Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Results
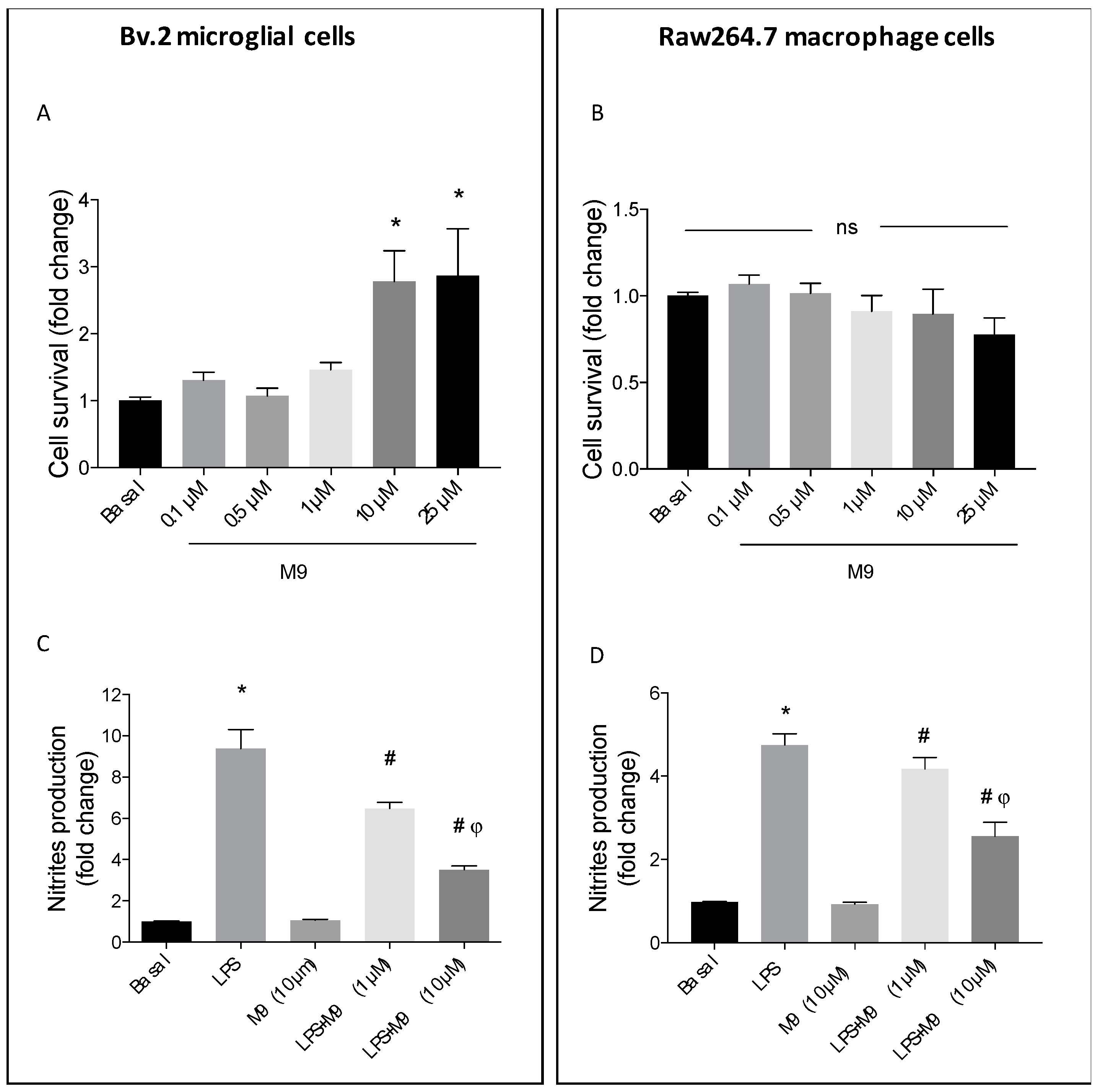
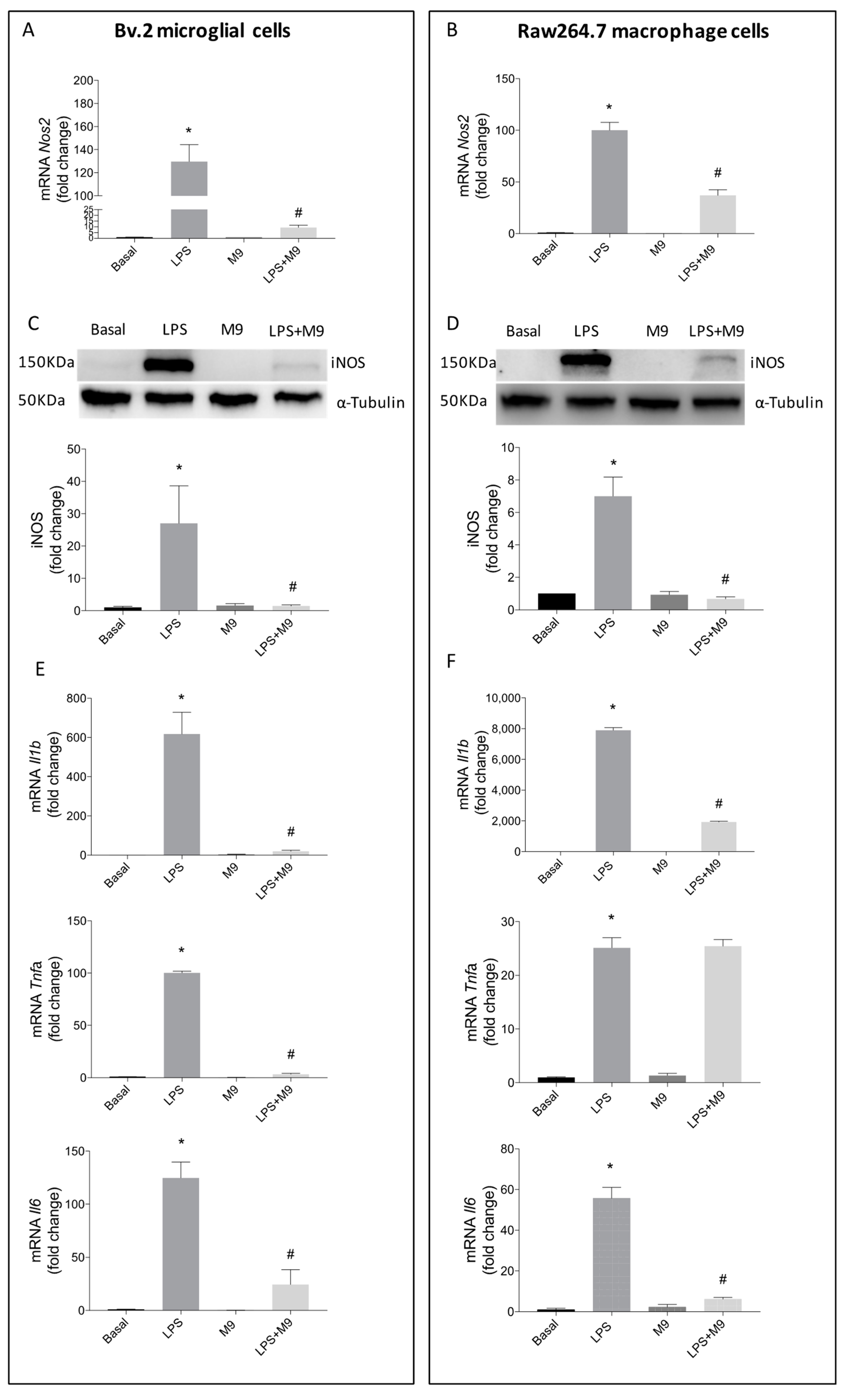
2.1. Deciphering the Anti-Inflammatory Effects of M9 in LPS-Stimulated Immune Cells
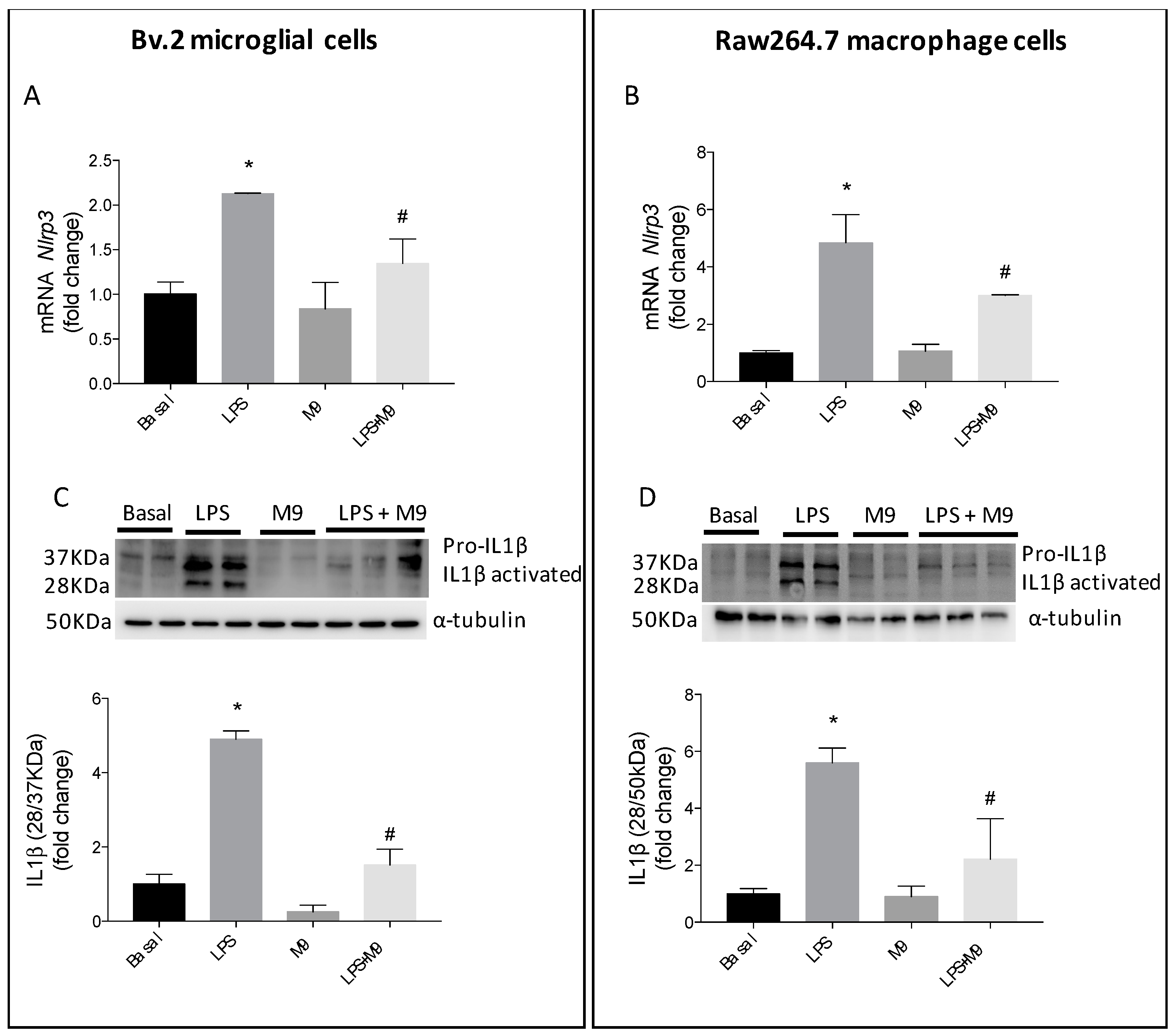
2.2. M9 Exerts Anti-Inflammatory Effects via Inhibition of Inflammasome Complex in Immune Cells
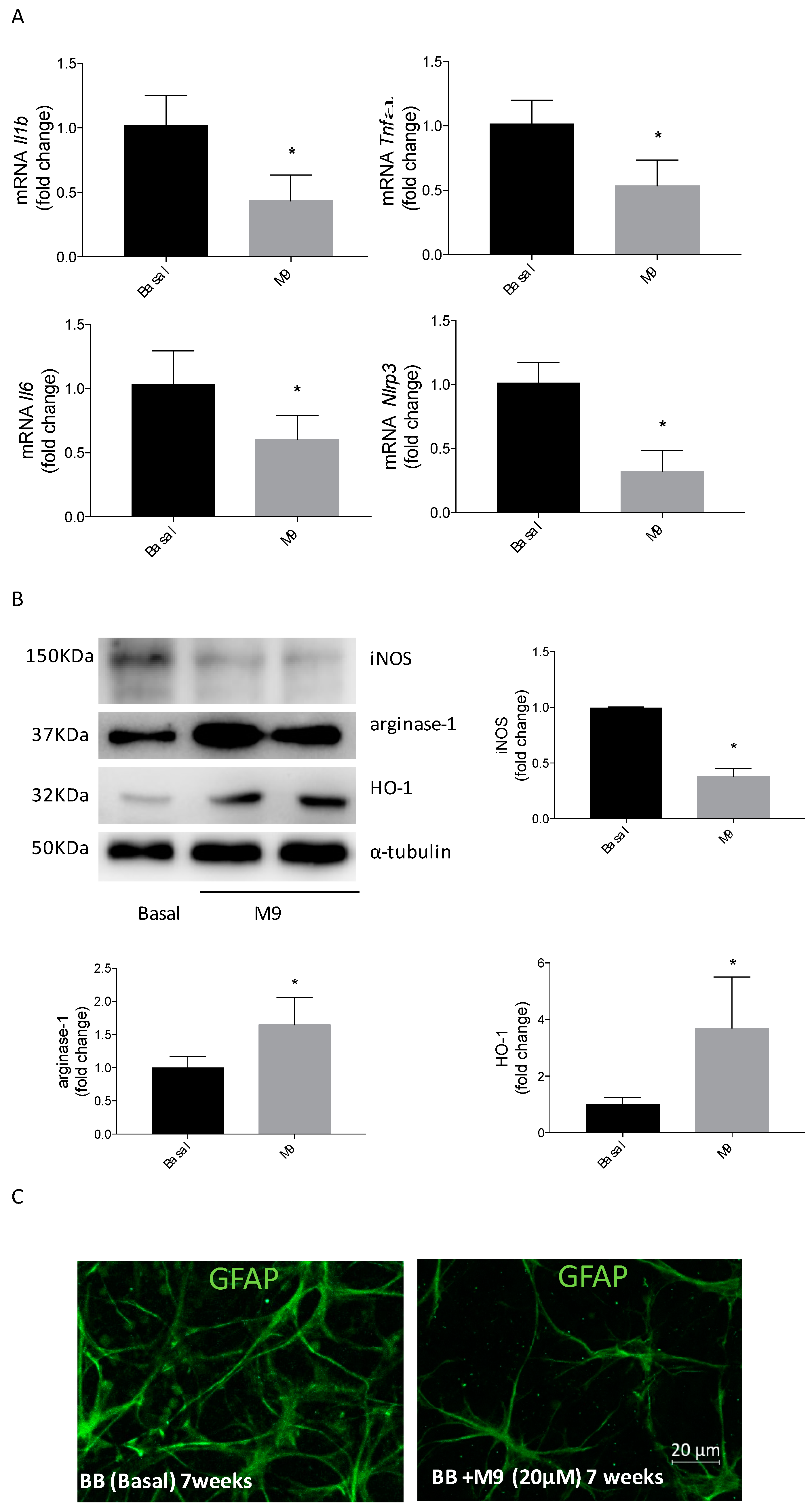
2.3. HO-1 Induction Involves the Arginase-1 Upregulation due to M9 in Bv.2 Microglial Cells
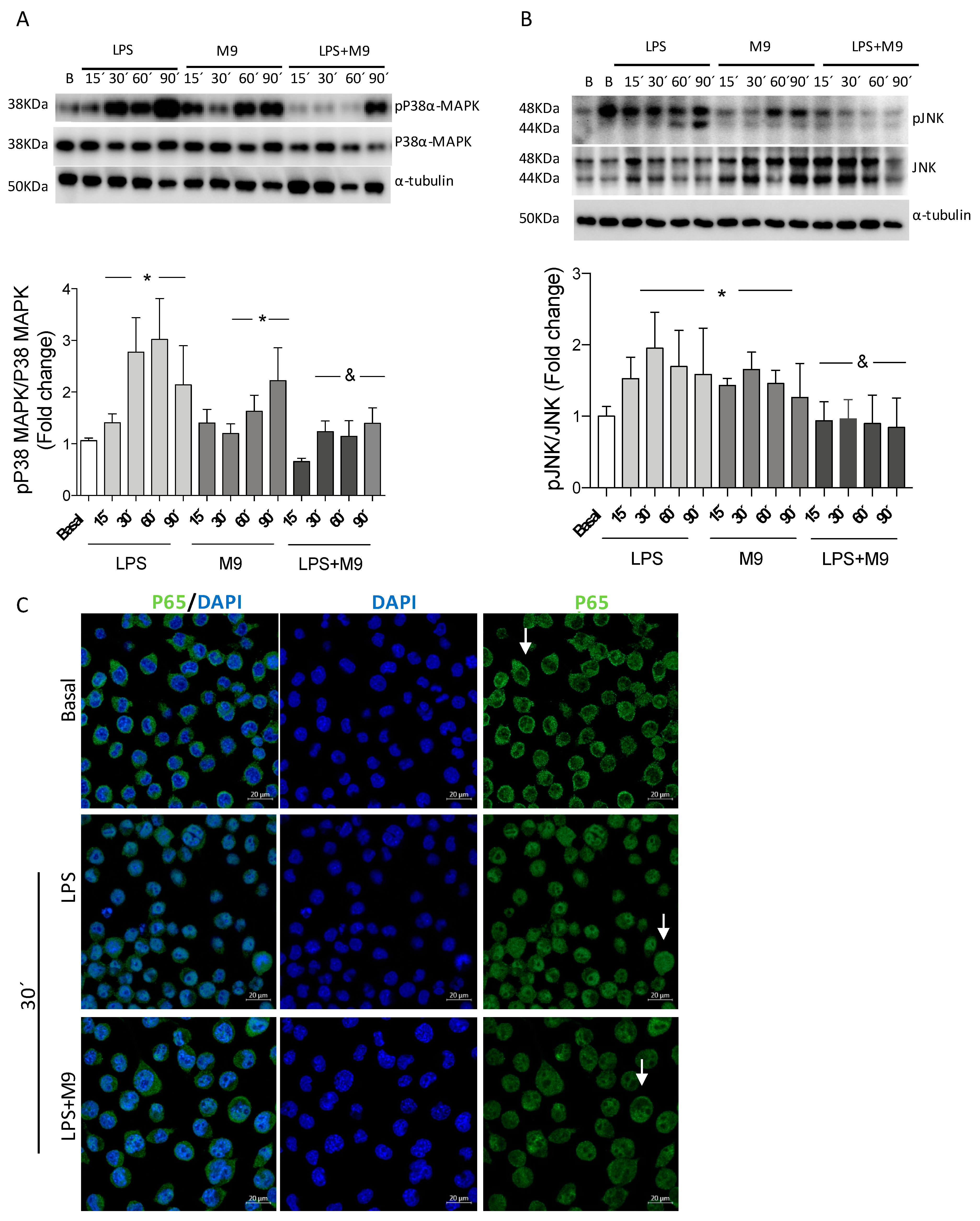
2.4. The M9 Immunomodulatory Effects Alter/Affect Kinase Stress Signaling Pathways in the Microglial Cell Line Bv.2
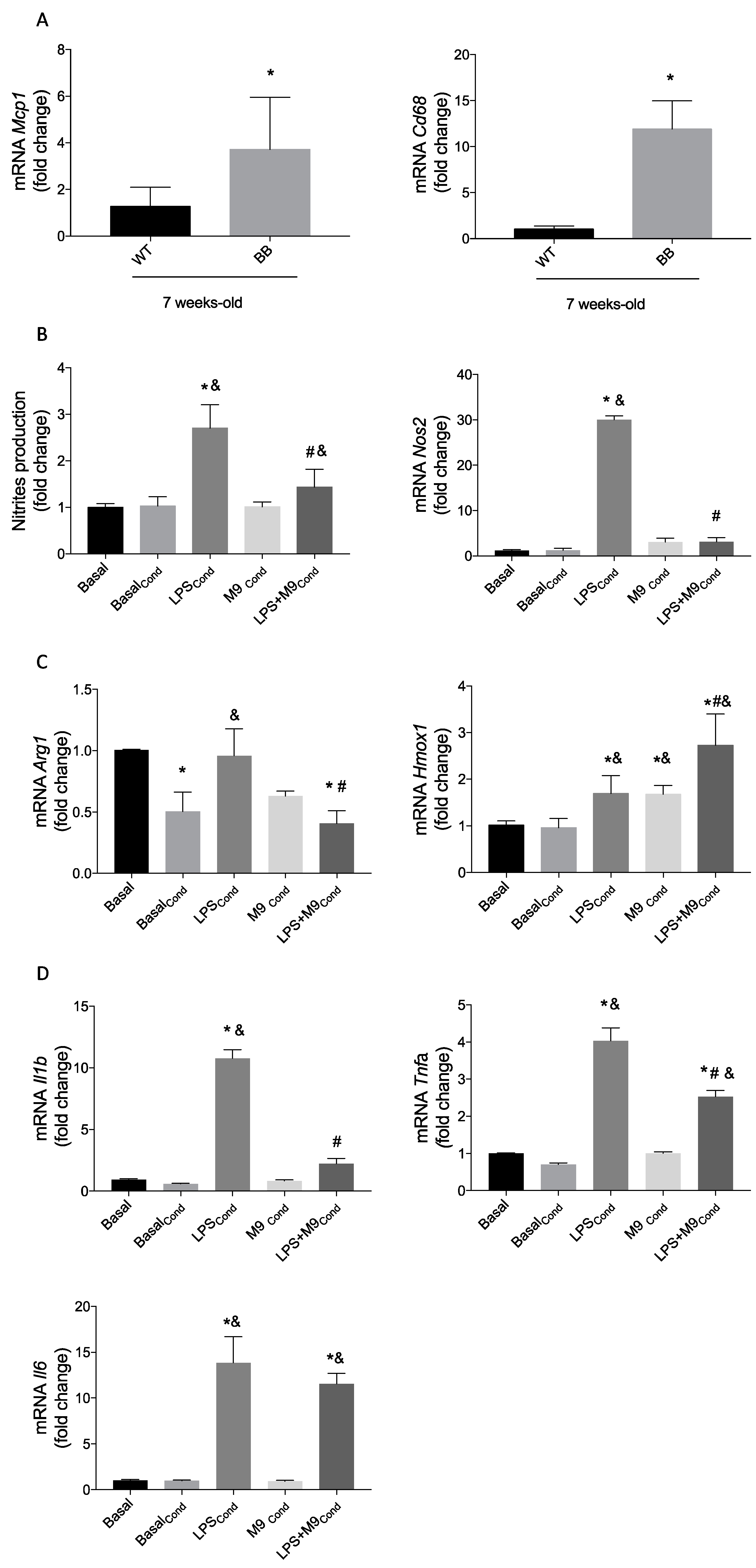
2.5. Macrophage–Microglia Interaction under Proinflammatory Environment and the M9 Effects
2.6. Retinal Explants from BB Rats Treated with M9 Reduce the Inflammatory Response and Potentially the M2 Response Mediated by Microglial Cells
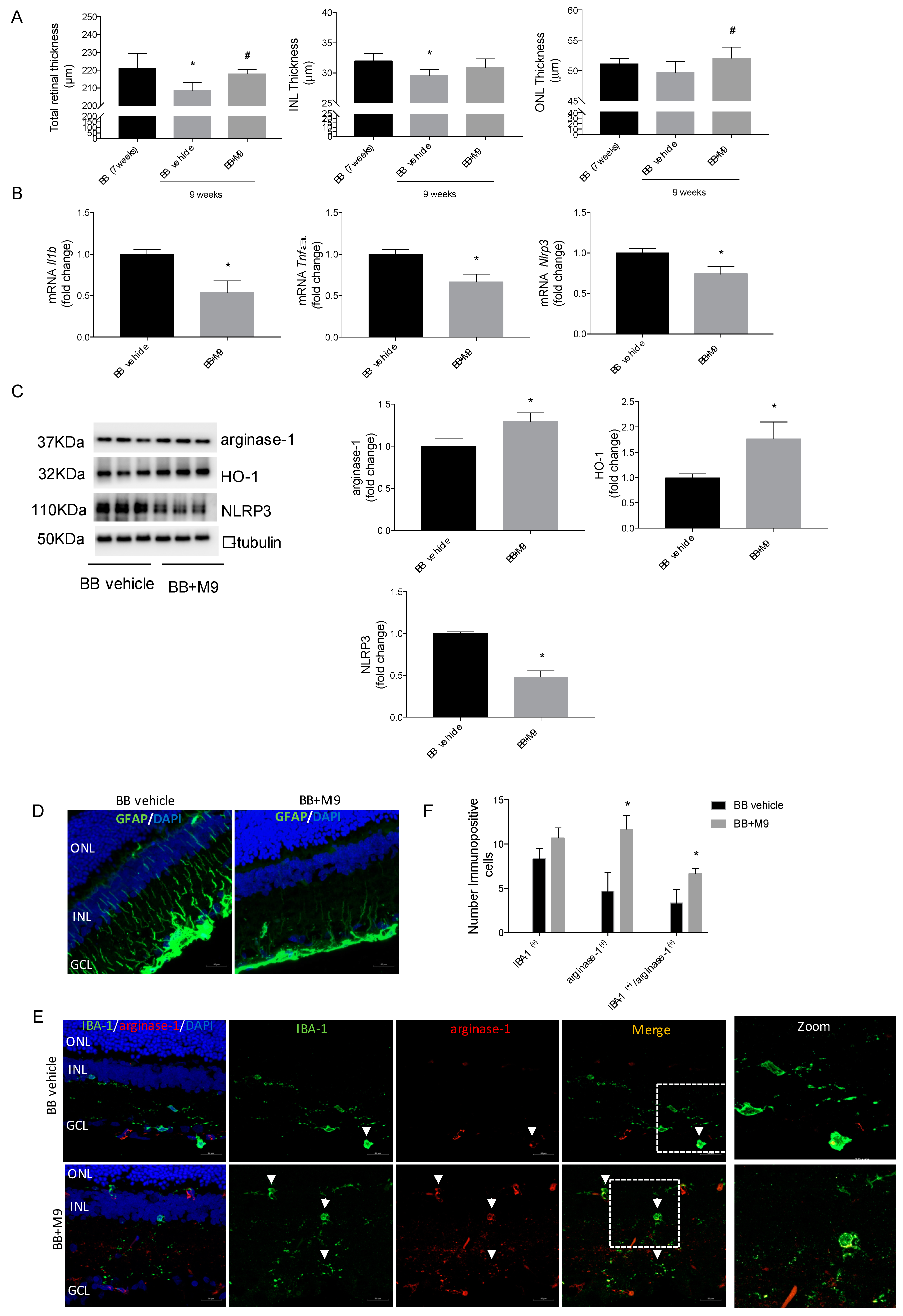
2.7. M9 Reduces the Inflammatory Phenomena and Modulates the Microglia Polarity towards an Anti-Inflammatory Phenotype during DR without Modifying the Metabolic Mechanisms of Diabetes
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Antibodies
4.3. Synthesis of the 3-arylphthalide M9
4.4. Cell Culture
4.5. Analysis of the Cellular Viability by Crystal Violet Staining
4.6. Analysis of Nitrite (NO2−)
4.7. Conditioned Medium from Immune Cells
4.8. Animals
4.9. Retinal Explants
4.10. Intraperitoneal Administration of M9
4.11. Spectral-Domain Optical Coherence Tomography (SD-OCT)
4.12. Immunofluorescence
4.13. Western Blot
4.14. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, M.; Bryl, A.; Zorena, K. Retinal vascular endothelial cell dysfunction and neuroretinal degeneration in diabetic patients. J. Clin. Med. 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Marium, R.; Khan, M.; Jia, Z.; Chua, Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of Vitreous Inflammatory Factors with Diabetic Macular Edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; Lanoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline reduces proinflammatory cytokine expression, microglial activation, and Caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Cancarini, A.; Dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic retinopathy: Vascular and inflammatory disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, K.; Van Hove, H.; Kancheva, D.; Scheyltjens, I.; Pombo Antunes, A.R.; Bastos, J.; Vara-Perez, M.; Ali, L.; Mampay, M.; Deneyer, L.; et al. Differential plasticity and fate of brain-resident and recruited macrophages during the onset and resolution of neuroinflammation. Immunity 2022, 55, 2085–2102.e9. [Google Scholar] [CrossRef] [PubMed]
- Matuleviciute, R.; Akinluyi, E.T.; Muntslag, T.A.O.; Dewing, J.M.; Long, K.R.; Vernon, A.C.; Tremblay, M.E.; Menassa, D.A. Microglial contribution to the pathology of neurodevelopmental disorders in humans. Acta Neuropathol. 2023, 146, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, S.; Carrillo, C.; Qi, Z.; Wilson, J.M.; Baucum, A.J.; Sorenson, C.M.; Sheibani, N.; Belecky-Adams, T.L. Retinal inflammation in murine models of type 1 and type 2 diabetes with diabetic retinopathy. Diabetologia 2023, 66, 2170–2185. [Google Scholar] [CrossRef]
- Karmakar, R.; Pahari, P.; Mal, D. Phthalides and phthalans: Synthetic methodologies and their applications in the total synthesis. Chem. Rev. 2014, 114, 6213–6284. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity; Springer: Cham, Switzerland, 2017; Volume 104, ISBN 9783319456188. [Google Scholar]
- Ortega, M.J.; Parra-Torrejón, B.; Cano-Cano, F.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Synthesis and Antioxidant/Anti-Inflammatory Activity of 3-Arylphthalides. Pharmaceuticals 2022, 15, 588. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Alcalde-Estevez, E.; García-Ramírez, M.; Cazzoni, D.; de la Villa, P.; Sánchez-Fernández, E.M.; Mellet, C.O.; García Fernández, J.M.; Hernández, C.; Simó, R.; et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1663–1674. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Arroba, A.I.; Sánchez-Fernández, E.M.; Mellet, C.O.; García Fernández, J.M.; Masgrau, L.; Valverde, Á.M. The sp2-iminosugar glycolipid 1-dodecylsulfonyl-5N,6O-oxomethylidenenojirimycin (DSO2-ONJ) as selective anti-inflammatory agent by modulation of hemeoxygenase-1 in Bv.2 microglial cells and retinal explants. Food Chem. Toxicol. 2018, 111, 454–466. [Google Scholar] [CrossRef]
- Pathak, S.; Vambutas, A. Autoimmune inner ear disease patient–associated 28-kDa proinflammatory IL-1β fragment results from caspase-7–mediated cleavage in vitro. JCI Insight 2020, 5, e130845. [Google Scholar] [CrossRef]
- Alcaraz, M.; Guillen, M.; Ferrandiz, M.; Megias, J.; Motterlini, R. Carbon Monoxide-Releasing Molecules: A Pharmacological Expedient to Counteract Inflammation. Curr. Pharm. Des. 2008, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Megías, J.; Guillén, M.I.; Clérigues, V.; Rojo, A.I.; Cuadrado, A.; Castejón, M.A.; Gomar, F.; Alcaraz, M.J. Heme oxygenase-1 induction modulates microsomal prostaglandin E synthase-1 expression and prostaglandin E2 production in osteoarthritic chondrocytes. Biochem. Pharmacol. 2009, 77, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cano, F.; Alcalde-Estévez, E.; Gómez-Jaramillo, L.; Iturregui, M.; Sánchez-Fernández, E.M.; García Fernández, J.M.; Ortiz Mellet, C.; Campos-Caro, A.; López-Tinoco, C.; Aguilar-Diosdado, M.; et al. Anti-Inflammatory (M2) Response Is Induced by a sp2-Iminosugar Glycolipid Sulfoxide in Diabetic Retinopathy. Front. Immunol. 2021, 12, 632132. [Google Scholar] [CrossRef]
- Padilla-Pérez, M.C.; Sánchez-Fernández, E.M.; González-Bakker, A.; Puerta, A.; Padrón, J.M.; Martín-Loro, F.; Arroba, A.I.; García Fernández, J.M.; Mellet, C.O. Fluoro-labelled sp2-iminoglycolipids with immunomodulatory properties. Eur. J. Med. Chem. 2023, 255, 115390. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Land Vatter, S.W.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Hasebe, R.; Murakami, K.; Sugawara, T.; Yamasaki, T.; Murakami, M. Gateway reflexes describe novel neuro-immune communications that establish immune cell gateways at specific vessels. Bioelectron. Med. 2023, 9, 24. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; Van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Luo, Y.P.; Jiang, L.; Kang, K.; Fei, D.S.; Meng, X.L.; Nan, C.C.; Pan, S.H.; Zhao, M.R.; Zhao, M.Y. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int. Immunopharmacol. 2014, 20, 24–32. [Google Scholar] [CrossRef]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Okazaki, R.; Morioka, K.; Nakamura, K.; Tanaka, S.; Ogata, T. Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J. Neurosci. Res. 2014, 92, 1647–1658. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.H.; Zhang, L.J.; Liu, X.; Li, S.; Zou, J.X. Identification of differential immune cells and related diagnostic genes in patients with diabetic retinopathy. Medicine 2023, 102, e35331. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Hernández, C.; Simó, R.; Simó-Servat, O. Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 8796. [Google Scholar] [CrossRef] [PubMed]
- Frizinsky, S.; Haj-Yahia, S.; MacHnes Maayan, D.; Lifshitz, Y.; Maoz-Segal, R.; Offengenden, I.; Kidon, M.; Agmon-Levin, N. The innate immune perspective of autoimmune and autoinflammatory conditions. Rheumatology 2019, 58, VI1–VI8. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; De La Rosa, L.R.; Murillo-Cuesta, S.; Vaquero-Villanueva, L.; Hurlé, J.M.; Varela-Nieto, I.; Valverde, Á.M. Autophagy resolves early retinal inflammation in Igf1-deficient mice. DMM Dis. Model. Mech. 2016, 9, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Arroba, A.I.; Bogdanov, P.; Ramos, H.; Simó-Servat, O.; Simó, R.; Valverde, A.M. Effect of topical administration of somatostatin on retinal inflammation and neurodegeneration in an experimental model of diabetes. J. Clin. Med. 2020, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Yokota, T.; Utsunomiya, K.; Taniguchi, K.; Gojo, A.; Kurata, H.; Tajima, N. Involvement of the Rho/Rho kinase signaling pathway in platelet-derived growth factor BB-induced vascular endothelial growth factor expression in diabetic rat retina. Jpn. J. Ophthalmol. 2007, 51, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.J.; Chen, D.; Mott, R.; Yu, Q.; Ma, J.-X.; Zhang, S.X. Systemic Administration of HMG CoA Reductase Inhibitor Protects the Blood-retinal Barrier and Ameliorates Retinal Inflammation in Type 2 diabetes. Exp. Eye Res. 2009, 89, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lypka, K.R.; Carmy-Bennun, T.; Garces, K.N.; Venanzi, A.W.; Hackam, A.S. Assessment of outer retinal thickness and function in mice after experimental optic nerve trauma. BMC Ophthalmol. 2022, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Bogdanov, P.; Sabater, D.; Huerta, J.; Valeri, M.; Hernández, C.; Simó, R. Neuromodulation induced by sitagliptin: A new strategy for treating diabetic retinopathy. Biomedicines 2021, 9, 1772. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Clare, M.; Ensinger, C.L.; Hood, M.M.; Lord, J.W.; Lu, W.P.; Miller, D.F.; Patt, W.C.; Smith, B.D.; Vogeti, L.; et al. Switch control pocket inhibitors of p38-MAP kinase. Durable type II inhibitors that do not require binding into the canonical ATP hinge region. Bioorganic Med. Chem. Lett. 2010, 20, 5793–5798. [Google Scholar] [CrossRef] [PubMed]
- Elms, S.C.; Toque, H.A.; Rojas, M.; Xu, Z.; Caldwell, R.W.; Caldwell, R.B. The role of arginase i in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia 2013, 56, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, H.; Ferrante, R.J.; Morris, S.M.; Ratan, R.R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. USA 2003, 100, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Latour, Y.L.; Gobert, A.P.; Wilson, K.T. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids 2020, 52, 151–160. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tanabu, R.; Monai, N.; Gonome, T.; Xie, Y.; Takahashi, S.; Ishiguro, S.I.; Nakazawa, M. The Spectral-Domain Optical Coherence Tomography Findings Associated with the Morphological and Electrophysiological Changes in a Rat Model of Retinal Degeneration, Rhodopsin S334ter-4 Rats. Biomed Res. Int. 2018, 2018, 5174986. [Google Scholar] [CrossRef] [PubMed]

| Administration Days. | Weight (g) | Glycemia (mg/dL) | ||
|---|---|---|---|---|
| Vehicle | M9 | Vehicle | M9 | |
| 0 | 155.33 ± 31.77 | 146.80 ± 15.36 | 111.67 ± 13.42 *# | 97.40 ± 8.17 |
| 1 | 160.00 ± 34.69 | 149.80 ± 16.52 | 106.67 ± 3.21 | 107.60 ± 10.47 *# |
| 3 | 168.67 ± 38.47 | 156.20 ± 20.10 | 98.33 ± 7.09 | 99.20 ± 3.89 |
| 6 | 174.66 ± 42.77 | 164.20 ± 21.62 | 90.67 ± 11.84 | 96.60 ± 4.21 |
| 8 | 177.67 ± 44.45 | 167.40 ± 24.06 | 100.00 ± 6 | 98.20 ± 7.59 |
| 10 | 185.66 ± 46.71 | 177.00 ± 27.09 | 92.00 ± 5.29 | 97.00 ± 6.28 |
| 13 | 195.33 ± 50.14 | 183.80 ± 28.46 | 102.67 ± 0.577 | 98.20 ± 6.76 |
| 15 | 198.33 ± 49.32 | 186.4 ± 30.82 | 86.00 ± 6.24 | 89.00 ± 4.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Loro, F.; Cano-Cano, F.; Ortega, M.J.; Cuevas, B.; Gómez-Jaramillo, L.; González-Montelongo, M.d.C.; Freisenhausen, J.C.; Lara-Barea, A.; Campos-Caro, A.; Zubía, E.; et al. Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 8440. https://doi.org/10.3390/ijms25158440
Martín-Loro F, Cano-Cano F, Ortega MJ, Cuevas B, Gómez-Jaramillo L, González-Montelongo MdC, Freisenhausen JC, Lara-Barea A, Campos-Caro A, Zubía E, et al. Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(15):8440. https://doi.org/10.3390/ijms25158440
Chicago/Turabian StyleMartín-Loro, Francisco, Fátima Cano-Cano, María J. Ortega, Belén Cuevas, Laura Gómez-Jaramillo, María del Carmen González-Montelongo, Jan Cedric Freisenhausen, Almudena Lara-Barea, Antonio Campos-Caro, Eva Zubía, and et al. 2024. "Arylphthalide Delays Diabetic Retinopathy via Immunomodulating the Early Inflammatory Response in an Animal Model of Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 15: 8440. https://doi.org/10.3390/ijms25158440






