Therapy-Induced Senescence: Novel Approaches for Markers Identification
Abstract
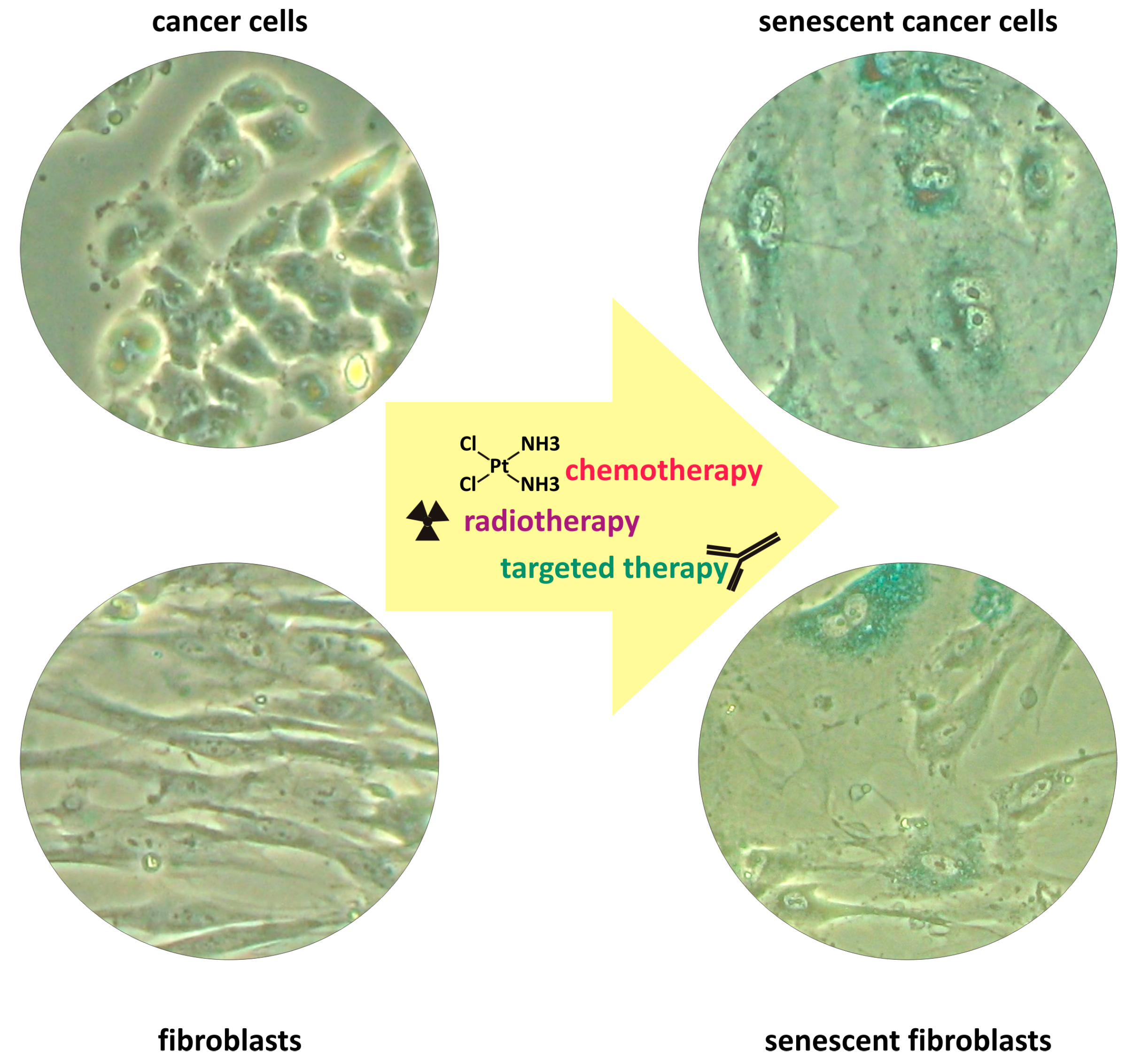
1. Therapy-Induced Senescence
2. Favorable Effects of TIS on Anti-Tumor Immunity
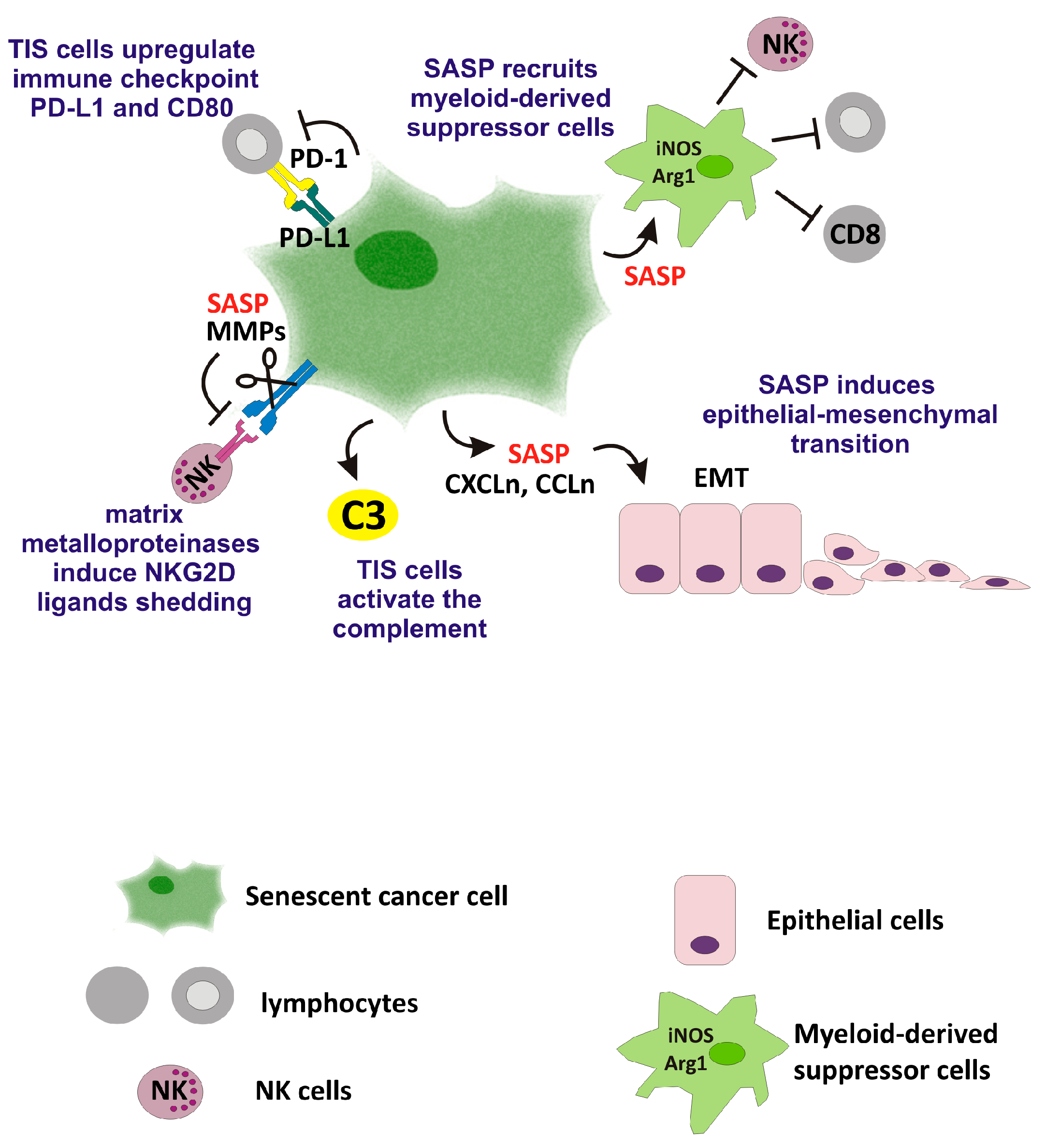
3. Adverse Effects of TIS
4. Therapeutic Approaches to Target TIS Cells
5. TIS Biomarkers
5.1. Senescence-Associated Beta-Galactosidase
5.2. Lipofuscin
5.3. Cell Surface Markers
5.4. Soluble Markers
5.5. Other Markers
5.6. Markers Panels
5.7. Morphology-Based Classifiers
5.8. Aptamers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, B.D.; Broude, E.V.; Dokmanovic, M.; Zhu, H.; Ruth, A.; Xuan, Y.; Kandel, E.S.; Lausch, E.; Christov, K.; Roninson, I.B. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999, 59, 3761–3767. [Google Scholar] [PubMed]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.Y.; Wu, D.Y. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, F.; Badolati, N.; Mellone, S.; Stornaiuolo, M.; Leonardi, A.; Crescenzi, E. Glutamine promotes escape from therapy-induced senescence in tumor cells. Aging 2021, 13, 20962–20991. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Altschuler, S.J.; Wu, L.F. Patterns of Early p21 Dynamics Determine Proliferation-Senescence Cell Fate after Chemotherapy. Cell 2019, 178, 361–373.e12. [Google Scholar] [CrossRef] [PubMed]
- Bojko, A.; Czarnecka-Herok, J.; Charzynska, A.; Dabrowski, M.; Sikora, E. Diversity of the Senescence Phenotype of Cancer Cells Treated with Chemotherapeutic Agents. Cells 2019, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell- nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Sidi, R.; Pasello, G.; Opitz, I.; Soltermann, A.; Tutic, M.; Rehrauer, H.; Weder, W.; Stahel, R.A.; Felley-Bosco, E. Induction of senescence markers after neo-adjuvant chemotherapy of malignant pleural mesothelioma and association with clinical outcome: An exploratory analysis. Eur. J. Cancer. 2011, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tato-Costa, J.; Casimiro, S.; Pacheco, T.; Pires, R.; Fernandes, A.; Alho, I.; Pereira, P.; Costa, P.; Castelo, H.B.; Ferreira, J.; et al. Therapy-Induced Cellular Senescence Induces Epithelial-to-Mesenchymal Transition and Increases Invasiveness in Rectal Cancer. Clin. Colorectal Cancer 2016, 15, 170–178.e3. [Google Scholar] [PubMed]
- Kim, J.H.; Brown, S.L.; Gordon, M.N. Radiation-induced senescence: Therapeutic opportunities. Radiat. Oncol. 2023, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.L.; Hoffmann, R.; González-López, C.; Doherty, G.J.; Korkola, J.E.; Muñoz-Espín, D. Cellular senescence in cancer: From mechanisms to detection. Mol. Oncol. 2021, 15, 2634–2671. [Google Scholar]
- Blagosklonny, M.V. Cellular senescence: When growth stimulation meets cell cycle arrest. Aging 2023, 15, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Zatulovskiy, E.; Swaffer, M.P.; Zhang, L.; Ilerten, I.; Zhang, S.; You, D.S.; Marinov, G.; McAlpine, P.; Elias, J.E.; et al. Increasing cell size remodels the proteome and promotes senescence. Mol. Cell 2022, 82, 3255–3269.e8. [Google Scholar] [CrossRef] [PubMed]
- Heckenbach, I.; Mkrtchyan, G.V.; Ezra, M.B.; Bakula, D.; Madsen, J.S.; Nielsen, M.H.; Oró, D.; Osborne, B.; Covarrubias, A.J.; Idda, M.L.; et al. Nuclear morphology is a deep learning biomarker of cellular senescence. Nat. Aging 2022, 2, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Flor, A.C.; Wolfgeher, D.; Wu, D.; Kron, S.J. A signature of enhanced lipid metabolism.; lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell Death Discov. 2017, 3, 17075. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Atanassov, I.; Dethloff, F.; Kroczek, L.; Langer, T. Time-resolved proteomic analyses of senescence highlight metabolic rewiring of mitochondria. Life Sci. Alliance 2023, 6, e202302127. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef] [PubMed]
- Jochems, F.; Thijssen, B.; De Conti, G.; Jansen, R.; Pogacar, Z.; Groot, K.; Wang, L.; Schepers, A.; Wang, C.; Jin, H.; et al. The Cancer SENESCopedia: A delineation of cancer cell senescence. Cell Rep. 2021, 36, 109441. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.; Lee, S.; Schmitt, C.A. Cellular senescence: Neither irreversible nor reversible. J. Exp. Med. 2024, 221, e20232136. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [PubMed]
- Colucci, M.; Zumerle, S.; Bressan, S.; Gianfanti, F.; Troiani, M.; Valdata, A.; D’Ambrosio, M.; Pasquini, E.; Varesi, A.; Cogo, F.; et al. Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells. Cancer Cell 2024, 42, 646–661.e9. [Google Scholar] [CrossRef] [PubMed]
- Lindell, E.; Zhong, L.; Zhang, X. Quiescent Cancer Cells-A Potential Therapeutic Target to Overcome Tumor Resistance and Relapse. Int. J. Mol. Sci. 2023, 24, 3762. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Hsu, C.C.; Pai, V.C.; Liao, W.Y.; Huang, S.S.; Tan, K.T.; Yen, C.J.; Hsu, S.C.; Chen, W.Y.; Shan, Y.S.; et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016, 213, 2967–2988. [Google Scholar] [PubMed]
- Peiris-Pagès, M.; Sotgia, F.; Lisanti, M.P. Chemotherapy induces the cancer-associated fibroblast phenotype, activating paracrine Hedgehog-GLI signalling in breast cancer cells. Oncotarget 2015, 6, 10728–10745. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Komarova, E.A.; Hill, J.E.; Browder, T.; Tararova, N.D.; Mavrakis, L.; DiCorleto, P.E.; Folkman, J.; Gudkov, A.V. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006, 66, 9356–9361. [Google Scholar] [CrossRef] [PubMed]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Z.; Luo, Q.; Wang, Y.; Li, Q.; Zhou, L.; Zuo, H. Radiotherapy modulates tumor cell fate decisions: A review. Radiat. Oncol. 2022, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Iannello, A.; Thompson, T.W.; Ardolino, M.; Lowe, S.W.; Raulet, D.H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013, 210, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.; Prats, N.; López-Domínguez, J.A.; et al. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef]
- Chen, H.A.; Ho, Y.J.; Mezzadra, R.; Adrover, J.M.; Smolkin, R.; Zhu, C.; Woess, K.; Bernstein, N.; Schmitt, G.; Fong, L.; et al. Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov. 2023, 13, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Soriani, A.; Ricci, B.; Ponzetta, A.; Benigni, G.; Morrone, S.; Bernardini, G.; Santoni, A. Natural killer cell recognition of in vivo drug-induced senescent multiple myeloma cells. Oncoimmunology 2016, 5, e1218105. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, E.; Pacifico, F.; Lavorgna, A.; De Palma, R.; D’Aiuto, E.; Palumbo, G.; Formisano, S.; Leonardi, A. NF-κB-dependent cytokine secretion controls Fas expression on chemotherapy-induced premature senescent tumor cells. Oncogene 2011, 30, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Kang, H.H.; Feng, Y.; Minikes, A.M.; Jiang, X. Autophagy inhibition signals through senescence to promote tumor suppression. Autophagy 2023, 19, 1764–1780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hawkins, O.E.; Su, Y.; Vilgelm, A.E.; Sobolik, T.; Thu, Y.M.; Kantrow, S.; Splittgerber, R.C.; Short, S.; Amiri, K.I.; et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-κB impairs this drug-induced senescence. EMBO Mol. Med. 2013, 5, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Morris, J.P., 4th; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhao, B.; Zhou, W.; Liu, H.; Fukumoto, T.; Gabrilovich, D.; Zhang, R. Sensitization of ovarian tumor to immune checkpoint blockade by boosting senescence-associated secretory phenotype. iScience 2020, 24, 102016. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pant, V.; Li, Q.; Chang, L.L.; Quintás-Cardama, A.; Garza, D.; Tavana, O.; Yang, P.; Manshouri, T.; Li, Y.; et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 2012, 21, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; LaPak, K.M.; Hennessey, R.C.; Yu, C.Y.; Shakya, R.; Zhang, J.; Burd, C.E. Stromal Senescence by Prolonged CDK4/6 Inhibition Potentiates Tumor Growth. Mol. Cancer Res. 2017, 15, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lou, Y.; Fang, H.; Sun, S.; Jin, R.; Ji, Y.; Chen, Z. Cancer associated fibroblasts under therapy induced senescence in the tumor microenvironment. Exp. Ther. Med. 2024, 27, 150. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.; Havas, A.P.; Lin, B.; Rajagopal, J.; Sen, P.; Adams, P.D.; Dou, Z. Upregulation of PD-L1 in Senescence and Aging. Mol. Cell Biol. 2022, 42, e0017122. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Chiu, F.Y.; Ungerleider, N.A.; Kvadas, R.; Mheidly, Z.; Sun, M.J.S.; Tian, D.; Waizman, D.A.; Anderson, A.Y.; Machado, H.L.; et al. Breast cancer cells survive chemotherapy by activating targetable immune-modulatory programs characterized by PD-L1 or CD80. Nat. Cancer. 2022, 3, 1513–1533. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.P.; Yannone, S.M.; Daemen, A.; Sun, Y.; Vakar-Lopez, F.; Kawahara, M.; Freund, A.M.; Rodier, F.; Wu, J.D.; Desprez, P.Y.; et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight. 2019, 5, e124716. [Google Scholar] [CrossRef] [PubMed]
- Abu-Humaidan, A.H.; Ismail, M.A.; Ahmad, F.M.; Al Shboul, S.; Barham, R.; Tadros, J.S.; Alhesa, A.; El-Sadoni, M.; Alotaibi, M.R.; Ababneh, N.A.; et al. Therapy-induced senescent cancer cells exhibit complement activation and increased complement regulatory protein expression. Immunol. Cell Biol. 2024, 102, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Revandkar, A.; Di Mitri, D.; Guccini, I.; Proietti, M.; Sarti, M.; Pinton, S.; Zhang, J.; Kalathur, M.; Civenni, G.; et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014, 9, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Guccini, I.; Revandkar, A.; D’Ambrosio, M.; Colucci, M.; Pasquini, E.; Mosole, S.; Troiani, M.; Brina, D.; Sheibani-Tezerji, R.; Elia, A.R.; et al. Senescence Reprogramming by TIMP1 Deficiency Promotes Prostate Cancer Metastasis. Cancer Cell 2021, 39, 68–82.e9. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Elmore, L.W.; Di, X.; Dumur, C.; Holt, S.E.; Gewirtz, D.A. Evasion of a single-step.; chemotherapy-induced senescence in breast cancer cells: Implications for treatment response. Clin. Cancer Res. 2005, 11, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Belogiannis, K.; Papaspyropoulos, A.; Petty, R.; Gorgoulis, V.G. Escape from senescence: Molecular basis and therapeutic ramifications. J. Pathol. 2023, 260, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Achuthan, S.; Santhoshkumar, T.R.; Prabhakar, J.; Nair, S.A.; Pillai, M.R. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J. Biol. Chem. 2011, 286, 37813–37829. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Gil, J. Targeting senescence as an anticancer therapy. Mol Oncol. 2022, 16, 3855–3880. [Google Scholar] [CrossRef]
- Samaraweera, L.; Adomako, A.; Rodriguez-Gabin, A.; McDaid, H.M. A Novel Indication for Panobinostat as a Senolytic Drug in NSCLC and HNSCC. Sci. Rep. 2017, 7, 1900. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef] [PubMed]
- Troiani, M.; Colucci, M.; D’Ambrosio, M.; Guccini, I.; Pasquini, E.; Varesi, A.; Valdata, A.; Mosole, S.; Revandkar, A.; Attanasio, G.; et al. Single-cell transcriptomics identifies Mcl-1 as a target for senolytic therapy in cancer. Nat. Commun. 2022, 13, 2177. [Google Scholar] [CrossRef] [PubMed]
- Pardella, E.; Pranzini, E.; Nesi, I.; Parri, M.; Spatafora, P.; Torre, E.; Muccilli, A.; Castiglione, F.; Fambrini, M.; Sorbi, F.; et al. Therapy-Induced Stromal Senescence Promoting Aggressiveness of Prostate and Ovarian Cancer. Cells 2022, 11, 4026. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sananikone, E.; Kanji, S.; Tomimatsu, N.; Di Cristofaro, L.F.M.; Kollipara, R.K.; Saha, D.; Floyd, J.R.; Sung, P.; Hromas, R.; Burns, T.C.; et al. Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma Recurrence. Cancer Res. 2021, 81, 5935–5947. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Wong, F.; Omori, S.; Donghia, N.M.; Zheng, E.J.; Collins, J.J. Discovering small-molecule senolytics with deep neural networks. Nat. Aging 2023, 3, 734–750. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.K. Hungry for biomarkers of aging. Aging Cell 2024, 23, e14158. [Google Scholar] [CrossRef] [PubMed]
- Severino, J.; Allen, R.G.; Balin, S.; Balin, A.; Cristofalo, V.J. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 2000, 257, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, K.H.; Cohen, S.N. Senescence-specific gene expression fingerprints reveal cell-type-dependent physical clustering of up-regulated chromosomal loci. Proc. Natl. Acad. Sci. USA 2003, 100, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Hu, M.L. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Guiho, R.; Herranz, N.; Uren, A.; Withers, D.J.; Martínez-Barbera, J.P.; Tietze, L.F.; Gil, J. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell 2020, 19, e13133. [Google Scholar] [CrossRef] [PubMed]
- Morsli, S.; Doherty, G.J.; Muñoz-Espín, D. Activatable senoprobes and senolytics: Novel strategies to detect and target senescent cells. Mech. Ageing Dev. 2022, 202, 111618. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.; Veroutis, D.; Pousias, A.; Papaspyropoulos, A.; Pippa, N.; Lougiakis, N.; Kambas, K.; Lagopati, N.; Polyzou, A.; Georgiou, M.; et al. A fluorophore-conjugated reagent enabling rapid detection, isolation and live tracking of senescent cells. Mol. Cell 2023, 83, 3558–3573.e7. [Google Scholar] [CrossRef] [PubMed]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M.; Lezina, L.; Carrera, S.; Jukes-Jones, R.; Giblett, S.M.; Antonov, A.; Barlev, N.; Saldanha, G.S.; Pritchard, C.A.; Cain, K.; et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.; Thompson, D.; Jones, R.; Cruz, A.G.; Poblocka, M.; Canfarotta, F.; Norman, R.; Macip, S.; Jones, D.J.L.; Piletsky, S. Snapshot imprinting as a tool for surface mapping and identification of novel biomarkers of senescent cells. Nanoscale Adv. 2022, 4, 5304–5311. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Sasaki, N.; Toyoda, M. Qualitative and quantitative alterations in intracellular and membrane glycoproteins maintain the balance between cellular senescence and human aging. Aging 2018, 10, 2190–2208. [Google Scholar] [CrossRef] [PubMed]
- Baldensperger, T.; Eggen, M.; Kappen, J.; Winterhalter, P.R.; Pfirrmann, T.; Glomb, M.A. Comprehensive analysis of posttranslational protein modifications in aging of subcellular compartments. Sci. Rep. 2020, 10, 7596. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.P.; Ahmed, E.K.; Baraibar, M.A.; Friguet, B. Proteome Oxidative Modifications and Impairment of Specific Metabolic Pathways During Cellular Senescence and Aging. Proteomics 2020, 20, e1800421. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef] [PubMed]
- Celard, P.; Iglesias, E.L.; Sorribes-Fdez, J.M.; Romero, R.; Vieira, A.S.; Borrajo, L. A survey on deep learning applied to medical images: From simple artificial neural networks to generative models. Neural Comput. Appl. 2023, 35, 2291–2323. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, D.; Seki, T.; Sawada, H.; Kunitomi, A.; Katsuki, T.; Kimura, M.; Ito, S.; Komuro, J.; Hashimoto, H.; Fukuda, K.; et al. Anti-senescent drug screening by deep learning-based morphology senescence scoring. Nat. Commun. 2021, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.; Pombo, J.; Sun, B.; Gallage, S.; Kudo, H.; McHugh, D.; Bousset, L.; Barragan Avila, J.E.; Forlano, R.; Manousou, P.; et al. Detection of senescence using machine learning algorithms based on nuclear features. Nat. Commun. 2024, 15, 1041. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, M.; Wang, X.; Wu, X.; Yue, G.; Wang, T.; Zhou, Y.; Lei, B.; Zhou, G. Morphology-based deep learning enables accurate detection of senescence in mesenchymal stem cell cultures. BMC Biol. 2024, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Celard, P.; Iglesias, E.L.; Cellina, M.; Cè, M.; Khenkina, N.; Sinichich, P.; Cervelli, M.; Poggi, V.; Boemi, S.; Ierardi, A.M.; et al. Artificial Intelligence in the Era of Precision Oncological Imaging. Technol. Cancer Res. Treat. 2022, 21, 15330338221141793. [Google Scholar]
- Li, Y.; Tam, W.W.; Yu, Y.; Zhuo, Z.; Xue, Z.; Tsang, C.; Qiao, X.; Wang, X.; Wang, W.; Li, Y.; et al. The application of Aptamer in biomarker discovery. Biomark. Res. 2023, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Pacifico, F.; Crescenzi, E. Potential Applications of Aptamers for Targeting Senescent Cells. In Senolytics in Disease, Ageing and Longevity; Muñoz-Espin, D., Demaria, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 11, pp. 181–200. [Google Scholar]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Shao, X.Y.; Gong, W.; Shi, T.S.; Dong, J.; Shi, Y.; Shen, S.Y.; He, Y.; Qin, J.H.; et al. Specific Clearance of Senescent Synoviocytes Suppresses the Development of Osteoarthritis based on Aptamer-Functionalized Targeted Drug Delivery System. Adv. Funct. Mater. 2022, 32, 2109460. [Google Scholar] [CrossRef]
- Matsui, T.; Higashimoto, Y.; Nishino, Y.; Nakamura, N.; Fukami, K.; Yamagishi, S.I. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes 2017, 66, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Ohara, M.; Terasaki, M.; Osaka, N.; Yashima, H.; Saito, T.; Otoyama-Kataoka, Y.; Omachi, T.; Higashimoto, Y.; Matsui, T.; et al. Subcutaneous Infusion of DNA-Aptamer Raised against Advanced Glycation End Products Prevents Loss of Skeletal Muscle Mass and Strength in Accelerated-Aging Mice. Biomedicines 2023, 11, 3112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Zhao, Z.; Xia, Y.; Xie, Y.; Hong, D.; Liu, Y.; Tan, W. Aptamer Conjugate-Based Ratiometric Fluorescent Probe for Precise Imaging of Therapy-Induced Cancer Senescence. Anal. Chem. 2024, 96, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Wang, L.; Xie, Y.; Zhang, L.; Han, X.; Tan, W.; Liu, Y. Engineering Hierarchical Recognition-Mediated Senolytics for Reliable Regulation of Cellular Senescence and Anti-Atherosclerosis Therapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214169. [Google Scholar] [CrossRef] [PubMed]


| Chemotherapeutic Agents | Targeted Drugs | In Vitro Model | In Vivo Model | References |
|---|---|---|---|---|
| KAT6A/B inhibitors (WM-8014; WM-1119) | Mouse embryonic fibroblasts (MEFs); B cell lymphoma cells EMRK1184 | EMRK1184 xenografts | [26] | |
| CDC7 inhibitor (XL413) | Human liver cancer cells (Hep3B, Huh7, HepG2, SNU182, SNU398, SNU449, Huh6, SK-Hep1); human non-transformed cells (BJ, RPE-1) | Huh7 and MHCC97H xenografts | [27] | |
| Retinoic-acid-receptor (RAR) agonist adapalene | Mouse embryonic fibroblasts (MEFs); mouse prostate tumor cells (TrampC1); human prostate tumor cells (PC3, LNCaP, 22RV1, LAPC4) | TrampC1 allografts; PC3 xenografts | [28] | |
| Doxorubicin; bleomycin | CDK4/6 inhibitor palbociclib; p53 activator nutlin-3A | Human IMR-90 fibroblasts; mouse embryonic fibroblasts (MEF); human melanoma cells SKMEL-103; mouse melanoma cells B16-F10 | B16-F10 allografts | [38] |
| Cisplatin | p53 activator nutlin; MEK inhibitor trametinib; CDK4/6 inhibitor palbociclib | Human liver cancer cells (HepG2, SK-Hep1); human lung cancer cells (A549, H460, H2030) | p53-restorable murine liver cancer cells orthotopic transplant model | [39] |
| Melphalan | Murine model of multiple myeloma (MM) | [40] | ||
| Doxorubicin | Human lung cancer cells (A549); Human breast cancer cells (MCF7) | [41] | ||
| Temozolomide | Human glioblastoma cells (A172, U87MG) | U87MG xenografts | [42] | |
| Aurora kinase inhibitors MLN8054 and MLN8237 (Alisertib) | Human melanoma cells (Hs294T, SK-Mel-5, SK-Mel-2 and SK-Mel-28); mouse melanoma cells MelA | Orthotopic implants of patients-derived melanoma | [43] | |
| MEK inhibitor trametinib; CDK4/6 inhibitor palbociclib | Fresh frozen sections of pancreas tumor tissue | Murine models of pancreatic ductal adenocarcinoma | [44] | |
| CDK4/6 inhibitor abemaciclib; CDK4/6 inhibitor palbociclib | MMTV-rtTA/tetO-HER2 transgenic mouse model of mammary carcinoma; patient-derived breast cancer xenografts | [45] | ||
| CDK4/6 inhibitor abemaciclib | Human melanoma cells (GR39, UACC62, A2058); mouse cells B16F10 | B16-F10 allografts | [46] | |
| Cisplatin; irinotecan | Mouse ovarian cancer cells (UPK10, ID8) | Orthotopic implants of UPK10 cells | [47] | |
| UV irradiation; mitomycin C | CDK4/6 inhibitor palbociclib. | Mouse embryonic fibroblasts (MEF); mouse melanoma cells (B16-F1, B16-F10) | [49] | |
| Doxorubicin | p53 activator nutlin | 4226 cells (from a spontaneous p53 WT MMTV-Wnt1 tumor); human breast cancer cells MCF-7 | MMTV-Wnt1 mice | [52] |
| Mitoxantrone; X-ray; etoposide | Human fibroblasts (WI-38, IMR-90, HCA2); human prostate cells (PC-3, BPH1, RWPE1, DU145); human breast cells (MCF10A) | Tumor samples from patients with prostate cancer before and after mitoxantrone treatment | [53] | |
| Etoposide; doxorubicin | Human lung cancer cells (A549); human breast cancer cells (MCF7); human pancreatic cancer cells (Panc-1) | [54] | ||
| Doxorubicin; paclitaxel; temozolomide; cisplatin | Mouse embryonic fibroblasts (MEF); mouse dermal fibroblasts (MDF); human dermal fibroblasts (HCA2, BJ) | p16-3MR mouse model | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacifico, F.; Magni, F.; Leonardi, A.; Crescenzi, E. Therapy-Induced Senescence: Novel Approaches for Markers Identification. Int. J. Mol. Sci. 2024, 25, 8448. https://doi.org/10.3390/ijms25158448
Pacifico F, Magni F, Leonardi A, Crescenzi E. Therapy-Induced Senescence: Novel Approaches for Markers Identification. International Journal of Molecular Sciences. 2024; 25(15):8448. https://doi.org/10.3390/ijms25158448
Chicago/Turabian StylePacifico, Francesco, Fulvio Magni, Antonio Leonardi, and Elvira Crescenzi. 2024. "Therapy-Induced Senescence: Novel Approaches for Markers Identification" International Journal of Molecular Sciences 25, no. 15: 8448. https://doi.org/10.3390/ijms25158448
APA StylePacifico, F., Magni, F., Leonardi, A., & Crescenzi, E. (2024). Therapy-Induced Senescence: Novel Approaches for Markers Identification. International Journal of Molecular Sciences, 25(15), 8448. https://doi.org/10.3390/ijms25158448







