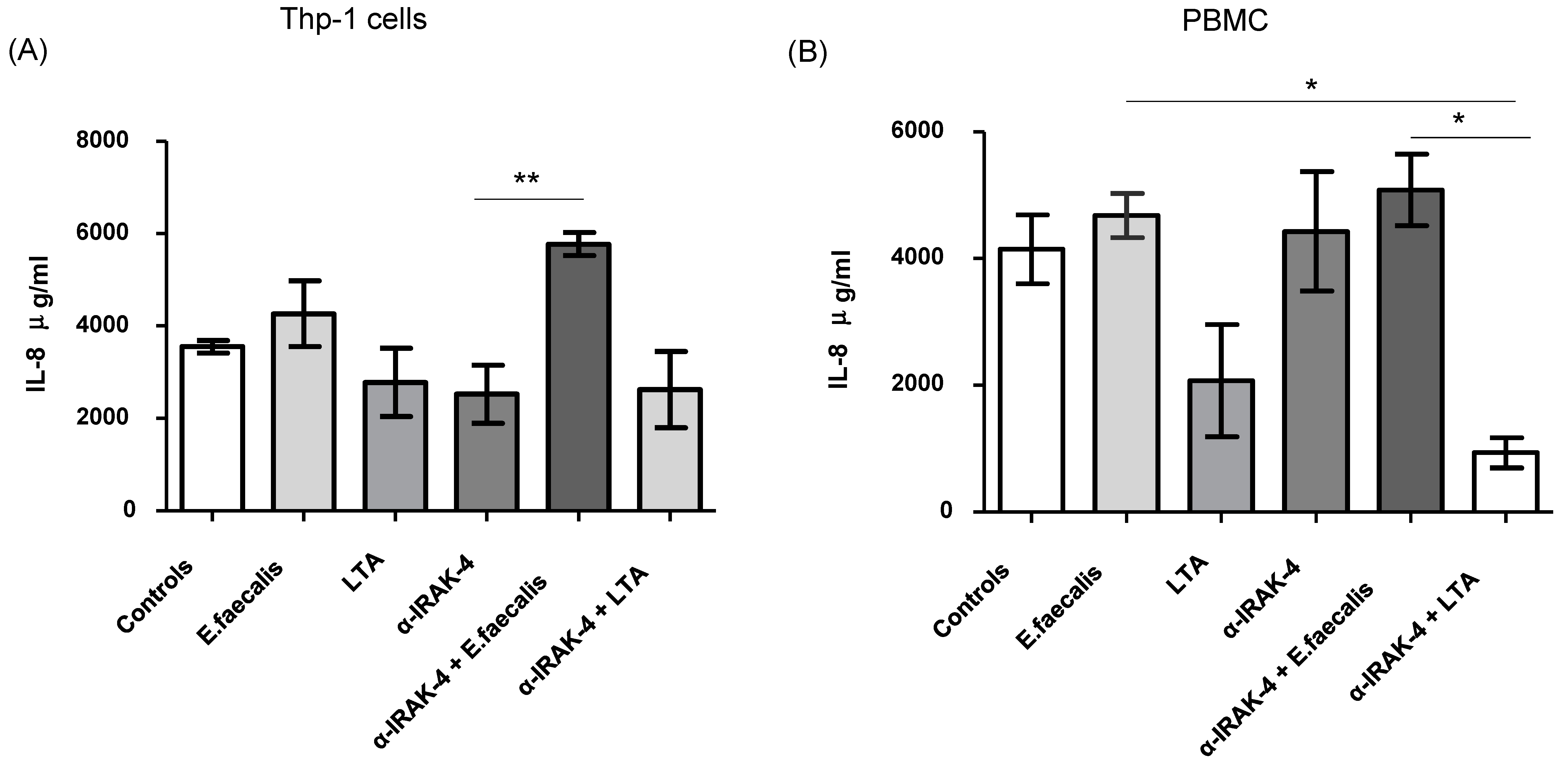
2.4. Quantification of Inflammatory Cytokines
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lina, R.M.; Sindhu, R.; Bharathwaj, V.V.; Dhamodhar, D.; Sathiyapriya, S.; Prabu, D.; Rajmohan, M. Systematic Review on Copper Calcium Hydroxide Nanoparticles in the Treatment of Apical Periodontitis. J. Pharm. Bioallied Sci. 2023, 15, S105–S109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Hu, J.; Chen, J.; Zhou, W. High-performing cross-dataset machine learning reveals robust microbiota alteration in secondary apical periodontitis. Front. Cell. Infect. Microbiol. 2024, 14, 1393108. [Google Scholar] [CrossRef] [PubMed]
- Elashiry, M.M.; Bergeron, B.E.; Tay, F.R. Enterococcus faecalis in secondary apical periodontitis: Mechanisms of bacterial survival and disease persistence. Microb. Pathog. 2023, 183, 106337. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, E.P.; Sampoerno, G.; Juniarti, D.E.; Cahyani, F.; Saraswati, W.; Kuntjoro, M.; Tjendronegoro, E. Effect of Lipopolysaccharide-Induced Apical Periodontitis in Diabetes Mellitus Rats on Periapical Inflammation. Eur. J. Dent. 2023, 17, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Seneviratne, C.J.; Li, X.; Cheung, G.S.; Jin, L.; Chu, C.H.; Zhang, C. Lipoteichoic acid from an Enterococcus faecalis clinical strain promotes TNF-α expression through the NF-κB and p38 MAPK signaling pathways in differentiated THP-1 macrophages. Biomed. Rep. 2015, 3, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lin, B.; Liu, F.; Zhao, W. Role of Enterococcus faecalis in refractory apical periodontitis: From pathogenicity to host cell response. J. Oral Microbiol. 2023, 15, 2184924. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The macrophage-osteoclast axis in osteoimmunity and osteo-related diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef]
- Zou, W.; Bar-Shavit, Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J. Bone Miner. Res. 2002, 7, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliot, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that’s regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A., Jr.; Medzhitov, R.; Flavell, R.A. IRAK-M Is a Negative Regulator of Toll-like Receptor Signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef]
- Hubbard, L.L.; Moore, B.B. IRAK-M regulation and function in host defense and immune homeostasis. Infect. Dis. Rep. 2010, 2, e9. [Google Scholar] [CrossRef] [PubMed]
- Li, X. IRAK4 in TLR/IL-1R signaling: Possible clinical applications. Eur. J. Immunol. 2008, 38, 614–618. [Google Scholar] [CrossRef]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef] [PubMed]
- Torres-Monjarás, A.P.; Sánchez-Gutiérrez, R.; Hernández-Castro, B.; González-Baranda, L.; Alvarado-Hernández, D.L.; Pozos-Guillén, A.; Muñoz-Ruiz, A.; Méndez-González, V.; González-Amaro, R.; Vitales-Noyola, M. Bacteria associated with apical periodontitis promotes in vitro the differentiation of macrophages to osteoclasts. Clin. Oral Investig. 2023, 27, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, Z.; Seneviratne, C.J.; Cheung, G.S.; Jin, L.; Zhao, B.; Zhang, C. Enterococcus faecalis promotes osteoclastogenesis and semaphorin 4D expression. Innate Immun. 2015, 21, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Teti, A. The tight relationship between osteoclasts and the immune system. Inflamm. Allergy Drug Targets 2012, 11, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. The unexpected link between osteoclasts and the immune system. Adv. Exp. Med. Biol. 2010, 658, 61–68. [Google Scholar] [PubMed]
- Park, O.J.; Han, J.Y.; Baik, J.E.; Jeon, J.H.; Kang, S.S.; Yun, C.H.; Oh, J.W.; Seo, H.S.; Han, S.H. Lipoteichoic acid of Enterococcus faecalis induces the expression of chemokines via TLR2 and PAFR signaling pathways. J. Leukoc. Biol. 2013, 94, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Yang, J.; Park, O.J.; Park, C.; Kim, J.; Lee, D.; Yun, C.H.; Han, S.H. Lipoteichoic acid inhibits osteoclast differentiation and bone resorption via interruption of gelsolin-actin dissociation. Cell Physiol. 2023, 238, 2425–2439. [Google Scholar] [CrossRef]
- Yang, J.; Park, O.J.; Kim, J.; Baik, J.E.; Yun, C.H.; Han, S.H. Lipoteichoic Acid of Enterococcus faecalis Inhibits the Differentiation of Macrophages into Osteoclasts. J. Endod. 2016, 42, 570–574. [Google Scholar] [CrossRef]
- Ma, R.Y.; Deng, Z.L.; Du, Q.Y.; Dai, M.Q.; Luo, Y.Y.; Liang, Y.E.; Da, X.Z.; Guo, S.M.; Zhao, W.H. Enterococcus faecalis extracellular vesicles promotes apical periodontitis. J. Dent. Res. 2024, 103, 672–682. [Google Scholar] [PubMed]
- Wang, S.; Heng, B.C.; Qiu, S.; Deng, J.; Cheung, G.S.P.; Jin, L.; Zhao, B.; Zhang, C. Lipoteichoic acid of Enterococcus faecalis inhibits osteoclastogenesis via transcription factor RBP-J. Innate Immun. 2019, 25, 13–21. [Google Scholar] [CrossRef]
- Park, O.J.; Yang, J.; Kim, J.; Yun, C.H.; Han, S.H. Enterococcus faecalis attenuates the differentiation of macrophages into osteoclasts. J. Endod. 2015, 41, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, J.H.; Martin, M.; Michalek, S.M.; Nahm, M.H. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 2003, 71, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Park, O.J.; Ha, Y.E.; Sim, J.R.; Lee, D.; Lee, E.H.; Kim, S.Y.; Yun, C.H.; Han, S.H. Butyrate potentiates Enterococcus faecalis lipoteichoic acid-induced inflammasome activation via histone deacetylase inhibition. Cell Death Discov. 2023, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, J.; Xu, A.; Xu, Y.; He, F.; Mao, Y. IRAK4 inhibition: An effective strategy for immunomodulating peri-implant osseointegration via reciprocally-shifted polarization in the monocyte-macrophage lineage cells. BMC Oral Health 2023, 23, 265. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.Y.; Shen, J.W.; He, F.M.; Liu, W. The Characterization and Osteogenic Activity of Nanostructured Strontium-Containing Oxide Layers on Titanium Surfaces. Int. J. Oral Maxillofac. Implant. 2016, 31, e102–e115. [Google Scholar] [CrossRef] [PubMed]
- Hattar, K.; Grandel, U.; Moeller, A.; Fink, L.; Iglhaut, J.; Hartung, T.; Morath, S.; Seeger, W.; Grimminger, F.; Sibelius, U. Lipoteichoic acid (LTA) from Staphylococcus aureus stimulates human neutrophil cytokine release by a CD14-dependent, Toll-like-receptor-independent mechanism: Autocrine role of tumor necrosis factor-[alpha] in mediating LTA-induced interleukin-8 generation. Crit. Care Med. 2006, 34, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, R.; Araujo-Pérez, J.; Alvarado-Hernández, D.L.; González-Amaro, A.M.; Méndez-González, V.; Rivas-Santiago, B.; González-Amaro, R.; Pozos-Guillén, A.; Vitales-Noyola, M. Increased IL-12p70 and IL-8 Produced by Monocytes in Response to Streptococcus spp. and Actinomyces spp. Causals of Endodontic Primary Infections. Int. J. Mol. Sci. 2023, 24, 16853. [Google Scholar] [CrossRef]
- Fokkema, S.J.; Loos, B.G.; de Slegte, C.; Burger, W.; Piscaer, M.; IJzerman, Y.; Van der Velden, U. Increased release of IL-12p70 by monocytes after periodontal therapy. J. Clin. Periodontol. 2003, 30, 1091–1106. [Google Scholar] [CrossRef]


| Demographic or Clinic Characteristics | Patients n = 20 |
|---|---|
| Gender (%) | |
| Female | 85% |
| Male | 15% |
| Age (years) | 45.0 ± 8.0 years |
| Patients with periradicular lesion (%) | 100% |
| Apical periodontitis (%) | |
| Primary infection | 40% |
| Secondary/persistent infection | 60% |
| Symptomatic patients (%) | 83% |
| Palpation 100% | |
| Percussion 100% | |
| Mastication 100% | |
| Mobility 100% | |
| Asymptomatic patients | 17% |
| Evolution time of endodontic treatment (years) | 4.5 ± 4.7 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sandoval, E.M.; Sánchez-Gutiérrez, R.; Torres-Monjarás, A.P.; Alvarado-Hernández, D.L.; Méndez-González, V.; Hernández-Castro, B.; Bernal-Silva, S.; Comas-García, A.; Martínez-Rider, R.; González-Amaro, R.; et al. α-IRAK-4 Suppresses the Activation of RANK/RANKL Pathway on Macrophages Exposed to Endodontic Microorganisms. Int. J. Mol. Sci. 2024, 25, 8434. https://doi.org/10.3390/ijms25158434
Hernández-Sandoval EM, Sánchez-Gutiérrez R, Torres-Monjarás AP, Alvarado-Hernández DL, Méndez-González V, Hernández-Castro B, Bernal-Silva S, Comas-García A, Martínez-Rider R, González-Amaro R, et al. α-IRAK-4 Suppresses the Activation of RANK/RANKL Pathway on Macrophages Exposed to Endodontic Microorganisms. International Journal of Molecular Sciences. 2024; 25(15):8434. https://doi.org/10.3390/ijms25158434
Chicago/Turabian StyleHernández-Sandoval, Elsa Montserrat, Raquel Sánchez-Gutiérrez, Ana Patricia Torres-Monjarás, Diana Lorena Alvarado-Hernández, Verónica Méndez-González, Berenice Hernández-Castro, Sofía Bernal-Silva, Andreu Comas-García, Ricardo Martínez-Rider, Roberto González-Amaro, and et al. 2024. "α-IRAK-4 Suppresses the Activation of RANK/RANKL Pathway on Macrophages Exposed to Endodontic Microorganisms" International Journal of Molecular Sciences 25, no. 15: 8434. https://doi.org/10.3390/ijms25158434







