NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review
Abstract
:1. Introduction
2. Methods
Project
3. Results
4. Non-Invasive Evaluation of Steatosis and Fibrosis
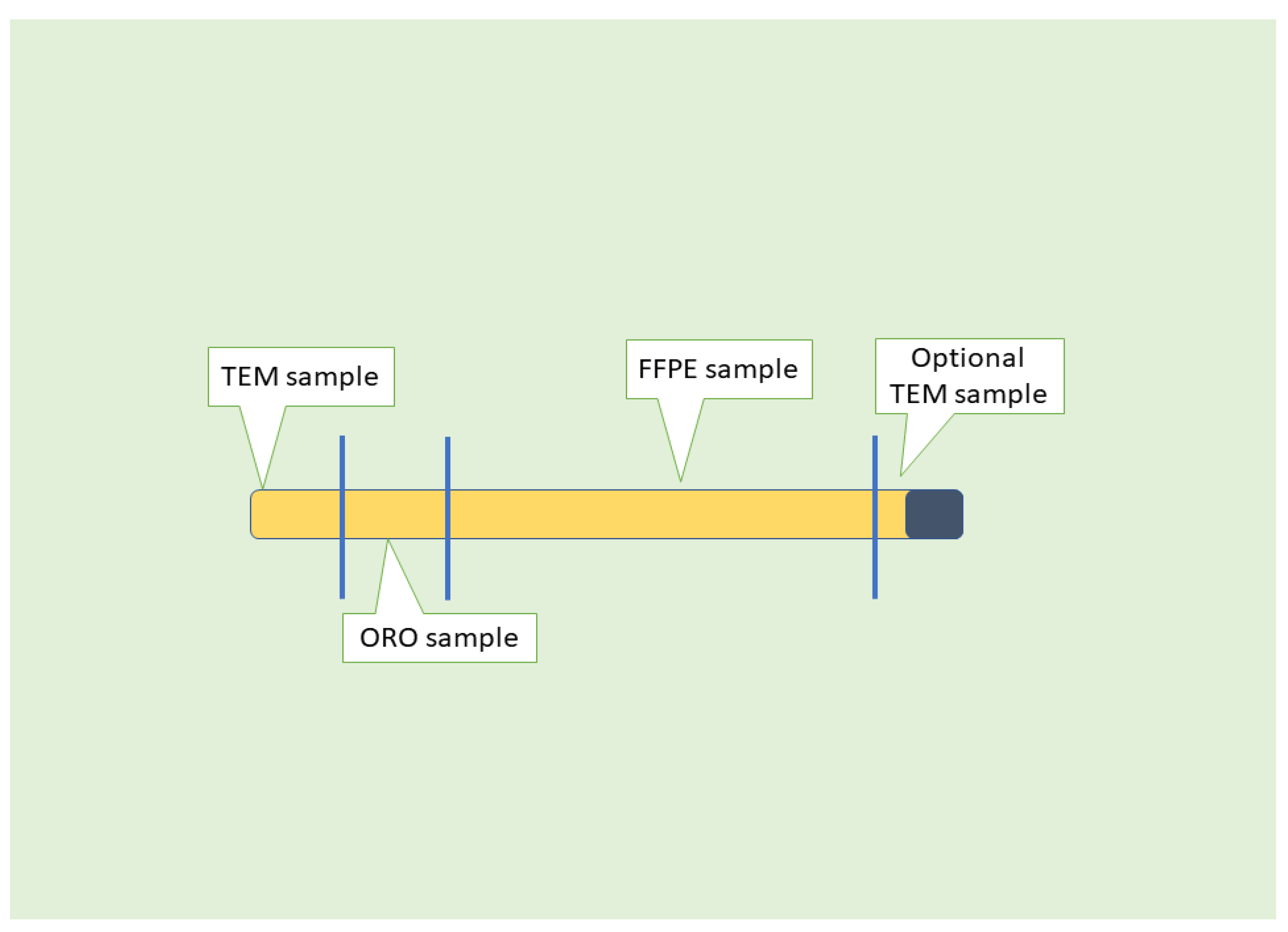
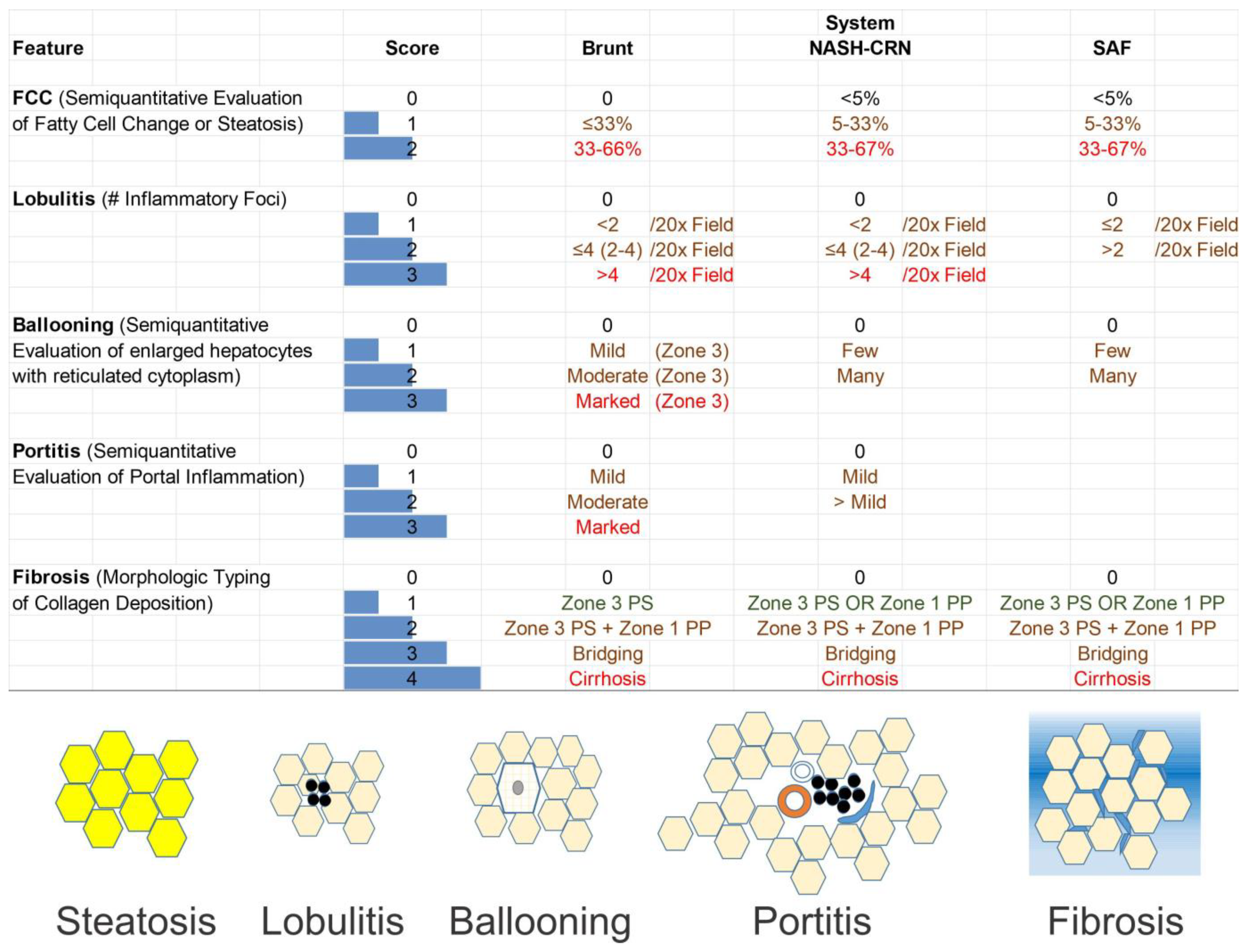
5. Assessment of NAFLD/MASLD Using Liver Biopsy
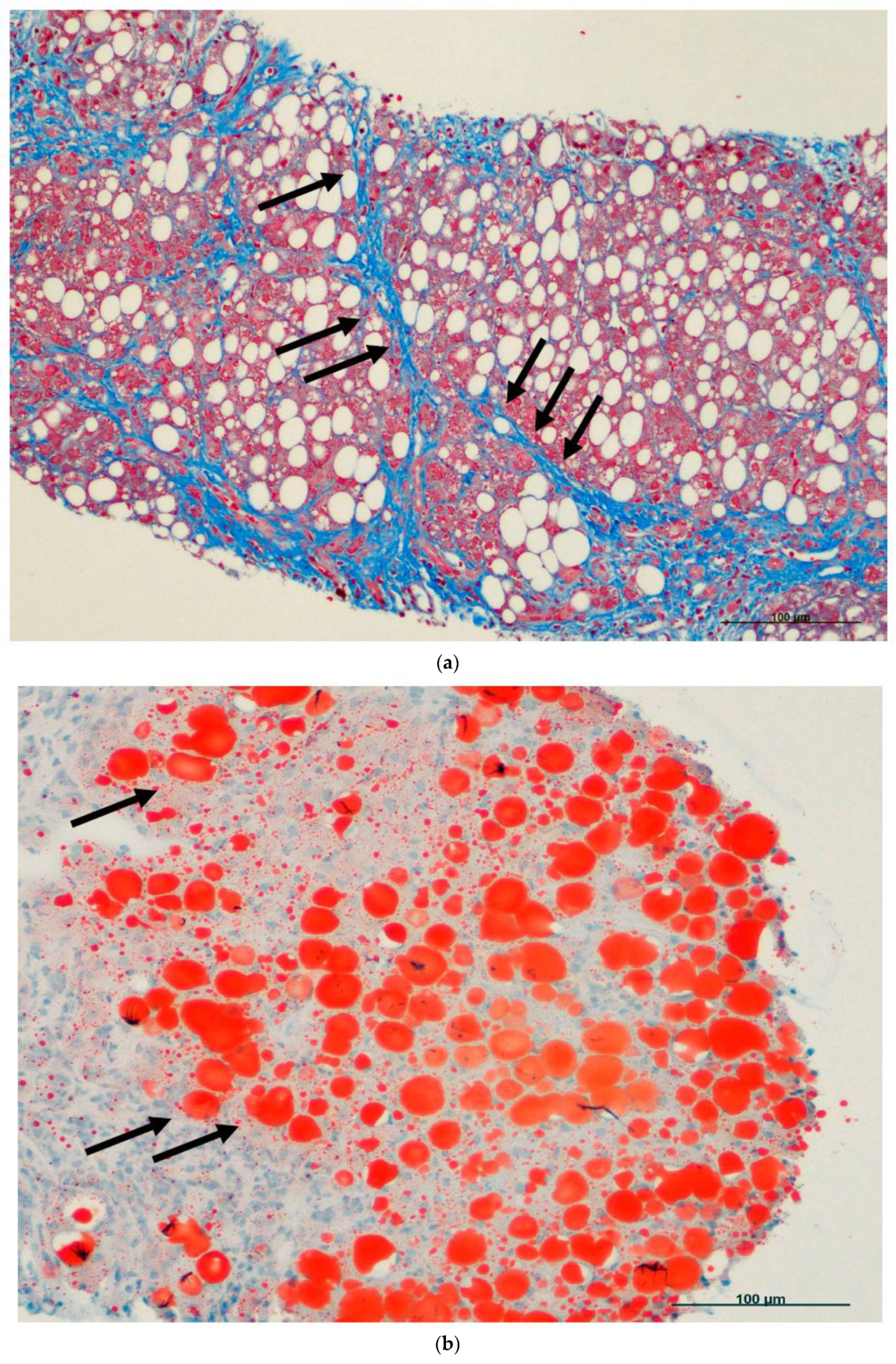
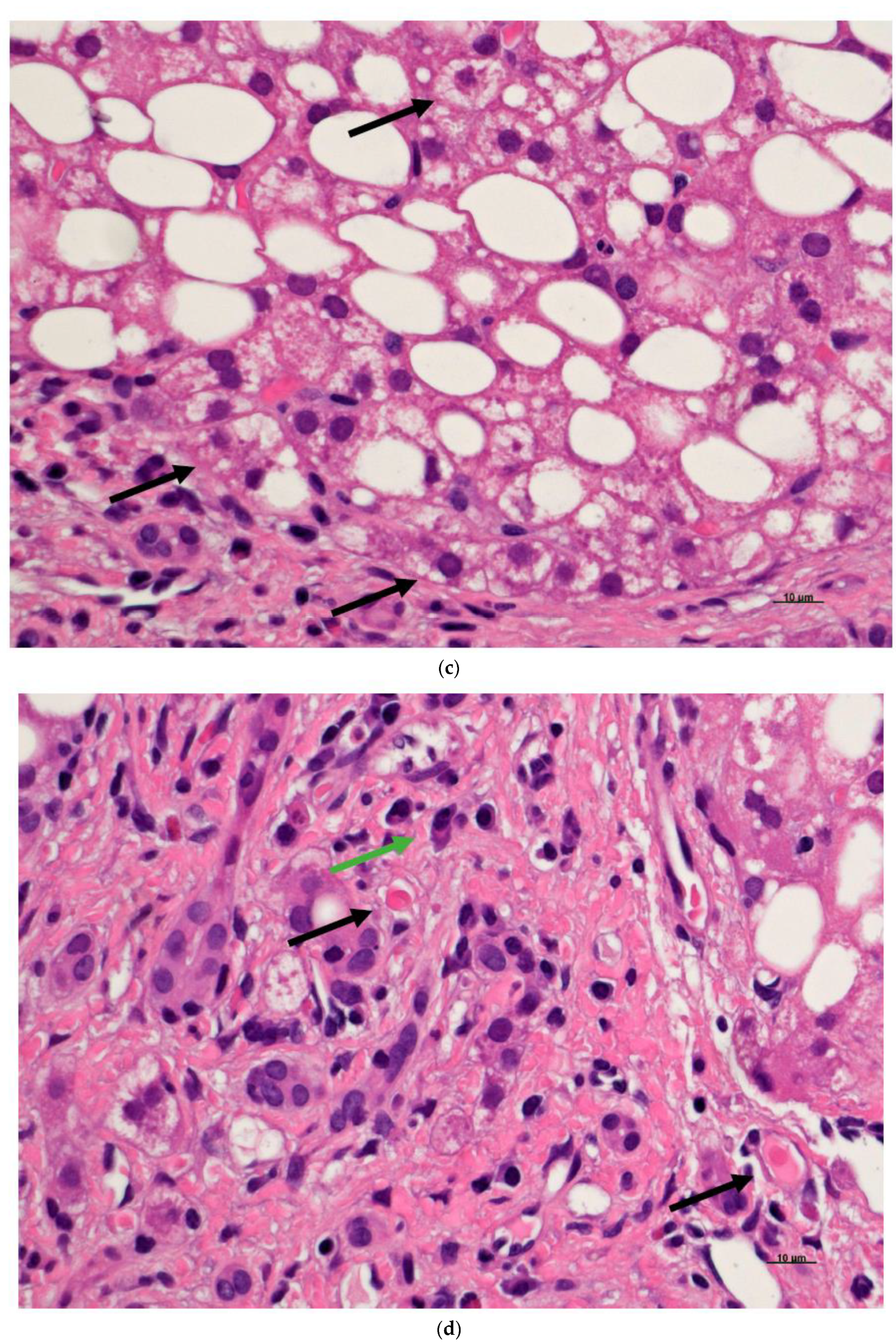
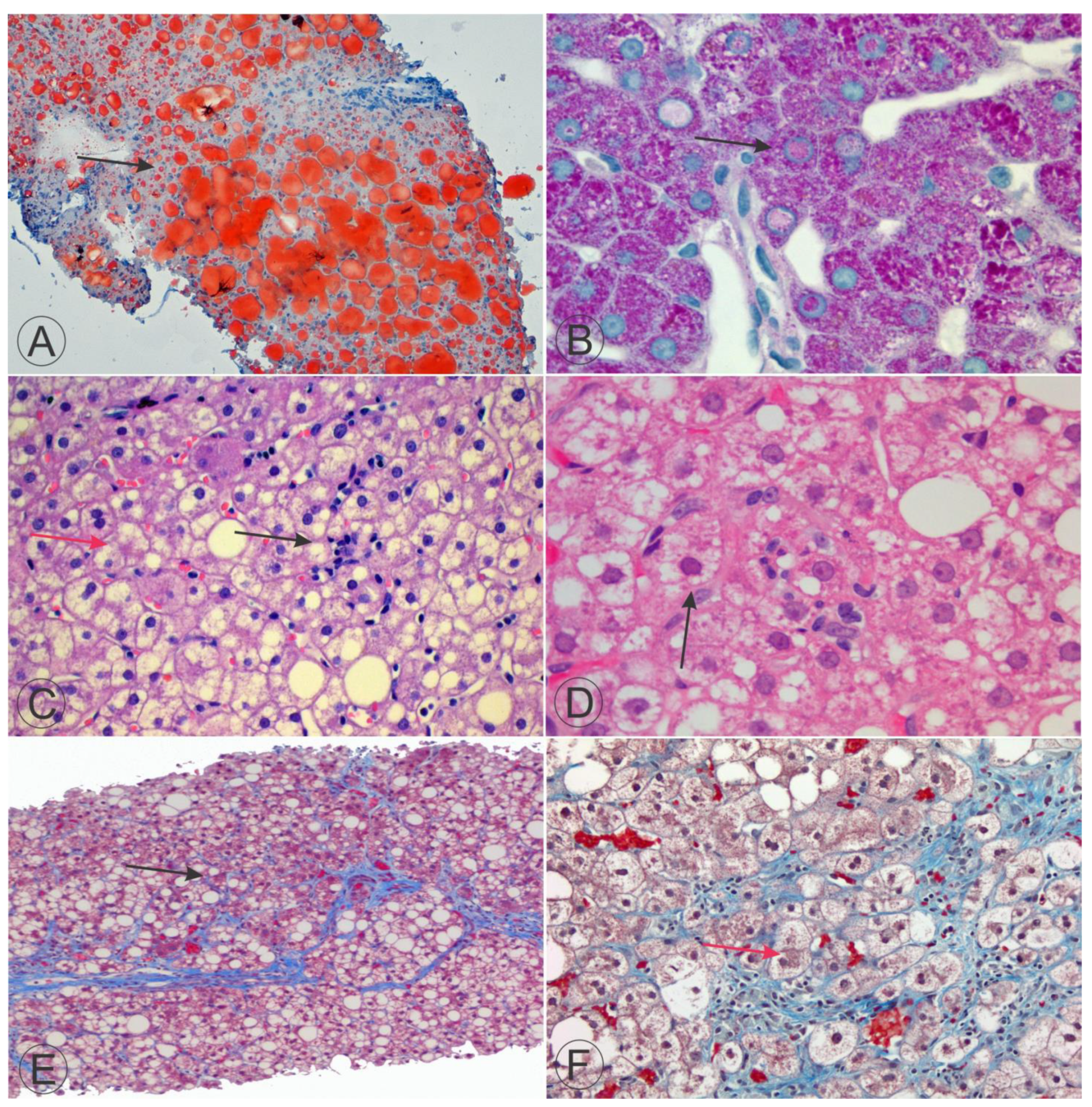
6. Fatty Cell Change with or without Inflammation
7. Steatohepatitis
8. Grading and Staging
9. The Brunt System
10. CRN System
11. Genetic Prediction of NAFLD/MASLD
12. Artificial Intelligence
13. Conclusive Remarks
Funding
Conflicts of Interest
References
- Lu, H. Inflammatory liver diseases and susceptibility to sepsis. Clin. Sci. 2024, 138, 435–487. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, in press. [Google Scholar] [CrossRef]
- Sergi, C.; Chiu, B.; Feulefack, J.; Shen, F.; Chiu, B. Usefulness of resveratrol supplementation in decreasing cardiometabolic risk factors comparing subjects with metabolic syndrome and healthy subjects with or without obesity: Meta-analysis using multinational, randomised, controlled trials. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e98–e111. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef]
- Fouad, Y. Metabolic-associated fatty liver disease: New nomenclature and approach with hot debate. World J Hepatol. 2023, 15, 123–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Moriarty, P.; et al. Nutraceutical approaches to non-alcoholic fatty liver disease (NAFLD): A position paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef]
- Sergi, C.M.; Kehar, M.; Jimenez-Rivera, C. Liver Biopsy Handling of Metabolic-Associated Fatty Liver Disease (MAFLD): The Children’s Hospital of Eastern Ontario grossing protocol. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241227766. [Google Scholar] [CrossRef]
- Kleiner, D.E. Histopathology, grading and staging of nonalcoholic fatty liver disease. Minerva Gastroenterol. Dietol. 2018, 64, 28–38. [Google Scholar] [CrossRef]
- Lal, B.B.; Sood, V.; Rastogi, A.; Mukund, A.; Khanna, R.; Sharma, M.K.; Alam, S. Safety, Feasibility, Yield, Diagnostic and Prognostic Implications of Transjugular Liver Biopsy in Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e109–e114. [Google Scholar] [CrossRef]
- Gill, R.M.; Allende, D.; Belt, P.H.; Behling, C.A.; Cummings, O.W.; Guy, C.D.; Carpenter, D.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; Tonascia, J.; et al. The nonalcoholic steatohepatitis extended hepatocyte ballooning score: Histologic classification and clinical significance. Hepatol. Commun. 2023, 7, e0033. [Google Scholar] [CrossRef]
- Allende, D.S.; Kleiner, D.E. Fatty liver disease that is neither metabolic nor alcoholic. Hum. Pathol. 2023, 141, 212–221. [Google Scholar] [CrossRef]
- JBI. Joanna Briggs Institute Reviewers’ Manual: 2015; The Joanna Briggs Institute: Adelaide, Australia, 2015; p. 24. [Google Scholar]
- Haring, M.P.D.; Cuperus, F.J.C.; Duiker, E.W.; de Haas, R.J.; de Meijer, V.E. Scoping review of clinical practice guidelines on the management of benign liver tumours. BMJ Open Gastroenterol. 2021, 8, e000592. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Neuberger, J.; Cain, O. The Need for Alternatives to Liver Biopsies: Non-Invasive Analytics and Diagnostics. Hepat. Med. 2021, 13, 59–69. [Google Scholar] [CrossRef]
- Thomaides-Brears, H.B.; Lepe, R.; Banerjee, R. Multiparametric DC. MR mapping in clinical decision-making for diffuse liver disease. Abdom. Radiol. 2020, 45, 3507–3522. [Google Scholar] [CrossRef]
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: An individual patient data meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 15, 30355–30357. [Google Scholar] [CrossRef]
- Pirmoazen, A.M.; Khurana, A.; El Kaffas, A.; Kamaya, A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar] [CrossRef]
- Pavlov, C.S.; Casazza, G.; Semenistaia, M.; Nikolova, D.; Tsochatzis, E.; Liusina, E.; Ivashkin, V.T.; Gluud, C. Ultrasonography for diagnosis of alcoholic cirrhosis in people with alcoholic liver disease. Cochrane Database Syst. Rev. 2016, 3, CD011602. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ratziu, V.; Boursier, J.; Francque, S.; Bedossa, P.; Majd, Z.; Cordonnier, G.; Sudrik, F.B.; Darteil, R.; Liebe, R.; et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Tetlow, L.; Dennis, A.; Shumbayawonda, E.; Mouchti, S.; Kendall, T.J.; Fryer, E.; Yamanaka, S.; Honda, Y.; Kessoku, T.; et al. Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort. World J. Gastroenterol. 2021, 27, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Roccarina, D.; Rosselli, M.; Genesca, J.; Tsochatzis, E.A. Elastography methods for the non-invasive assessment of portal hypertension. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Adams, L.A. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wong, G.L.; Wong, V.W. Application of transient elastography in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2020, 26, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; de Saedeleer, A.; Kibble, G.; et al. Breath biopsy assessment of liver disease using an exogenous volatile organic compound-toward improved detection of liver impairment. Clin. Transl. Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef]
- Herrmann, T.; Muckenthaler, M.; van der Hoeven, F.; Brennan, K.; Gehrke, S.G.; Hubert, N.; Sergi, C.; Grone, H.J.; Kaiser, I.; Gosch, I.; et al. Iron overload in adult Hfe-deficient mice independent of changes in the steady-state expression of the duodenal iron transporters DMT1 and Ireg1/ferroportin. J. Mol. Med. 2004, 82, 39–48. [Google Scholar] [CrossRef]
- Fargion, S.; Bissoli, F.; Fracanzani, A.L.; Suigo, E.; Sergi, C.; Taioli, E.; Ceriani, R.; Dimasi, V.; Piperno, A.; Sampietro, M.; et al. No association between genetic hemochromatosis and alpha1-antitrypsin deficiency. Hepatology 1996, 24, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Allende, D.S.; Gawrieh, S.; Cummings, O.W.; Belt, P.; Wilson, L.; Van Natta, M.; Behling, C.A.; Carpenter, D.; Gill, R.M.; Kleiner, D.E.; et al. Glycogenosis is common in nonalcoholic fatty liver disease and is independently associated with ballooning, but lower steatosis and lower fibrosis. Liver Int. 2021, 41, 996–1011. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Loomba, R.; Anstee, Q.M.; Ratziu, V.; Kowdley, K.V.; Rinella, M.E.; Harrison, S.A.; Resnick, M.B.; Capozza, T.; Sawhney, S.; et al. Utility of pathologist panels for achieving consensus in NASH histologic scoring in clinical trials: Data from a phase 3 study. Hepatol. Commun. 2024, 8, e0325. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Kleiner, D.E.; Goodman, Z.; Brunt, E.; Avigan, M.I.; Regev, A.; Hayashi, P.H.; Lewis, J.H.; Mehta, R.; Harrison, S.A.; et al. Liver biopsy for assessment of suspected drug-induced liver injury in metabolic dysfunction-associated steatohepatitis clinical trials: Expert consensus from the Liver Forum. Aliment. Pharmacol. Ther. 2024, 59, 201–216. [Google Scholar] [CrossRef]
- Kudaravalli, P.; John, S. Nonalcoholic Fatty Liver; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Antunes, C.; Azadfard, M.; Hoilat, G.J.; Gupta, M. Fatty Liver; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lahiri, P.; Schmidt, V.; Smole, C.; Kufferath, I.; Denk, H.; Strnad, P.; Rülicke, T.; Fröhlich, L.F.; Zatloukal, K. p62/Sequestosome-1 Is Indispensable for Maturation and Stabilization of Mallory-Denk Bodies. PLoS ONE 2016, 11, e0161083. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Jha, P.; Kleiner, D.E. Digital pathology for nonalcoholic steatohepatitis assessment. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Martinez, R.; Torres, A.M.; Torres, B.; Mateo, J. A Machine Learning Method to Identify the Risk Factors for Liver Fibrosis Progression in Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2023, 68, 3801–3809. [Google Scholar] [CrossRef]
- Esparza, J.; Shrestha, U.; Kleiner, D.E.; Crawford, J.M.; Vanatta, J.; Satapathy, S.; Tipirneni-Sajja, A. Automated Segmentation and Morphological Characterization of Hepatic Steatosis and Correlation with Histopathology. J. Clin. Exp. Hepatol. 2023, 13, 468–478. [Google Scholar] [CrossRef]
- Iyer, J.S.; Pokkalla, H.; Biddle-Snead, C.; Carrasco-Zevallos, O.; Lin, M.; Shanis, Z.; Le, Q.; Juyal, D.; Pouryahya, M.; Pedawi, A.; et al. AI-based histologic scoring enables automated and reproducible assessment of enrollment criteria and endpoints in NASH clinical trials. medRxiv 2023. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zheng, T.L.; Xiao, S.Y.; Wang, P.; Yang, W.J.; Jiang, L.L.; Chen, L.L.; Sha, J.C.; Jin, Y.; Chen, S.D.; et al. Hepatocytic ballooning in non-alcoholic steatohepatitis: Dilemmas and future directions. Liver Int. 2023, 43, 1170–1182. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tincopa, M.A.; Anstee, Q.M.; Loomba, R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024, 36, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Singh, S.P.; Desmukh, A.; Sahoo, B.; Eslam, M. Post-Liver Transplant Metabolic Syndrome. J. Clin. Exp. Hepatol. 2024, 14, 101368. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Dong, J.; Zhu, L.; Mao, J.; Dong, J.; Zhao, Y.; Zou, Y.; Guo, M.; Ding, G. Non-alcoholic fatty liver disease: Pathogenesis and models. Am. J. Transl. Res. 2024, 16, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Khajehahmadi, Z.; Nikeghbalian, S.; Roshanaei, G.; Mohagheghi, S. Increasing Prevalence and High Survival Rate of Liver Transplanted Patients with NASH and PSC Cirrhosis. Arch. Iran. Med. 2024, 27, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Al Shabeeb, R.; Eberly, K.E.; Shah, D.; Nguyen, V.; Ong, J.; Henry, L.; Alqahtani, S.A. The changing epidemiology of adult liver transplantation in the United States in 2013–2022: The dominance of metabolic dysfunction-associated steatotic liver disease and alcohol-associated liver disease. Hepatol Commun 2024, 8, e0352. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Dhampalwar, S.; Saraf, N.; Rastogi, A.; Bhangui, P.; Soin, A.S. Post-transplant Non-alcoholic Fatty Liver Disease and Metabolic Syndrome After Living Donor Liver Transplantation in Indians. J. Clin. Exp. Hepatol. 2024, 14, 101281. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Allen, A.M.; Arab, J.P.; Carrieri, P.; Noureddin, M.; Alazawi, W.; Alkhouri, N.; Alqahtani, S.A.; Anstee, Q.M.; et al. A global action agenda for turning the tide on fatty liver disease. Hepatology 2024, 79, 502–523. [Google Scholar] [CrossRef]
- Mosca, A.; Volpe, L.D.; Sartorelli, M.R.; Comparcola, D.; Veraldi, S.; Alisi, A.; Maggiore, G. Metabolic Associated Fatty Liver Disease in Children and Adolescents: Mechanisms of a Silent Epidemic and Therapeutic Options. Curr. Pediatr. Rev. 2024, 20, 296–304. [Google Scholar] [CrossRef]
- Bahitham, W.; Alghamdi, S.; Omer, I.; Alsudais, A.; Hakeem, I.; Alghamdi, A.; Abualnaja, R.; Sanai, F.M.; Rosado, A.S.; Sergi, C.M. Double Trouble: How Microbiome Dysbiosis and Mitochondrial Dysfunction Drive Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Biomedicines 2024, 12, 550. [Google Scholar] [CrossRef]
- Choi, W.T.; Jen, K.Y.; Wang, D.; Tavakol, M.; Roberts, J.P.; Gill, R.M. Donor Liver Small Droplet Macrovesicular Steatosis Is Associated With Increased Risk for Recipient Allograft Rejection. Am. J. Surg. Pathol. 2017, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Ünalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 55, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Clark, J.M.; Bass, N.M.; Van Natta, M.L.; Unalp-Arida, A.; Tonascia, J.; Zein, C.O.; Brunt, E.M.; Kleiner, D.E.; McCullough, A.J.; et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010, 52, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitch, J.H.; Haythe, J.H.; Regent, N. Kupffer cell aggregation and perivenular distribution in steatohepatitis. Mod. Pathol. 2002, 15, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Unalp, A.; Behling, C.E.; Lavine, J.E.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): A histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009, 49, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, C.; Stål, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010, 51, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Haflidadottir, S.; Jonasson, J.G.; Norland, H.; Einarsdottir, S.O.; Kleiner, D.E.; Lund, S.H.; Bjornsson, E.S. Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014, 14, 166. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Lee, V.D.; Kleiner, D.E.; Al-Osaimi, A.M.; Argo, C.K.; Northup, P.G.; Berg, C.L. NASH and cryptogenic cirrhosis: A histological analysis. Ann. Hepatol. 2009, 8, 346–352. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Behling, C.; Newbury, R.; Deutsch, R.; Nievergelt, C.; Schork, N.J.; Lavine, J.E. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005, 42, 641–649. [Google Scholar] [CrossRef]
- Patton, H.M.; Lavine, J.E.; Van Natta, M.L.; Schwimmer, J.B.; Kleiner, D.; Molleston, J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology 2008, 135, 1961–1971.e1962. [Google Scholar] [CrossRef] [PubMed]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.B.; Lavine, J.E. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am. J. Gastroenterol. 2010, 105, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Africa, J.A.; Behling, C.A.; Brunt, E.M.; Zhang, N.; Luo, Y.; Wells, A.; Hou, J.; Belt, P.H.; Kohil, R.; Lavine, J.E.; et al. In Children With Nonalcoholic Fatty Liver Disease, Zone 1 Steatosis Is Associated With Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2018, 16, 438–446.e1. [Google Scholar] [CrossRef] [PubMed]
- Schranz, M.; Talasz, H.; Graziadei, I.; Winder, T.; Sergi, C.; Bogner, K.; Vogel, W.; Zoller, H. Diagnosis of hepatic iron overload: A family study illustrating pitfalls in diagnosing hemochromatosis. Diagn. Mol. Pathol. 2009, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Scholl-Buergi, S.; Sergi, C.; Theurl, I.; Weiss, G.; Unsinn, K.M.; Trawoger, R. An unusual case of intrauterine symptomatic neonatal liver failure. Klin. Padiatr. 2008, 220, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Himbert, U.; Weinhardt, F.; Heilmann, W.; Meyer, P.; Beedgen, B.; Zilow, E.; Hofmann, W.J.; Linderkamp, O.; Otto, H.F. Hepatic failure with neonatal tissue siderosis of hemochromatotic type in an infant presenting with meconium ileus. Case report and differential diagnosis of the perinatal iron storage disorders. Pathol. Res. Pr. 2001, 197, 699–709; discussion 711–713. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Nonalcoholic steatohepatitis clinical research network. Hepatology 2003, 37, 244. [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; Network, N.C.R. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Seth, D.; Day, C.P. Genetic factors that affect risk of alcoholic and nonalco-holic fatty liver disease. Gastroenterology 2016, 150, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Liebe, R.; Lammert, F. Toward Genetic Prediction of Nonalcoholic Fatty Liver Disease Trajectories: PNPLA3 and Beyond. Gastroenterology 2020, 158, 1865–1880.e1861. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, S.; Li, J.Z.; Seo, Y.K.; Osborne, T.F.; Cohen, J.C.; Hobbs, H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. USA 2010, 107, 7892–7897. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Smagris, E.; Gilyard, S.; BasuRay, S.; Cohen, J.C.; Hobbs, H.H. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2016, 291, 10659–10676. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef]
- Fabbretti, G.; Sergi, C.; Consalez, G.; Faa, G.; Brisigotti, M.; Romeo, G.; Callea, F. Genetic variants of alpha-1-antitrypsin (AAT). Liver 1992, 12, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.M. Carcinoma of the Liver in Children and Adolescents. In Liver Cancer; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar] [CrossRef]
- Sergi, C.; Consalez, G.G.; Fabbretti, G.; Brisigotti, M.; Faa, G.; Costa, V.; Romeo, G.; Callea, F. Immunohistochemical and genetic characterization of the M Cagliari alpha-1-antitrypsin molecule (M-like alpha-1-antitrypsin deficiency). Lab. Investig. 1994, 70, 130–133. [Google Scholar] [PubMed]
- Strnad, P.; Buch, S.; Hamesch, K.; Fischer, J.; Rosendahl, J.; Schmelz, R.; Brueckner, S.; Brosch, M.; Heimes, C.V.; Woditsch, V.; et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut 2019, 68, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.; Loomba, R. Advances in the genetics of nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2023, 39, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, X.; Kuppa, A.; Feitosa, M.F.; Bielak, L.F.; O’Connell, J.R.; Musani, S.K.; Guo, X.; Kahali, B.; Chen, V.L.; et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat. Genet. 2023, 55, 1640–1650. [Google Scholar] [CrossRef]
- Dutta, T.; Sasidharan, K.; Ciociola, E.; Pennisi, G.; Noto, F.R.; Kovooru, L.; Kroon, T.; Lindblom, A.; Du, Y.; Pirmoradian, M.; et al. Mitochondrial amidoxime-reducing component 1 p.Ala165Thr increases protein degradation mediated by the proteasome. Liver Int. 2024, 44, 1219–1232. [Google Scholar] [CrossRef]
- Lewis, L.C.; Chen, L.; Hameed, L.S.; Kitchen, R.R.; Maroteau, C.; Nagarajan, S.R.; Norlin, J.; Daly, C.E.; Szczerbinska, I.; Hjuler, S.T.; et al. Hepatocyte mARC1 promotes fatty liver disease. JHEP Rep. 2023, 5, 100693. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yoon, E.L.; Chung, G.E.; Choe, E.K.; Bae, J.H.; Choi, S.H.; Kim, M.; Hwang, W.; Kim, H.L.; Yang, S.Y.; et al. Genetic and Metabolic Characteristics of Lean Nonalcoholic Fatty Liver Disease in a Korean Health Examinee Cohort. Gut Liver 2024, 18, 316–327. [Google Scholar] [CrossRef]
- Sohal, A.; Chaudhry, H.; Kowdley, K.V. Genetic Markers Predisposing to Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2023, 27, 333–352. [Google Scholar] [CrossRef]
- Rea, T.; Guillari, A.; Sergi, C.; Serra, N. Mathematical models in nursing research. J. Public Health Res. 2020, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Solez, K.; Bernier, A.; Crichton, J.; Graves, H.; Kuttikat, P.; Lockwood, R.; Marovitz, W.F.; Monroe, D.; Pallen, M.; Pandya, S.; et al. Bridging the gap between the technological singularity and mainstream medicine: Highlighting a course on technology and the future of medicine. Glob. J. Health Sci. 2013, 5, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Mikuz, G. External quality assurance as a revalidation method for pathologists in pediatric histopathology: Comparison of four international programs. BMC Clin. Pathol. 2008, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C. Digital Pathology: The Time Is Now to Bridge the Gap between Medicine and Technological Singularity. In Interactive Multimedia; Chapter 9; Dragan, C., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Ratziu, V.; Francque, S.; Behling, C.A.; Cejvanovic, V.; Cortez-Pinto, H.; Iyer, J.S.; Krarup, N.; Le, Q.; Sejling, A.S.; Tiniakos, D.; et al. Artificial intelligence scoring of liver biopsies in a phase II trial of semaglutide in nonalcoholic steatohepatitis. Hepatology 2024, 80, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.J.; Kwok, J.T.; Yang, Q. Transfer learning via dimensionality reduction. In Proceedings of the 23rd National Conference on Artificial Intelligence—Volume 2, Chicago, IL, USA, 13–17 July 2008; pp. 677–682. [Google Scholar]
- Luo, Z.; Wang, R.; Sun, Y.; Liu, J.; Chen, Z.; Zhang, Y.J. Interpretable feature extraction and dimensionality reduction in ESM2 for protein localization prediction. Brief. Bioinform. 2024, 25, bbad534. [Google Scholar] [CrossRef] [PubMed]
- Hagendorff, T. The Ethics of AI Ethics: An Evaluation of Guidelines. Minds Mach. 2020, 30, 99–120. [Google Scholar] [CrossRef]
- Donato, I.; Velpula, K.K.; Tsung, A.J.; Tuszynski, J.A.; Sergi, C.M. Demystifying neuroblastoma malignancy through fractal dimension, entropy, and lacunarity. Tumori 2023, 109, 370–378. [Google Scholar] [CrossRef]

|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, C.M. NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review. Int. J. Mol. Sci. 2024, 25, 8462. https://doi.org/10.3390/ijms25158462
Sergi CM. NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review. International Journal of Molecular Sciences. 2024; 25(15):8462. https://doi.org/10.3390/ijms25158462
Chicago/Turabian StyleSergi, Consolato M. 2024. "NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review" International Journal of Molecular Sciences 25, no. 15: 8462. https://doi.org/10.3390/ijms25158462






