Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters
Abstract
1. Introduction
2. Results
2.1. NP Cell Surface Marker Expression
| Category | No | Age (Years) | Sex | Pfirrmann Grades [57] | Level(s) | Indication | Tie2 (%) | GD2 (%) | CD24 (%) |
|---|---|---|---|---|---|---|---|---|---|
| YOUNG | 1 | 16 | M | 2 | L4–L5 | LDH | 20.7 | 12.3 | 17.8 |
| 2 † | 14 | F | / | / | LDH | 24.0 | 16.0 | 6.1 | |
| 3 | 23 | M | 3 | L5-S1 | LDH | 17.7 | 11.6 | 10.6 | |
| Average | 18 ± 5 | 20.8 ± 3.2 | 13.3 ± 2.4 | 11.5 ± 5.9 | |||||
| OLD | 4 | 64 | M | 4 | L3–L5 | LCS | 14.5 | 46.4 | 10.4 |
| 5 | 65 | M | 5 | L3–L5 | LCS | 21.6 | 58.5 | 10.9 | |
| 6 | 66 | F | 4 | L4–L5 | LCS | 16.3 | 39.6 | 8.6 | |
| Average | 65 ± 1 | 17.5 ± 3.7 | 48.2 ± 9.6 | 10.0 ± 1.2 | |||||
| p-value * | <0.0001 | 0.300 | 0.004 | 0.682 | |||||
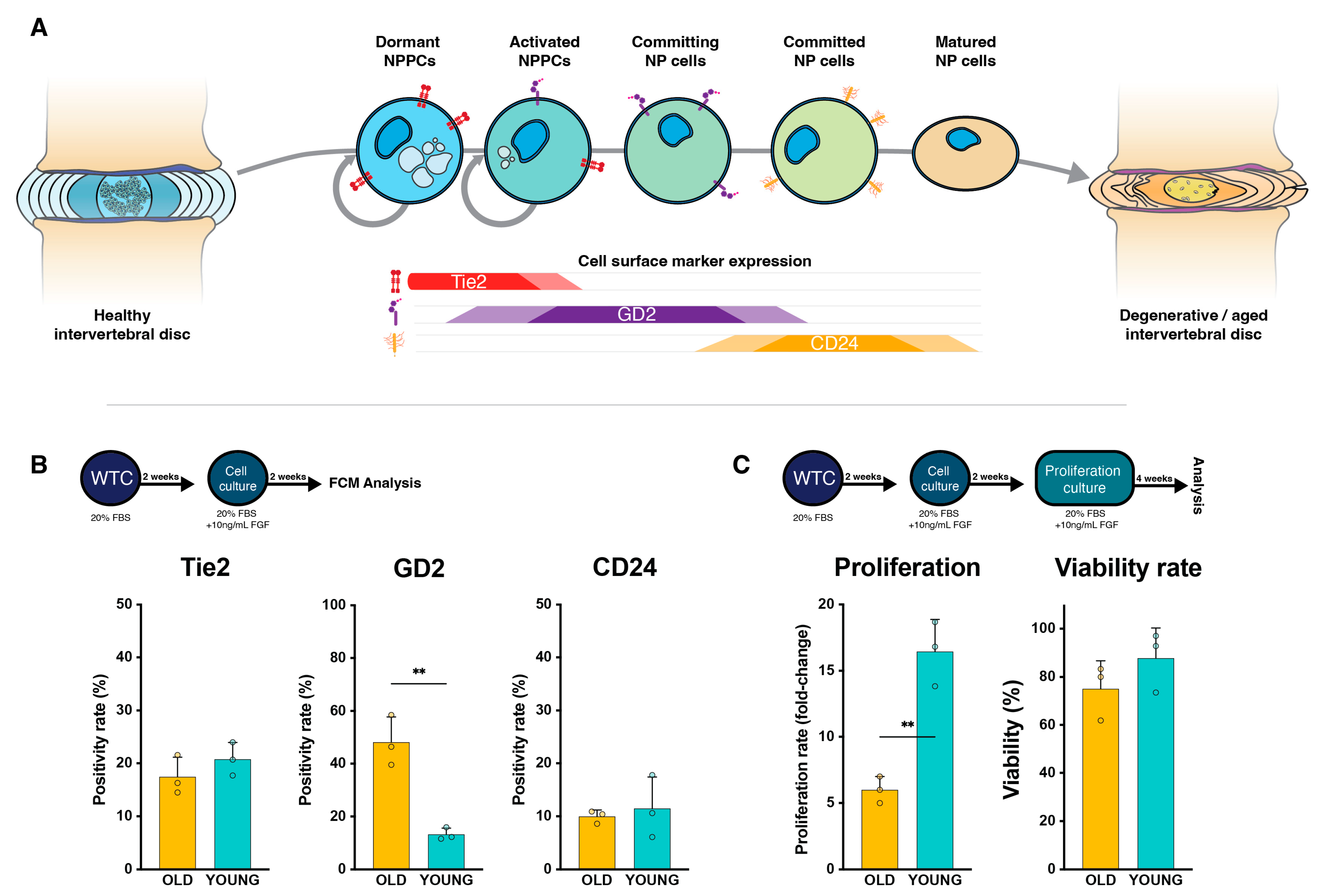
2.2. NP Cells’ Proliferation Rate and Viability
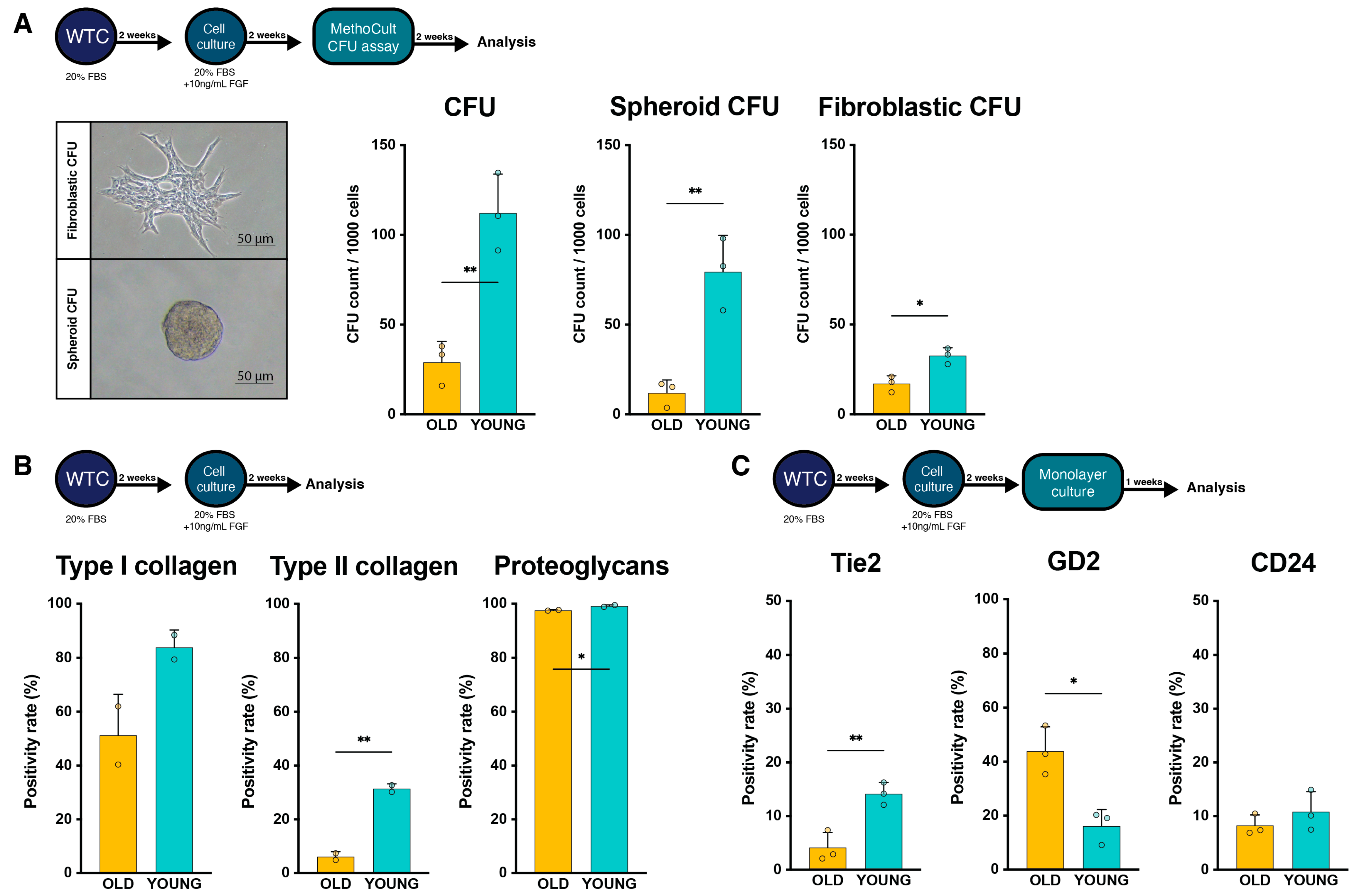
2.3. Regenerative Potential Assessment
2.4. Change in Cell Surface Markers with Extended Culture
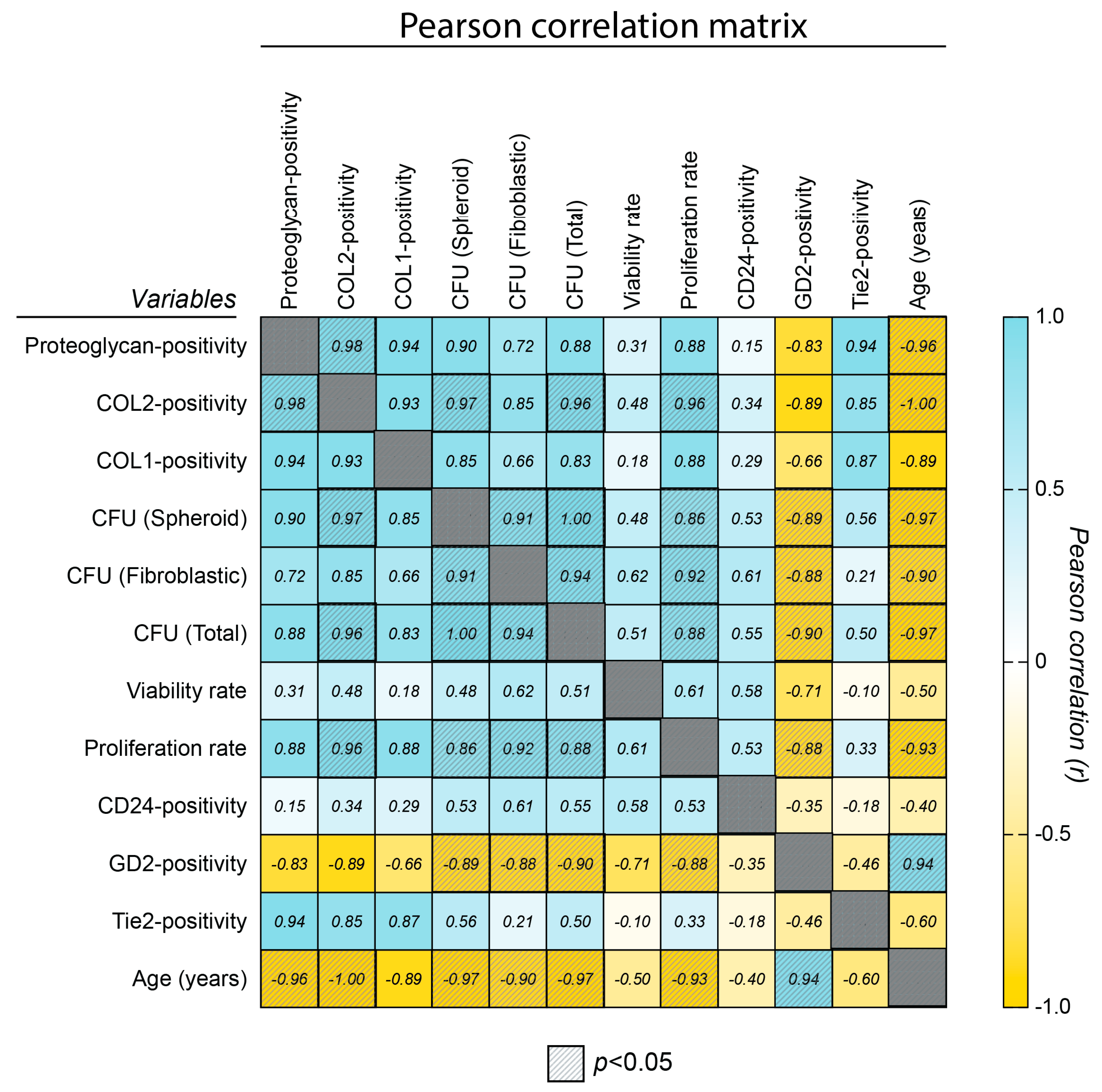
2.5. Correlation Assessment
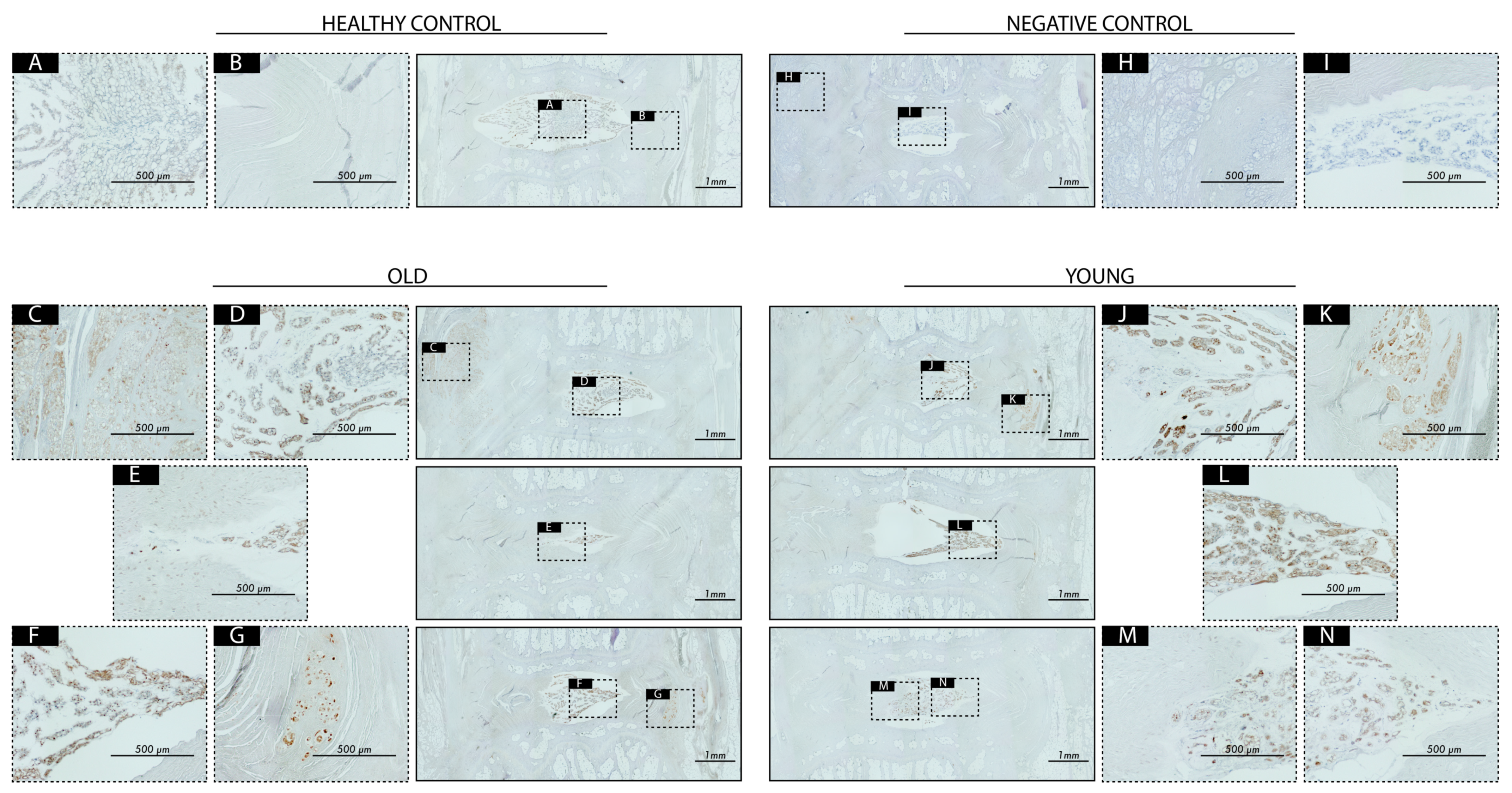
2.6. Transplantation Surgery
3. Discussion
Limitations and Considerations
4. Materials and Methods
4.1. Human NP Cell Isolation and Culture
4.2. Flow Cytometry Analysis
4.3. Proliferation Assessment and Viability Assay
4.4. Methylcellulose-Based Colony-Forming Unit Assay
4.5. Rat Disc Degeneration Model and Cell Transplantation
4.6. Radiographic Assessment
4.7. Disc Explantation and Processing
4.8. Histological Assessment
4.9. Statistic Analysis, Randomization, and Data Presentation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Härtl, R.; Bonassar, L.; Bonassar, L.J. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians; Thieme Medical Publishers, Incorporated: New York, NY, USA, 2017. [Google Scholar]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef] [PubMed]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordonana, J.R.; Ferreira, P.H. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J. 2015, 15, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Theologis, A.A.; O’Connell, G.D. Understanding the etiopathogenesis of lumbar intervertebral disc herniation: From clinical evidence to basic scientific research. JOR Spine 2024, 7, e1289. [Google Scholar] [CrossRef]
- Fainor, M.; Orozco, B.S.; Muir, V.G.; Mahindroo, S.; Gupta, S.; Mauck, R.L.; Burdick, J.A.; Smith, H.E.; Gullbrand, S.E. Mechanical crosstalk between the intervertebral disc, facet joints, and vertebral endplate following acute disc injury in a rabbit model. JOR Spine 2023, 6, e1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, S.; Li, B.; Tian, W.; Zhou, Z.; Liu, S. Intradiscal injection for the management of low back pain. JOR Spine 2022, 5, e1186. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, D.G. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr. Opin. Rheumatol. 2000, 12, 143–149. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 2007, 9, R45. [Google Scholar] [CrossRef]
- Roughley, P.J. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine 2004, 29, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Ohtori, S.; Nakagawa, K.; Nakajima, A.; Inoue, G.; Miyagi, M.; Takahashi, K. Neural Mechanisms of Discogenic Back Pain: How Does Nerve Growth Factor Play a Key Role? Korean J. Spine 2011, 8, 83–87. [Google Scholar] [CrossRef]
- Jiang, W.; Glaeser, J.D.; Kaneda, G.; Sheyn, J.; Wechsler, J.T.; Stephan, S.; Salehi, K.; Chan, J.L.; Tawackoli, W.; Avalos, P.; et al. Intervertebral disc human nucleus pulposus cells associated with back pain trigger neurite outgrowth in vitro and pain behaviors in rats. Sci. Transl. Med. 2023, 15, eadg7020. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Faraj, S.S.A.; Ket, J.C.F.; Emanuel, K.S.; Smit, T.H. Prognostic factors in the progression of intervertebral disc degeneration: Which patient should be targeted with regenerative therapies? JOR Spine 2019, 2, e1063. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.A.; Smit, T.H. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.; et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Wu, H.; Shang, Y.; Yu, J.; Zeng, X.; Lin, J.; Tu, M.; Cheang, L.H.; Zhang, J. Regenerative potential of human nucleus pulposus resident stem/progenitor cells declines with ageing and intervertebral disc degeneration. Int. J. Mol. Med. 2018, 42, 2193–2202. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheung, C.W.; Wong, S.S.C. Regenerative medicine for the treatment of chronic low back pain: A narrative review. J. Int. Med. Res. 2023, 51, 3000605231155777. [Google Scholar] [CrossRef]
- Schol, J.; Tamagawa, S.; Volleman, T.N.E.; Ishijima, M.; Sakai, D. A comprehensive review of cell transplantation and platelet-rich plasma therapy for the treatment of disc degeneration-related back and neck pain: A systematic evidence-based analysis. JOR Spine 2024, 7, e1348. [Google Scholar] [CrossRef]
- Gornet, M.F.; Beall, D.P.; Davis, T.T.; Coric, D.; LaBagnara, M.; Krull, A.; DePalma, M.J.; Hsieh, P.C.; Mallempati, S.; Schranck, F.W.; et al. Allogeneic Disc Progenitor Cells Safely Increase Disc Volume and Improve Pain, Disability, and Quality of Life in Patients with Lumbar Disc Degeneration-Results of an FDA-Approved Biologic Therapy Randomized Clinical Trial. Int. J. Spine Surg. 2024, 18, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Tryfonidou, M.A.; Snuggs, J.W.; Le Maitre, C.L. Cell sources proposed for nucleus pulposus regeneration. JOR Spine 2021, 4, e1175. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Hoyland, J.A.; Fujii, K.; Pandit, A.; Iatridis, J.C.; Grad, S. Critical aspects and challenges for intervertebral disc repair and regeneration-Harnessing advances in tissue engineering. JOR Spine 2018, 1, e1029. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, S.; Sakai, D.; Schol, J.; Sako, K.; Nakamura, Y.; Matsushita, E.; Warita, T.; Hazuki, S.; Nojiri, H.; Sato, M.; et al. N-acetylcysteine attenuates oxidative stress-mediated cell viability loss induced by dimethyl sulfoxide in cryopreservation of human nucleus pulposus cells: A potential solution for mass production. JOR Spine 2022, 5, e1223. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Petrucci, G.; Russo, F.; Cicione, C.; Papalia, R.; Vadala, G.; Denaro, V. Why clinical trials in disc regeneration strive to achieve completion: Insights from publication status and funding sources. JOR Spine 2024, 7, e1329. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Laagland, L.T.; Bach, F.C.; Ward, L.; Chan, W.; Tam, V.; Medzikovic, A.; Basatvat, S.; Paillat, L.; Vedrenne, N.; et al. Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization. JOR Spine 2023, 6, e1272. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.C.; Poramba-Liyanage, D.W.; Riemers, F.M.; Guicheux, J.; Camus, A.; Iatridis, J.C.; Chan, D.; Ito, K.; Le Maitre, C.L.; Tryfonidou, M.A. Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration. Front. Cell Dev. Biol. 2021, 9, 780749. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Schol, J.; Ruiz-Fernández, C.; Tamagawa, S.; Joyce, K.; Nomura, A.; de Rinaldis, E.; Sakai, D.; Papalia, R.; Vadalà, G.; et al. Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus. J. Dev. Biol. 2024, 12, 18. [Google Scholar] [CrossRef]
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, X.; Yu, J.; Shang, Y.; Tu, M.; Cheang, L.H.; Zhang, J. Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 2017, 361, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, T.; Zhang, W.; Yang, M.; Li, Z. Autologous cultured adipose derived mesenchymal stem cells combined with hyaluronic acid hydrogel in the treatment of discogenic low back pain: A study protocol for a phase II randomised controlled trial. BMJ Open 2022, 12, e063925. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Knowles, R.; Tyler, J.; Mobasheri, A.; Hoyland, J.A. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem. Cell Biol. 2008, 129, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battie, M.C.; Seguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; An, H.J.; Yeo, H.; Jeong, Y.; Lee, H.; Lee, J.; Nam, K.; Lee, J.; Shin, D.E.; Lee, S. Activation of Hypoxia-Inducible Factor-1alpha Signaling Pathway Has the Protective Effect of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2021, 22, 11355. [Google Scholar] [CrossRef]
- Kirnaz, S.; Capadona, C.; Lintz, M.; Kim, B.; Yerden, R.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B., Jr.; Bonassar, L.J.; et al. Pathomechanism and Biomechanics of Degenerative Disc Disease: Features of Healthy and Degenerated Discs. Int. J. Spine Surg. 2021, 15, 10–25. [Google Scholar] [CrossRef]
- Sako, K.; Sakai, D.; Nakamura, Y.; Schol, J.; Matsushita, E.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Effect of Whole Tissue Culture and Basic Fibroblast Growth Factor on Maintenance of Tie2 Molecule Expression in Human Nucleus Pulposus Cells. Int. J. Mol. Sci. 2021, 22, 4723. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Schol, J.; Bach, F.C.; Tekari, A.; Sagawa, N.; Nakamura, Y.; Chan, S.C.W.; Nakai, T.; Creemers, L.B.; Frauchiger, D.A.; et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine 2018, 1, e1018. [Google Scholar] [CrossRef]
- Soma, H.; Sakai, D.; Nakamura, Y.; Tamagawa, S.; Warita, T.; Schol, J.; Matsushita, E.; Naiki, M.; Sato, M.; Watanabe, M. Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 16713. [Google Scholar] [CrossRef]
- Bischof, M.C.; Häckel, S.; Oberli, A.; Croft, A.S.; Oswald, K.A.C.; Albers, C.E.; Gantenbein, B.; Guerrero, J. Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype. Appl. Sci. 2021, 11, 7144. [Google Scholar] [CrossRef]
- Nukaga, T.; Sakai, D.; Schol, J.; Suyama, K.; Nakai, T.; Hiyama, A.; Watanabe, M. Minimal Sustainability of Dedifferentiation by ROCK Inhibitor on Rat Nucleus Pulposus Cells In Vitro. Spine Surg. Relat. Res. 2019, 3, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Hackel, S.; Croft, A.S.; Albers, C.E.; Gantenbein, B. The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency. JOR Spine 2021, 4, e1131. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Sakai, D.; Schol, J.; Nakai, T.; Suyama, K.; Watanabe, M. Sciatic nerve regeneration by transplantation of in vitro differentiated nucleus pulposus progenitor cells. Regen. Med. 2017, 12, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806.e2795. [Google Scholar] [CrossRef] [PubMed]
- Tekari, A.; Chan, S.C.W.; Sakai, D.; Grad, S.; Gantenbein, B. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res. Ther. 2016, 7, 75. [Google Scholar] [CrossRef]
- Ambrosio, L.; Schol, J.; Ruiz-Fernadez, C.; Tamagawa, S.; Soma, H.; Tilotta, V.; Di Giacomo, G.; Cicione, C.; Nakayama, S.; Kamiya, K.; et al. ISSLS PRIZE in Basic Science 2024: Superiority of nucleus pulposus cell-versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur. Spine J. 2024, 33, 1713–1727. [Google Scholar] [CrossRef]
- Schol, J.; Sakai, D.; Warita, T.; Nukaga, T.; Sako, K.; Wangler, S.; Tamagawa, S.; Zeiter, S.; Alini, M.; Grad, S. Homing of vertebral-delivered mesenchymal stromal cells for degenerative intervertebral discs repair—An in vivo proof-of-concept study. JOR Spine 2023, 6, e1228. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gong, J.; Zhang, H.; Qin, J.; Li, C.; Zhang, J.; Tang, Y.; Zhang, Y.; Chen, J.; Zhou, Y.; et al. Cartilage Endplate Stem Cells Transdifferentiate into Nucleus Pulposus Cells via Autocrine Exosomes. Front. Cell Dev. Biol. 2021, 9, 648201. [Google Scholar] [CrossRef]
- Xia, K.S.; Li, D.D.; Wang, C.G.; Ying, L.W.; Wang, J.K.; Yang, B.; Shu, J.W.; Huang, X.P.; Zhang, Y.A.; Yu, C.; et al. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023, 21, 69–85. [Google Scholar] [CrossRef]
- Takahashi, T.; Donahue, R.P.; Nordberg, R.C.; Hu, J.C.; Currall, S.C.; Athanasiou, K.A. Commercialization of regenerative-medicine therapies. Nat. Rev. Bioeng. 2023, 1, 906–929. [Google Scholar] [CrossRef]
- Sako, K.; Sakai, D.; Nakamura, Y.; Matsushita, E.; Schol, J.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Optimization of Spheroid Colony Culture and Cryopreservation of Nucleus Pulposus Cells for the Development of Intervertebral Disc Regenerative Therapeutics. Appl. Sci. 2021, 11, 3309. [Google Scholar] [CrossRef]
- Munesada, D.; Sakai, D.; Nakamura, Y.; Schol, J.; Matsushita, E.; Tamagawa, S.; Sako, K.; Ogasawara, S.; Sato, M.; Watanabe, M. Investigation of the Mitigation of DMSO-Induced Cytotoxicity by Hyaluronic Acid following Cryopreservation of Human Nucleus Pulposus Cells. Int. J. Mol. Sci. 2023, 24, 12289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guerrero, J.; Croft, A.S.; Albers, C.E.; Hackel, S.; Gantenbein, B. Spheroid-Like Cultures for Expanding Angiopoietin Receptor-1 (aka. Tie2) Positive Cells from the Human Intervertebral Disc. Int. J. Mol. Sci. 2020, 21, 9423. [Google Scholar] [CrossRef]
- Silverman, L.I.; Dulatova, G.; Tandeski, T.; Erickson, I.E.; Lundell, B.; Toplon, D.; Wolff, T.; Howard, A.; Chintalacharuvu, S.; Foley, K.T. In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration. Spine J. 2020, 20, 138–149. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.M. The Angiopoietin-Tie2 Signaling Axis in Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 1973–1982. [Google Scholar] [CrossRef]
- Richards, R.M.; Sotillo, E.; Majzner, R.G. CAR T Cell Therapy for Neuroblastoma. Front. Immunol. 2018, 9, 2380. [Google Scholar] [CrossRef]
- Gilliam, D.T.; Menon, V.; Bretz, N.P.; Pruszak, J. The CD24 surface antigen in neural development and disease. Neurobiol. Dis. 2017, 99, 133–144. [Google Scholar] [CrossRef]
- Hiraishi, S.; Schol, J.; Sakai, D.; Nukaga, T.; Erickson, I.; Silverman, L.; Foley, K.; Watanabe, M. Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model. JOR Spine 2018, 1, e1013. [Google Scholar] [CrossRef]
- Barcellona, M.N.; McDonnell, E.E.; Samuel, S.; Buckley, C.T. Rat tail models for the assessment of injectable nucleus pulposus regeneration strategies. JOR Spine 2022, 5, e1216. [Google Scholar] [CrossRef]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.; Bishop, P.B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef]
- Lai, A.; Gansau, J.; Gullbrand, S.E.; Crowley, J.; Cunha, C.; Dudli, S.; Engiles, J.B.; Fusellier, M.; Goncalves, R.M.; Nakashima, D.; et al. Development of a standardized histopathology scoring system for intervertebral disc degeneration in rat models: An initiative of the ORS spine section. JOR Spine 2021, 4, e1150. [Google Scholar] [CrossRef]
- Leppanen, V.M.; Saharinen, P.; Alitalo, K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proc. Natl. Acad. Sci. USA 2017, 114, 4376–4381. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Huang, X.L.; Naruse, T.; Hamaguchi, I.; Dumont, D.J.; Yancopoulos, G.D.; Suda, T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998, 9, 677–686. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.M.; Reinardy, J.L.; Mueller, S.B.; McCord, T.J.; Kontos, C.D.; Brown, D.A.; Hussain, S.N.; Schmidt, C.A.; Ryan, T.E.; Green, T.D. Muscle cell derived angiopoietin-1 contributes to both myogenesis and angiogenesis in the ischemic environment. Front. Physiol. 2015, 6, 161. [Google Scholar] [CrossRef]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Ito, K.; Koh, G.Y.; Suda, T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Dallabrida, S.M.; Ismail, N.; Oberle, J.R.; Himes, B.E.; Rupnick, M.A. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ. Res. 2005, 96, e8–e24. [Google Scholar] [CrossRef]
- Croft, A.S.; Guerrero, J.; Oswald, K.A.C.; Hackel, S.; Albers, C.E.; Gantenbein, B. Effect of different cryopreservation media on human nucleus pulposus cells’ viability and trilineage potential. JOR Spine 2021, 4, e1140. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, W.; Tu, X.X.; Zhang, T.; Hou, Z.J.; Guo, T.; Shu, X.; Luo, X.; Liu, Y.; Peng, F.; et al. Neural ganglioside GD2+ cells define a subpopulation of mesenchymal stem cells in adult murine bone marrow. Cell. Physiol. Biochem. 2013, 32, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Machy, P.; Mortier, E.; Birkle, S. Biology of GD2 ganglioside: Implications for cancer immunotherapy. Front. Pharmacol. 2023, 14, 1249929. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, Y.; Yang, J.; Liu, Q.; Shi, M.; Zhang, R.; Shi, H.; Ren, Q.; Ma, J.; Guo, H.; et al. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC Cancer 2011, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Liu-Bordes, W.Y.; Londono-Vallejo, A.; Vernot, J.P. CD24 expression and stem-associated features define tumor cell heterogeneity and tumorigenic capacities in a model of carcinogenesis. Cancer Manag. Res. 2018, 10, 5767–5784. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Binch, A.L.; Creemers, L.B.; Sammon, C.; Le Maitre, C.L. Nucleus pulposus phenotypic markers to determine stem cell differentiation: Fact or fiction? Oncotarget 2016, 7, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Vadala, G.; Russo, F.; Di Martino, A.; Denaro, V. Intervertebral disc regeneration: From the degenerative cascade to molecular therapy and tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lyu, F.J.; Wang, H.; Zheng, Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 2022, 5, e1196. [Google Scholar] [CrossRef] [PubMed]
- Heggli, I.; Laux, C.J.; Mengis, T.; Karol, A.; Cornaz, F.; Herger, N.; Aradi-Vegh, B.; Widmer, J.; Burkhard, M.D.; Farshad-Amacker, N.A.; et al. Modic type 2 changes are fibroinflammatory changes with complement system involvement adjacent to degenerated vertebral endplates. JOR Spine 2023, 6, e1237. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Zhang, Y.; Liu, C.; Zhuang, Y.; Liu, M.; Ai, X.; Long, D.; Huang, B.; Li, C.; et al. Dynamics of N6-methyladenosine modification during aging and their potential roles in the degeneration of intervertebral disc. JOR Spine 2024, 7, e1316. [Google Scholar] [CrossRef]
- Rider, S.M.; Mizuno, S.; Kang, J.D. Molecular Mechanisms of Intervertebral Disc Degeneration. Spine Surg. Relat. Res. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Carazzo, C.A.; Peletti-Figueiro, M.; Fontana Nicoletti, N.; Scariot, F.J.; Echeverrigaray, S.; Falavigna, A. Genotoxic parameters of human degenerated intervertebral discs are linked to the pathogenesis of disc degeneration. J. Neurosurg. Sci. 2024, 68, 310–319. [Google Scholar] [CrossRef]
- Buisman, S.C.; de Haan, G. Epigenetic Changes as a Target in Aging Haematopoietic Stem Cells and Age-Related Malignancies. Cells 2019, 8, 868. [Google Scholar] [CrossRef]
- Picerno, A.; Stasi, A.; Franzin, R.; Curci, C.; di Bari, I.; Gesualdo, L.; Sallustio, F. Why stem/progenitor cells lose their regenerative potential. World J. Stem Cells 2021, 13, 1714–1732. [Google Scholar] [CrossRef]
- Srinageshwar, B.; Maiti, P.; Dunbar, G.L.; Rossignol, J. Role of Epigenetics in Stem Cell Proliferation and Differentiation: Implications for Treating Neurodegenerative Diseases. Int. J. Mol. Sci. 2016, 17, 199. [Google Scholar] [CrossRef]
- Armstrong, L.; Al-Aama, J.; Stojkovic, M.; Lako, M. Concise review: The epigenetic contribution to stem cell ageing: Can we rejuvenate our older cells? Stem Cells 2014, 32, 2291–2298. [Google Scholar] [CrossRef]
- Dompe, C.; Janowicz, K.; Hutchings, G.; Moncrieff, L.; Jankowski, M.; Nawrocki, M.J.; Jozkowiak, M.; Mozdziak, P.; Petitte, J.; Shibli, J.A.; et al. Epigenetic Research in Stem Cell Bioengineering-Anti-Cancer Therapy, Regenerative and Reconstructive Medicine in Human Clinical Trials. Cancers 2020, 12, 1016. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Qiu, T.; Zhang, H.; Liao, L.; Su, X. Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases. Stem Cell Res. Ther. 2022, 13, 201. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J.; Watanabe, M. Clinical Development of Regenerative Medicine Targeted for Intervertebral Disc Disease. Medicina 2022, 58, 267. [Google Scholar] [CrossRef]
- Lu, L.; Xu, A.; Gao, F.; Tian, C.; Wang, H.; Zhang, J.; Xie, Y.; Liu, P.; Liu, S.; Yang, C.; et al. Mesenchymal Stem Cell-Derived Exosomes as a Novel Strategy for the Treatment of Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2021, 9, 770510. [Google Scholar] [CrossRef]
- Lu, K.; Li, H.Y.; Yang, K.; Wu, J.L.; Cai, X.W.; Zhou, Y.; Li, C.Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 108. [Google Scholar] [CrossRef]
- Croft, A.S.; Illien-Junger, S.; Grad, S.; Guerrero, J.; Wangler, S.; Gantenbein, B. The Application of Mesenchymal Stromal Cells and Their Homing Capabilities to Regenerate the Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 3519. [Google Scholar] [CrossRef]
- Cunha, C.; Almeida, C.R.; Almeida, M.I.; Silva, A.M.; Molinos, M.; Lamas, S.; Pereira, C.L.; Teixeira, G.Q.; Monteiro, A.T.; Santos, S.G.; et al. Systemic Delivery of Bone Marrow Mesenchymal Stem Cells for In Situ Intervertebral Disc Regeneration. Stem Cells Transl. Med. 2017, 6, 1029–1039. [Google Scholar] [CrossRef]
- Tilotta, V.; Vadala, G.; Ambrosio, L.; Di Giacomo, G.; Cicione, C.; Russo, F.; Darinskas, A.; Papalia, R.; Denaro, V. Wharton’s Jelly mesenchymal stromal cell-derived extracellular vesicles promote nucleus pulposus cell anabolism in an in vitro 3D alginate-bead culture model. JOR Spine 2024, 7, e1274. [Google Scholar] [CrossRef]
- DiStefano, T.J.; Vaso, K.; Danias, G.; Chionuma, H.N.; Weiser, J.R.; Iatridis, J.C. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv. Healthc. Mater. 2022, 11, e2100596. [Google Scholar] [CrossRef]
- Samanta, A.; Lufkin, T.; Kraus, P. Intervertebral disc degeneration-Current therapeutic options and challenges. Front. Public Health 2023, 11, 1156749. [Google Scholar] [CrossRef]
- Costachescu, B.; Niculescu, A.G.; Teleanu, R.I.; Iliescu, B.F.; Radulescu, M.; Grumezescu, A.M.; Dabija, M.G. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. Int. J. Mol. Sci. 2022, 23, 6460. [Google Scholar] [CrossRef]
- McDonnell, E.E.; Wilson, N.; Barcellona, M.N.; Ni Neill, T.; Bagnall, J.; Brama, P.A.J.; Cunniffe, G.M.; Darwish, S.L.; Butler, J.S.; Buckley, C.T. Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration. JOR Spine 2023, 6, e1279. [Google Scholar] [CrossRef]
- Shalash, W.; Ahrens, S.R.; Bardonova, L.A.; Byvaltsev, V.A.; Giers, M.B. Patient-specific apparent diffusion maps used to model nutrient availability in degenerated intervertebral discs. JOR Spine 2021, 4, e1179. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Liang, C.; Han, B.; Li, F.; Chen, G.; Chen, Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs 2013, 198, 266–277. [Google Scholar] [CrossRef]
- Feng, G.; Li, L.; Liu, H.; Song, Y.; Huang, F.; Tu, C.; Shen, B.; Gong, Q.; Li, T.; Liu, L.; et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthr. Cartil. 2013, 21, 582–588. [Google Scholar] [CrossRef]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Poletto, D.L.; Crowley, J.D.; Tanglay, O.; Walsh, W.R.; Pelletier, M.H. Preclinical in vivo animal models of intervertebral disc degeneration. Part 1: A systematic review. JOR Spine 2023, 6, e1234. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.A.; Herzog, R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. BioMed Res. Int. 2016, 2016, 5952165. [Google Scholar] [CrossRef]
- Ruiz-Fernandez, C.; Francisco, V.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Jaumard, N.V.; Leung, J.; Gokhale, A.J.; Guarino, B.B.; Welch, W.C.; Winkelstein, B.A. Relevant Anatomic and Morphological Measurements of the Rat Spine: Considerations for Rodent Models of Human Spine Trauma. Spine 2015, 40, E1084–E1092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, H.L.; Li, Z.; Li, B.B.; Zhu, M.; Chen, D.; Ye, F.H.; Yu, B.S.; Huang, Y.C. Species variation in the cartilaginous endplate of the lumbar intervertebral disc. JOR Spine 2022, 5, e1218. [Google Scholar] [CrossRef] [PubMed]
- Mosley, G.E.; Wang, M.; Nasser, P.; Lai, A.; Charen, D.A.; Zhang, B.; Iatridis, J.C. Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci. Rep. 2020, 10, 15120. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, J.Q.; Kaplar, Z. Increased low back pain prevalence in females than in males after menopause age: Evidences based on synthetic literature review. Quant. Imaging Med. Surg. 2016, 6, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, S.H.; Suzuki-Narita, M.; Gregoire, S.; Millecamps, M.; Stone, L.S. Voluntary running attenuates behavioural signs of low back pain: Dimorphic regulation of intervertebral disc inflammation in male and female SPARC-null mice. Osteoarthr. Cartil. 2022, 30, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, H.M.; Nunez, M.I.; Morales, X.; Lisiewski, L.E.; Burt, K.G.; Kim, M.K.M.; Campos, L.; Kiridly, N.; Hung, C.T.; Chahine, N.O. Sex differences in the biomechanical and biochemical responses of caudal rat intervertebral discs to injury. JOR Spine 2023, 6, e1299. [Google Scholar] [CrossRef] [PubMed]
- Leopold, S.S.; Hensinger, R.N.; Schoenfeld, A.J.; Swiontkowski, M.; Rossi, M.J.; Templeton, K.J.; Sex and Gender Research in Orthopaedic Journals Group. Improving how orthopedic journals report research outcomes based on sex and gender. JOR Spine 2024, 7, e1334. [Google Scholar] [CrossRef] [PubMed]
- Vadala, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2012, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sancho, J.; Sanchez, A.; Vega, A.; Noriega, D.C.; Nocito, M. Influence of HLA Matching on the Efficacy of Allogeneic Mesenchymal Stromal Cell Therapies for Osteoarthritis and Degenerative Disc Disease. Transplant. Direct 2017, 3, e205. [Google Scholar] [CrossRef] [PubMed]
- Nukaga, T.; Sakai, D.; Schol, J.; Sato, M.; Watanabe, M. Annulus fibrosus cell sheets limit disc degeneration in a rat annulus fibrosus injury model. JOR Spine 2019, 2, e1050. [Google Scholar] [CrossRef]
- Boyd, L.M.; Carter, A.J. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur. Spine J. 2006, 15 (Suppl. S3), S414–S421. [Google Scholar] [CrossRef]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. [Google Scholar] [CrossRef]
- Wu, T.; Song, H.X.; Dong, Y.; Li, J.H. Cell-Based Therapies for Lumbar Discogenic Low Back Pain: Systematic Review and Single-Arm Meta-analysis. Spine 2018, 43, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Schol, J.; Sakai, D. Comprehensive narrative review on the analysis of outcomes from cell transplantation clinical trials for discogenic low back pain. N. Am. Spine Soc. J. 2023, 13, 100195. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine 2011, 36, E131–E138. [Google Scholar] [CrossRef] [PubMed]
- Basatvat, S.; Bach, F.C.; Barcellona, M.N.; Binch, A.L.; Buckley, C.T.; Bueno, B.; Chahine, N.O.; Chee, A.; Creemers, L.B.; Dudli, S.; et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR Spine 2023, 6, e1238. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Miyamoto, T.; Imai, J.; Hosogane, N.; Suzuki, T.; Yagi, M.; Morita, K.; Ninomiya, K.; Miyamoto, K.; Takaishi, H.; et al. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem. Biophys. Res. Commun. 2005, 338, 1890–1896. [Google Scholar] [CrossRef]
- Guan, X.; Ma, X.; Zhang, L.; Feng, H.; Ma, Z. Evaluation of CD24 as a marker to rapidly define the mesenchymal stem cell phenotype and its differentiation in human nucleus pulposus. Chin. Med. J. 2014, 127, 1474–1481. [Google Scholar]
- Morita, K.; Schol, J.; Volleman, T.N.E.; Sakai, D.; Sato, M.; Watanabe, M. Screening for Growth-Factor Combinations Enabling Synergistic Differentiation of Human MSC to Nucleus Pulposus Cell-Like Cells. Appl. Sci. 2021, 11, 3673. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.; Zhang, X.; Zhu, Z.; Yan, S.; Sun, T.; Guo, A.; Jones, J.; Steen, R.G.; Shan, B.; et al. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: A multicenter, randomized, double-blind, 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Res. Ther. 2015, 17, 51. [Google Scholar] [CrossRef]
- Mochizuki, T.; Ikari, K.; Yano, K.; Okazaki, K. Comparison of patient-reported outcomes of treatment with low- and intermediate molecular weight hyaluronic acid in Japanese patients with symptomatic knee osteoarthritis: A prospective, randomized, single-blind trial. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2020, 21, 22–26. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, Y.; Schol, J.; Sakai, D.; Nakamura, Y.; Sako, K.; Warita, T.; Tamagawa, S.; Ambrosio, L.; Munesada, D.; Ogasawara, S.; et al. Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters. Int. J. Mol. Sci. 2024, 25, 8335. https://doi.org/10.3390/ijms25158335
Otani Y, Schol J, Sakai D, Nakamura Y, Sako K, Warita T, Tamagawa S, Ambrosio L, Munesada D, Ogasawara S, et al. Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters. International Journal of Molecular Sciences. 2024; 25(15):8335. https://doi.org/10.3390/ijms25158335
Chicago/Turabian StyleOtani, Yuto, Jordy Schol, Daisuke Sakai, Yoshihiko Nakamura, Kosuke Sako, Takayuki Warita, Shota Tamagawa, Luca Ambrosio, Daiki Munesada, Shota Ogasawara, and et al. 2024. "Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters" International Journal of Molecular Sciences 25, no. 15: 8335. https://doi.org/10.3390/ijms25158335
APA StyleOtani, Y., Schol, J., Sakai, D., Nakamura, Y., Sako, K., Warita, T., Tamagawa, S., Ambrosio, L., Munesada, D., Ogasawara, S., Matsushita, E., Kawachi, A., Naiki, M., Sato, M., & Watanabe, M. (2024). Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters. International Journal of Molecular Sciences, 25(15), 8335. https://doi.org/10.3390/ijms25158335









