Influence of Lithium Triflate Salt Concentration on Structural, Thermal, Electrochemical, and Ionic Conductivity Properties of Cassava Starch Solid Biopolymer Electrolytes
Abstract
1. Introduction
2. Results
2.1. FTIR Spectroscopy of Cassava Starch SBPE Films
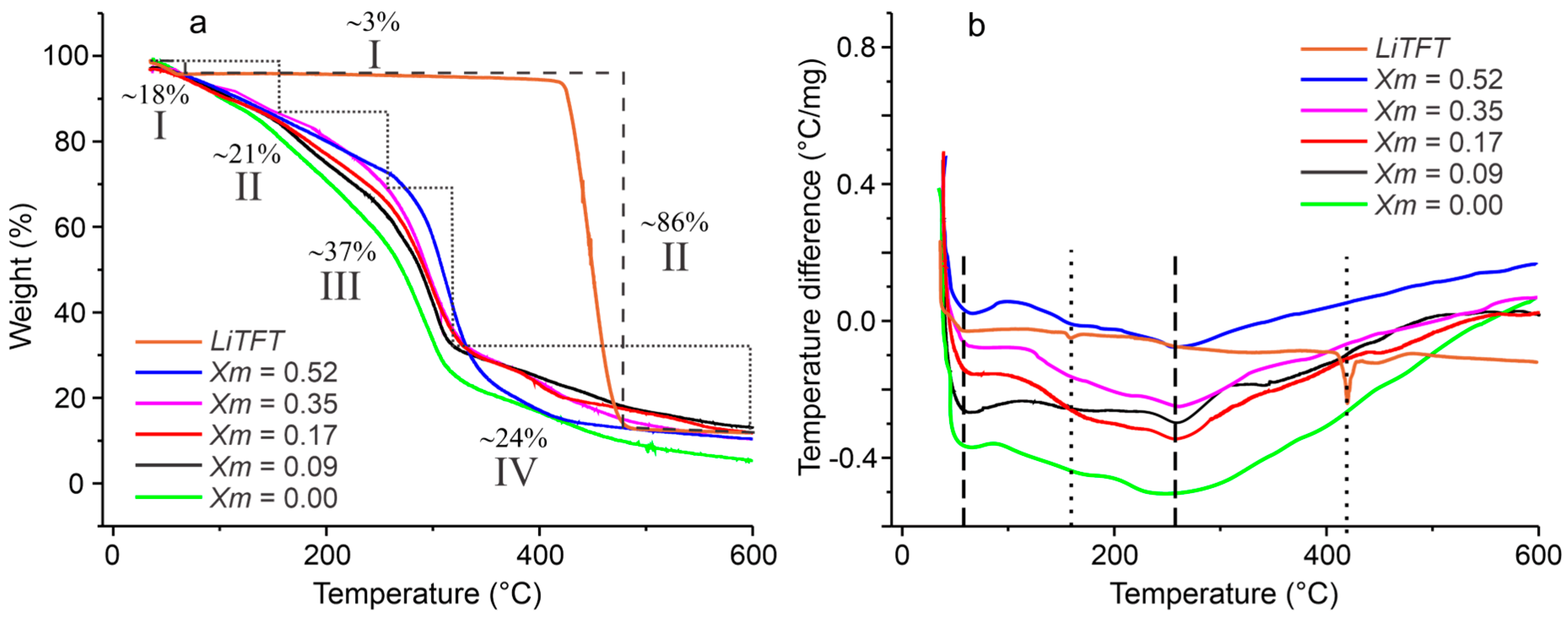
2.2. Thermal Characterization of SBPE Films
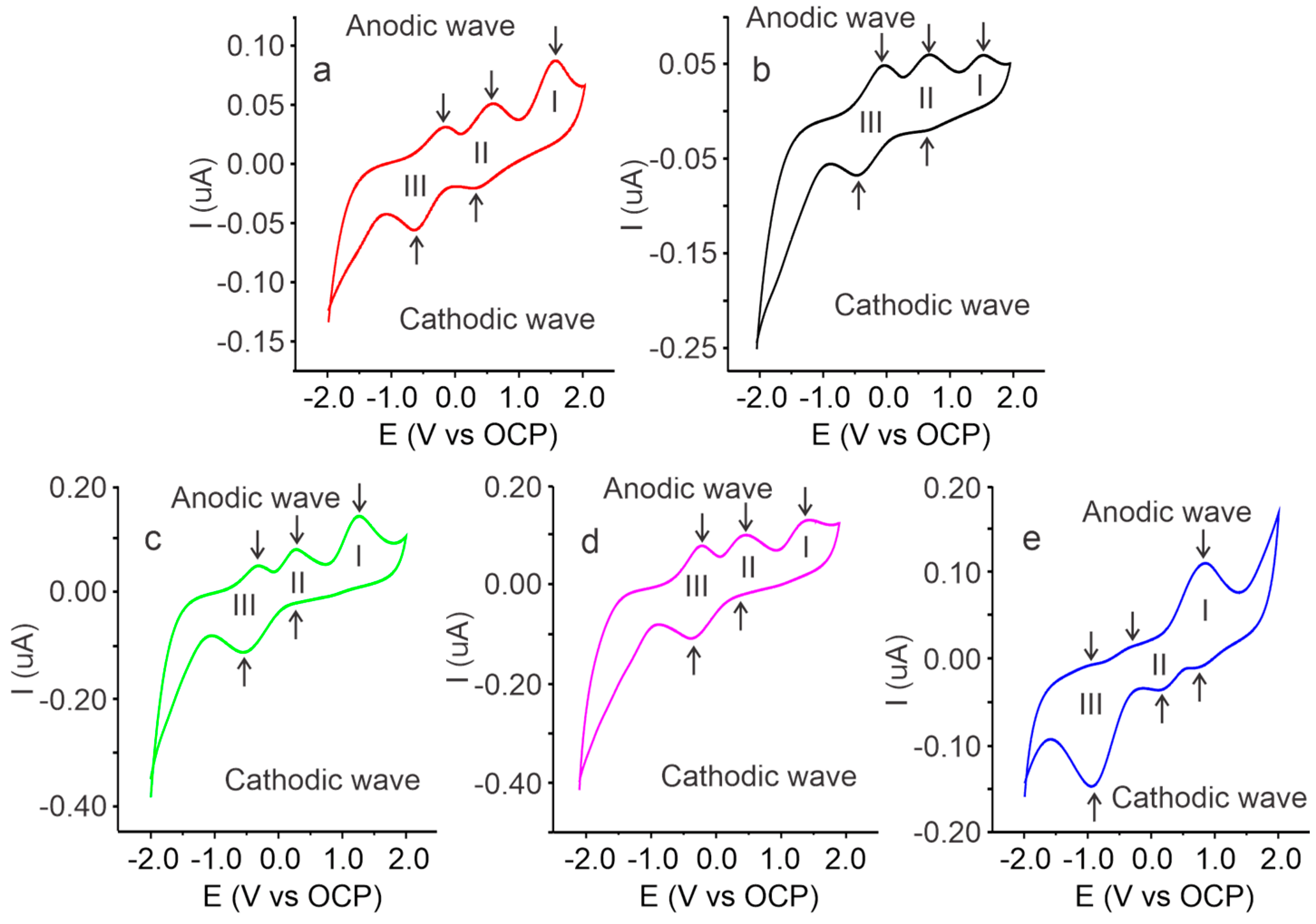
2.3. Voltammetric Characterization of Cassava Starch SBPE Films
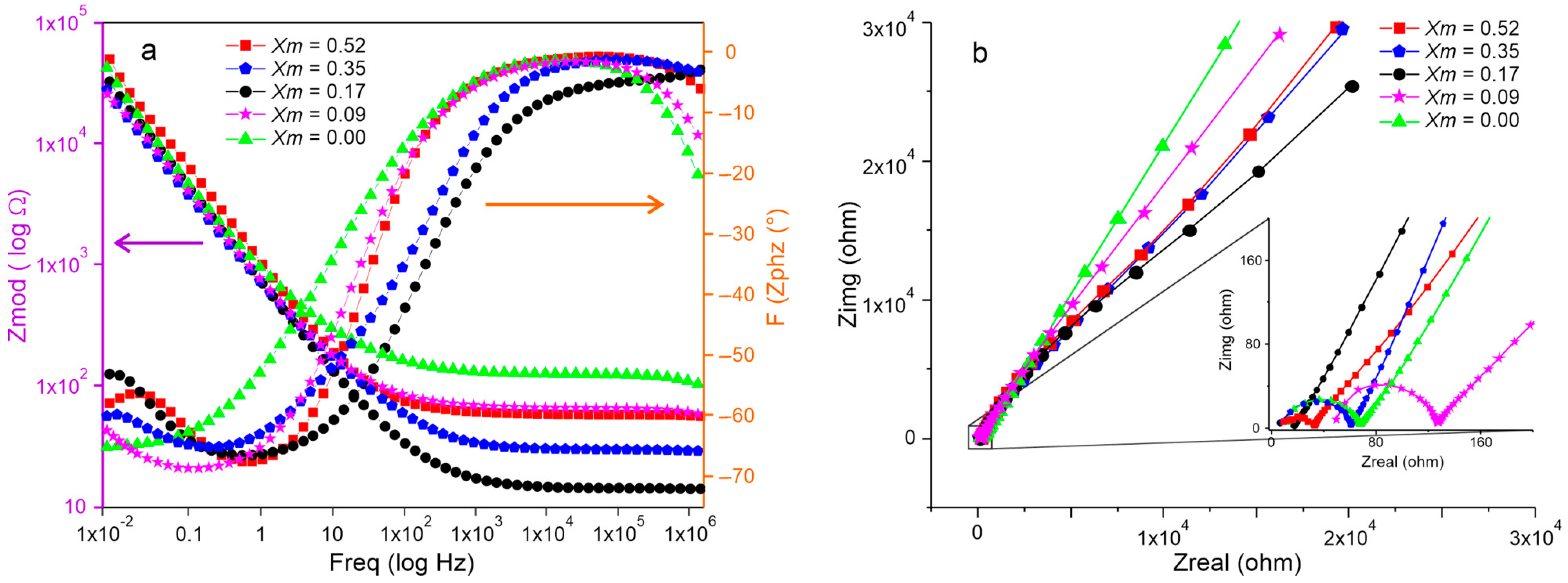
2.4. Electrochemical Impedance Spectroscopy Characterization of SBPE Films
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Cassava Starch SBPE Films with Different LiTFT Salt Concentrations
3.3. Characterization of Cassava Starch SBPEs
3.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.2. Thermal Analysis
3.3.3. Cyclic Voltammetry
3.3.4. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical conductivity in ionic complexes of poly (ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, T.; Jung, B.M.; Lee, S.B.; Choi, U.H. Multifunctional Epoxy-Based Solid Polymer Electrolytes for Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 35108–35117. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, R.D.; Shembel’, E.M.; Talyosef, I.; Grinblat, J.; Markovsky, B.; Aurbach, D. Study of electrolytic cobalt sulfide Co9S8 as an electrode material in lithium accumulator prototypes. Russ. J. Electrochem. 2009, 45, 311–319. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; Wang, C.; Zhao, C.; Vijayaraghavan, R.; MacFarlane, D.R.; Forsyth, M.; Wallace, G.G. Biocompatible Ionic Liquid–Biopolymer Electrolyte-Enabled Thin and Compact Magnesium–Air Batteries. ACS Appl. Mater. Interfaces 2014, 6, 21110–21117. [Google Scholar] [CrossRef] [PubMed]
- Xuesong, X.; Jingjing, L.; Zhengyue, X.; Bingan, L.; Shuquan, L.; Jiang, Z. Biocompatible zinc battery with programmable electro-cross-linked electrolyte. Natl. Sci. Rev. 2023, 10, nwac281. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Gañán, P.F.; Márquez, S.E.; Zuluaga, R. Electrically Conductive Bioplastics from Cassava Starch. J. Braz. Chem. Soc. 2011, 22, 1170–1176. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 2014, 20, 977–999. [Google Scholar] [CrossRef]
- Koliyoor, J.; Ismayil; Hegde, S.; Vasachar, R.; Sanjeev, G. Novel solid biopolymer electrolyte based on methyl cellulose with enhanced ion transport properties. J. Appl. Polym. Sci. 2021, 139, 51826. [Google Scholar] [CrossRef]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Franklin Benial, A.M.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Diana, M.I.; Selvin, P.C.; Selvasekarapandian, S.; Vengadesh Krishna, M. Investigations on Na-ion conducting electrolyte based on sodium alginate biopolymer for all-solid-state sodium-ion batteries. J. Solid State Electrochem. 2021, 25, 2009–2020. [Google Scholar] [CrossRef]
- Tamilisai, R.; Palanisamy, P.N.; Selvasekarapandian, S.; Maheshwari, T. Sodium alginate incorporated with magnesium nitrate as a novel solid biopolymer electrolyte for magnesium-ion batteries. J. Mater. Sci. Mater. Electron. 2021, 32, 22270–22285. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Shanthi, C.; Selvasekarapandian, S.; Manjuladevi, R.; Perumal, P.; Selvin, P.C. Synthesis and characterization of pectin-based biopolymer electrolyte for electrochemical applications. Ionics 2019, 25, 203–214. [Google Scholar] [CrossRef]
- Harikumar, M.E.; Batabyal, S.K. Fabrication of pectin biopolymer-based biocompatible freestanding electrodes for supercapacitor applications. Polym. Adv. Technol. 2023, 34, 2890–2902. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Structure, functionality and applications of debranched starch: A review. Trends Food Sci. Technol. 2017, 63, 70–79. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Nuñez de la Rosa, Y.; Palencia, M. Electrochemistry Study of Bio-Based Composite Biopolymer Electrolyte Starch/Cardol. Polymers 2023, 15, 1994. [Google Scholar] [CrossRef]
- Thrisha, K.; Saratha, R. Natural polymer-based electrolytes for energy storage devices-an overview. Ionics 2024, 30, 1245–1266. [Google Scholar] [CrossRef]
- Krishna Koduru, H.; Georgiev Marinov, Y.; Scaramuzza, N. Review on Microstructural and Ion-conductivity Properties of Biodegradable Starch-Based Solid Polymer Electrolyte Membranes. Starch 2022, 74, 2100170. [Google Scholar] [CrossRef]
- Hapuarachchi, S.N.S.; Wasalathilake, K.C.; Nerkar, J.Y.; Jaatinen, E.; O’Mullane, A.P.; Yan, C. Mechanically Robust Tapioca Starch Composite Binder with Improved Ionic Conductivity for Sustainable Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 9857–9865. [Google Scholar] [CrossRef]
- Mohammed, L.; Tamakloe, W.; Doodo-Arhin, D.; Adomah, R.; Zainudeen, M.N.; Opoku, J.; Nyamful, A.; Agyei-Tuffour, B.; Nyarko, A.; Mohammed, M.; et al. Exploratory study of the electrochemical properties of local cassava starch as a potential green and sustainable polymer binder for energy storage application. MRS Adv. 2023, 8, 708–713. [Google Scholar] [CrossRef]
- Priya, C.N.; Muthuvinayagam, M.; Ramesh, S.; Ramesh, K. Studies on structural, electrical and electrochemical properties of biodegradable PVP/starch blend polymer electrolytes with ammonium ceric nitrate for energy storage devices. Energy Environ. 2023, 34, 2740–2757. [Google Scholar] [CrossRef]
- Acosta Humánez, M.F.; Arrieta Almario, A.A.; Ávila Gómez, A.E.; Martínez Guarín, A.R.; Palacio Vega, M.A.; Nova Casares, V.A. Development of Charge Electrochemical Accumulator of Conductive Polypyrrole/Cassava Starch Biopolymers. In Proceedings of the ASME—International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13 November 2015. Volume 6A: Energy—V06AT07A044. [Google Scholar] [CrossRef]
- Núñez, Y.E.; Arrieta, A.A.; Segura, J.A.; Bertel, S.D. Synthesis of an air-working trilayer artificial muscle using a conductive cassava starch biofilm (manihot esculenta, cranz) and polypyrrole (PPy). J. Phys. Conf. Ser. 2016, 687, 012042. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Ionic conductivity and dielectric properties of potato starch-magnesium acetate biopolymer electrolytes: The effect of glycerol and 1-butyl-3-methylimidazolium chloride. Ionics 2016, 22, 1113–1123. [Google Scholar] [CrossRef]
- Vernon-Carter, E.; Alvarez-Ramirez, J.; Bello-Perez, L.; Roldan-Cruz, C.; Garcia-Hernandez, A.; Huerta, L. The order of addition of corn starch/lithium perchlorate/glycerol affects the optical, mechanical, and electrical properties of a solid polymer electrolyte. Ionics 2017, 23, 3111–3123. [Google Scholar] [CrossRef]
- Teoh, K.H.; Chin-Shen, L.; Ramesh, S. Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement 2014, 48, 87–95. [Google Scholar] [CrossRef]
- Ayala, G.; Agudelo, A.; Vargas, R. Effect of Glycerol on the Electrical Properties and Phase Behavior of Cassava Starch Biopolymers. Dyna 2012, 79, 138–147. [Google Scholar]
- Ramly, K.; Khiar, A.S.A. Electrical Properties of Starch/PEO Blend Polymer Electrolytes. Appl. Mech. Mater. 2015, 754-755, 29–33. [Google Scholar] [CrossRef]
- Khiar, A.S.A.; Anuar, M.R.S.; Md Parid, M.A. Effect of 1-Ethyl-3-Methylimidazolium Nitrate on the Electrical Properties of Starch/Chitosan Blend Polymer Electrolyte. Mater. Sci. Forum 2016, 846, 510–516. [Google Scholar] [CrossRef]
- Azahar, S.S.; Hamidon, T.S.; Latip, A.F.A.; Hussin, M.H. Physicochemical and conductivity studies of chitosan-tapioca flour-LiBF4 gel polymer electrolytes. Chem. Phys. Impact 2021, 3, 100055. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Calabokis, O.P.; Mendoza, J.M. Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes. Polymers 2023, 15, 4150. [Google Scholar] [CrossRef]
- Priya, C.N.; Muthuvinayagam, M.; Vahini, M. Environmental friendly solid blend polymer electrolytes based on PVA:starch:ceric ammonium nitrate for electric double layer capacitor (EDLC) applications. J. Mater. Sci. 2023, 58, 773–786. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Castillo, L.A.; López, O.V.; García, M.A.; Barbosa, S.E.; Villar, M.A. Crystalline morphology of thermoplastic starch/talc nanocomposites induced by thermal processing. Heliyon 2019, 5, e01877. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Dong Chung, T. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Finkenstadt, V.; Willett, J.L. Preparation and Characterization of Electroactive Biopolymers. Macromol. Symp. 2005, 227, 367–372. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.-W.; Arof, A.Ñ. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Non-Cryst. Solids 2011, 357, 3654–3660. [Google Scholar] [CrossRef]
- Bementa, E.; Jothi Rajan, M.A. Polymer Crust Electrolyte Based on Starch-Chitosan Blend Mixed with KI by Gelation Method. J. Appl. Sci. Eng. Methodol. 2016, 2, 336–341. [Google Scholar]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The Effect of Plasticization on Conductivity and Other Properties of Starch/Chitosan Blend Biopolymer Electrolyte Incorporated with Ammonium Iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]

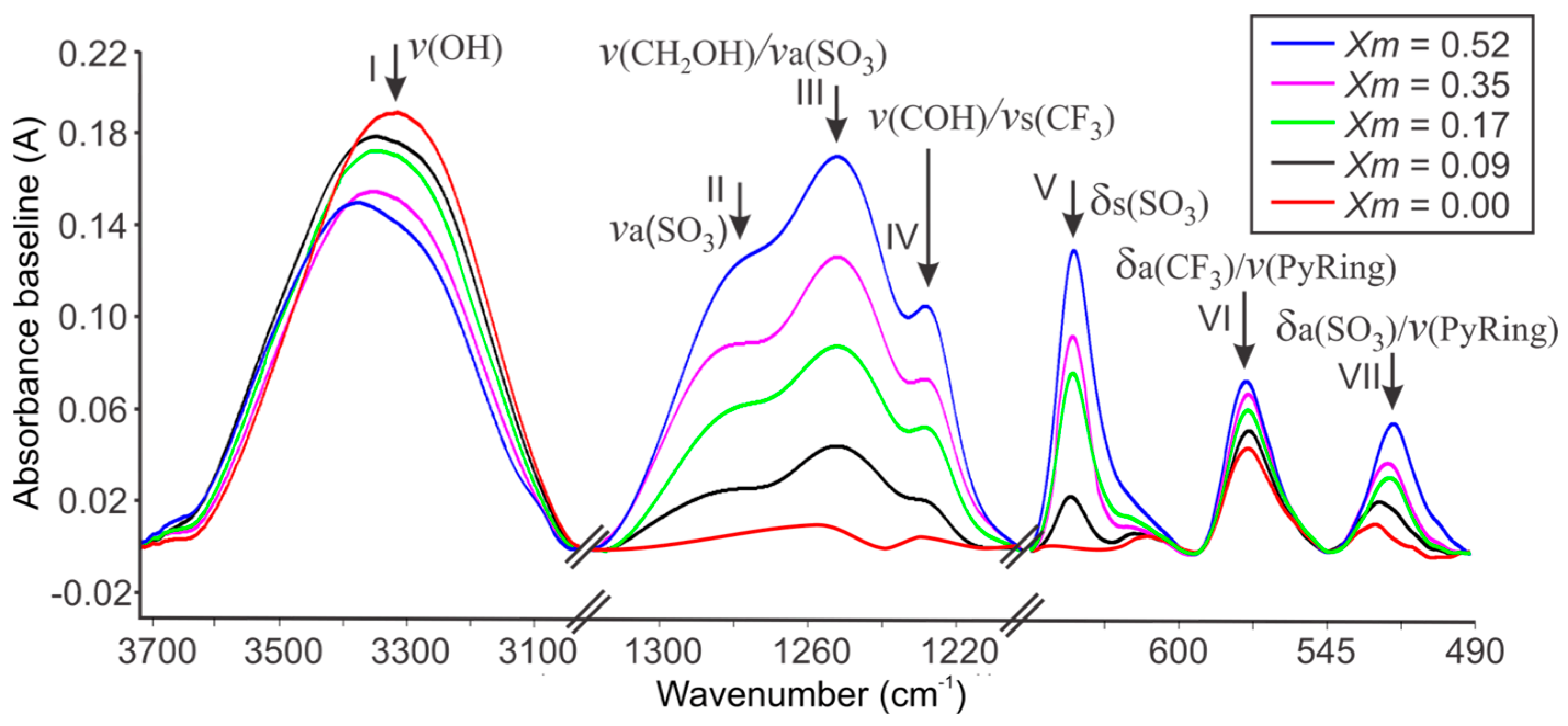
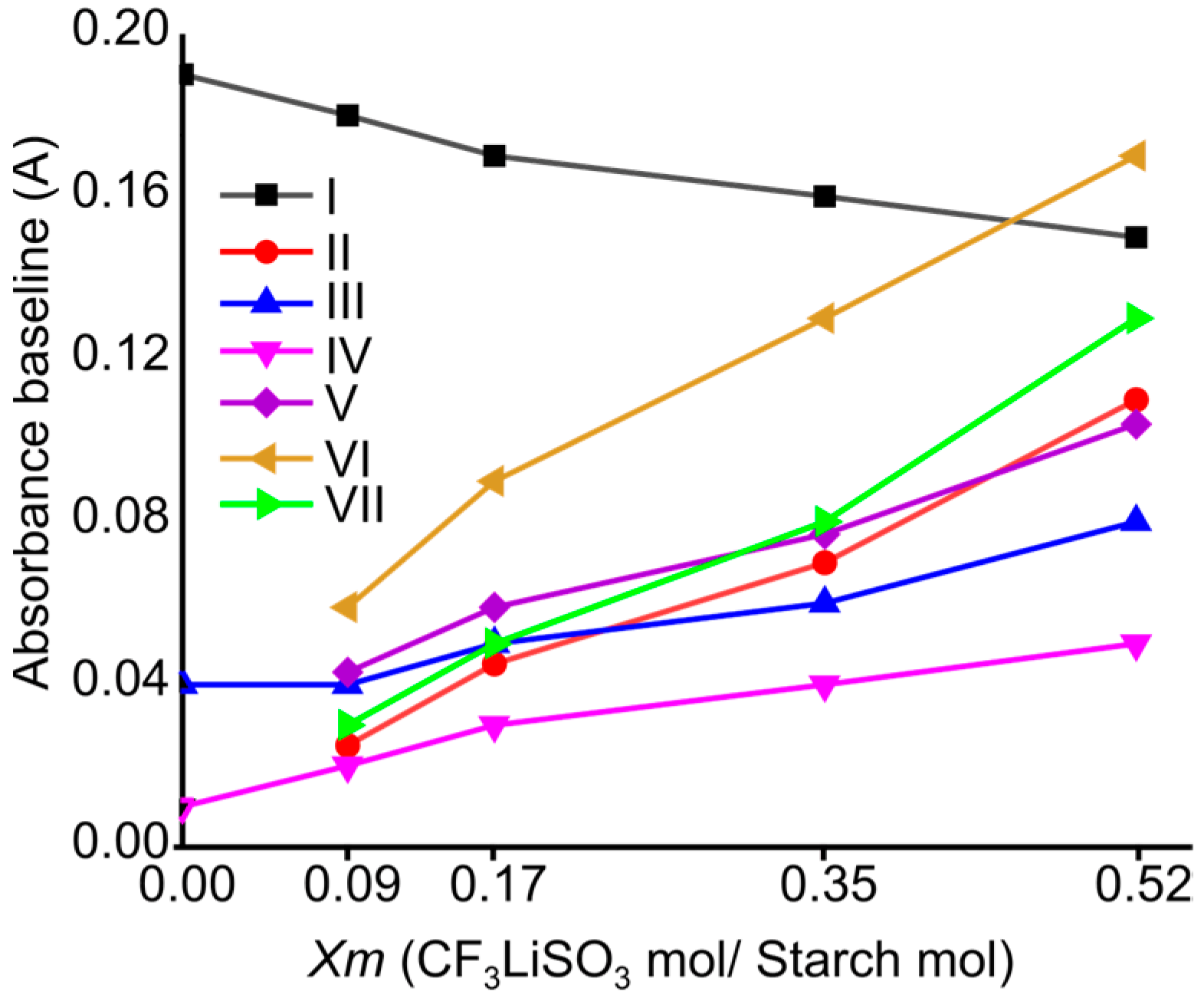


| Assignments | ) of SBPEs Xm = [LiTFT mol/starch mol] | LiTFT | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.09 | 0.17 | 0.35 | 0.52 | ||
| O–H stretching | 3364 | 3371 | 3377 | 3385 | 3399 | 3486 |
| C–H stretching | 2926–2878 | 2927–2878 | 2927–2878 | 2927–2878 | 2927–2878 | - |
| C=O stretching vibration | 1713 | 1713 | 1713 | 1713 | 1713 | - |
| O–H (water) bending/O-H bending (sulfonic) | 1649 | 1648 | 1648 | 1648 | 1648 | 1647 |
| C–H bending | 1456 | 1456 | 1456 | 1456 | 1456 | |
| O–H bending | 1407 | 1407 | 1408 | 1408 | 1408 | |
| COH bending/S=O stretching | 1350 | 1350 | 1350 | 1350 | 1350 | |
| CH2OH related modes/Asymmetric stretching mode of SO3 | 1247 | 1279–1250 | 1279–1250 | 1279–1250 | 1279–1250 | 1292–1248 |
| COH deformation/Symmetric stretching mode CF3 | 1202 | 1219 | 1219 | 1219 | 1219 | 1229 |
| Asymmetric stretching mode of CF3 | - | - | - | - | - | 1182 |
| CO antisymmetric bridge stretching | 1146 | 1145 | 1143 | 1142 | 1142 | - |
| COH antisymmetric stretching in plane ring | 1103 | 1103 | 1102 | 1102 | 1103 | - |
| C–OH bending | 1077 | 1078 | 1077 | 1077 | 1076 | - |
| Symmetric stretching mode of SO3 | - | - | - | - | - | 1039 |
| COC ring vibration of Carbohydrate | 1018 | 1021 | 1021 | 1021 | 1021 | - |
| COH solved | 995 | 998 | 998 | 998 | 998 | - |
| C-H bending modes | 844 | 843 | 843 | 843 | 843 | - |
| Symmetric bending mode CF3 | - | - | - | - | - | 773 |
| CH2 rocking | 757 | 757 | 757 | 757 | 757 | |
| Symmetric bending mode SO3 | 639 | 639 | 638 | 638 | 627 | |
| Asymmetric bending mode CF3/pyranose ring vibration | 570 | 571 | 571 | 571 | 571 | 574 |
| Pyranose ring vibration | 522 | 520 | 517 | 516 | 515 | |
| Asymmetric bending mode SO3 | - | - | - | - | - | 512 |
| Process | Peaks | Cassava Starch SBPEs with Different Salt Concentrations (Lithium Triflate) | |||||
|---|---|---|---|---|---|---|---|
| Xm = 0.00 | Xm = 0.09 | Xm = 0.17 | Xm = 0.35 | Xm = 0.52 | |||
| I | Anodic | Ep (V) | 1.52 | 1.54 | 1.26 | 1.50 | 0.85 |
| Ip (µA) | 0.096 | 0.067 | 0.144 | 0.132 | 0.111 | ||
| Cathodic | Ep (V) | - | - | - | - | 0.80 | |
| Ip (µA) | - | - | - | - | −0.010 | ||
| II | Anodic | Ep (V) | 0.56 | 0.70 | 0.26 | 0.54 | −0.21 |
| Ip (µA) | 0.055 | 0.069 | 0.083 | 0.104 | 0.014 | ||
| Cathodic | Ep (V) | 0.31 | 0.64 | 0.20 | 0.43 | 0.12 | |
| Ip (µA) | −0.028 | −0.012 | −0.022 | −0.019 | −0.038 | ||
| III | Anodic | Ep (V) | −0.19 | −0.02 | −0.32 | 0.13 | −0.85 |
| Ip (µA) | 0.032 | 0.058 | 0.052 | 0.087 | −0.005 | ||
| Cathodic | Ep (V) | −0.66 | −0.41 | −0.55 | −0.26 | −0.90 | |
| Ip (µA) | −0.070 | −0.059 | −0.112 | −0.108 | −0.014 | ||
| Salt Concentration (Xm) | Electrochemical Parameters | ||||
|---|---|---|---|---|---|
| Cdl (F) | Rct (Ω) | Rre (Ω) | CPE (S*sa) | Rf (Ω) | |
| 0.00 | 2.27 × 10−4 | 5.19 × 102 | 1.26 × 105 | 2.10 × 10−6 | 8.96 × 102 |
| 0.09 | 2.37 × 10−4 | 6.70 × 104 | 7.73 × 105 | 1.17 × 10−4 | 3.75 × 102 |
| 0.17 | 2.75 × 10−4 | 3.10 × 107 | 1.01 × 107 | 1.67 × 10−2 | 1.91 × 102 |
| 0.35 | 3.38 × 10−4 | 1.89 × 106 | 1.41 × 104 | 6.13 × 10−2 | 2.48 × 102 |
| 0.52 | 3.54 × 10−4 | 1.16 × 106 | 1.12 × 104 | 5.00 × 10−1 | 2.75 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta, A.A.; Calabokis, O.P.; Vanegas, C. Influence of Lithium Triflate Salt Concentration on Structural, Thermal, Electrochemical, and Ionic Conductivity Properties of Cassava Starch Solid Biopolymer Electrolytes. Int. J. Mol. Sci. 2024, 25, 8450. https://doi.org/10.3390/ijms25158450
Arrieta AA, Calabokis OP, Vanegas C. Influence of Lithium Triflate Salt Concentration on Structural, Thermal, Electrochemical, and Ionic Conductivity Properties of Cassava Starch Solid Biopolymer Electrolytes. International Journal of Molecular Sciences. 2024; 25(15):8450. https://doi.org/10.3390/ijms25158450
Chicago/Turabian StyleArrieta, Alvaro A., Oriana Palma Calabokis, and Carlos Vanegas. 2024. "Influence of Lithium Triflate Salt Concentration on Structural, Thermal, Electrochemical, and Ionic Conductivity Properties of Cassava Starch Solid Biopolymer Electrolytes" International Journal of Molecular Sciences 25, no. 15: 8450. https://doi.org/10.3390/ijms25158450
APA StyleArrieta, A. A., Calabokis, O. P., & Vanegas, C. (2024). Influence of Lithium Triflate Salt Concentration on Structural, Thermal, Electrochemical, and Ionic Conductivity Properties of Cassava Starch Solid Biopolymer Electrolytes. International Journal of Molecular Sciences, 25(15), 8450. https://doi.org/10.3390/ijms25158450







