Natural Products in the Treatment of Retinopathy of Prematurity: Exploring Therapeutic Potentials
Abstract
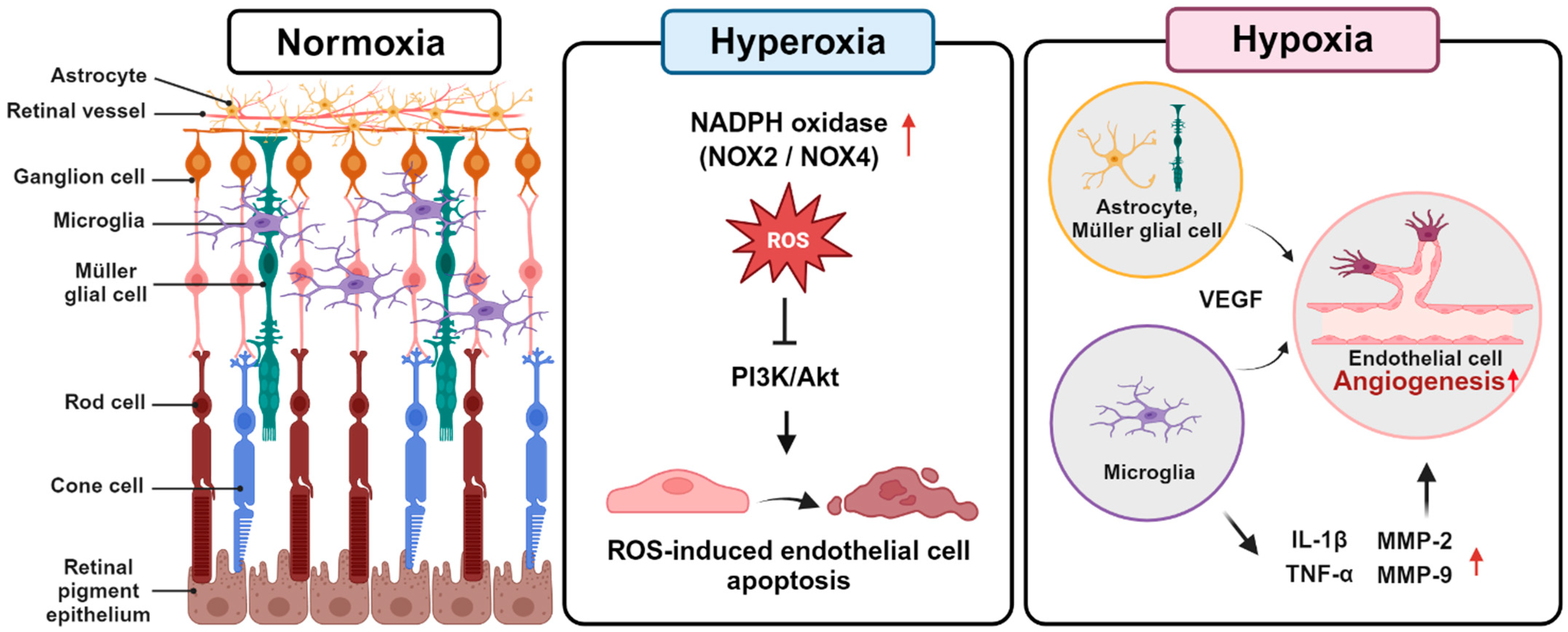
1. Retinopathy of Prematurity
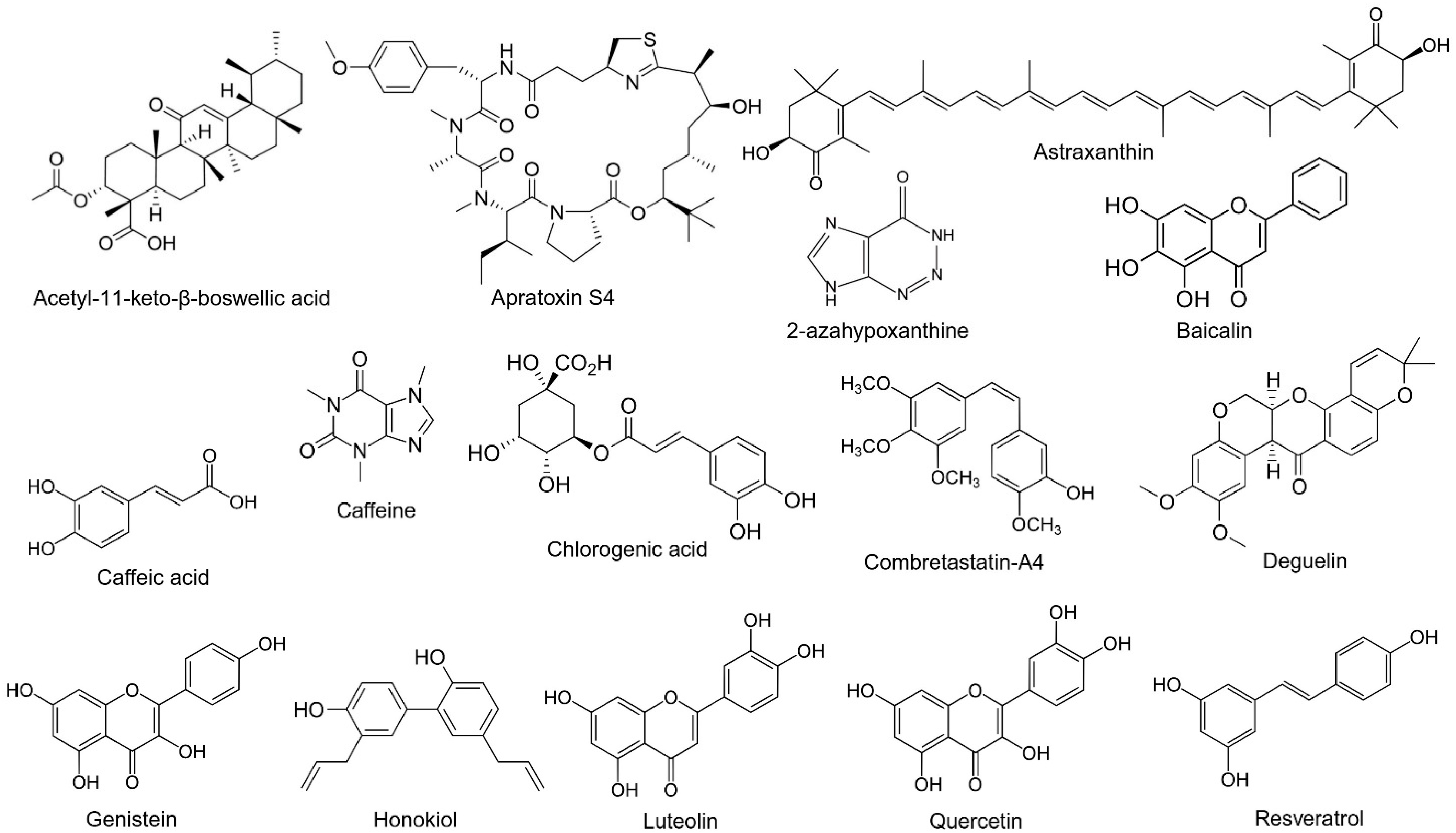
2. Natural Compounds
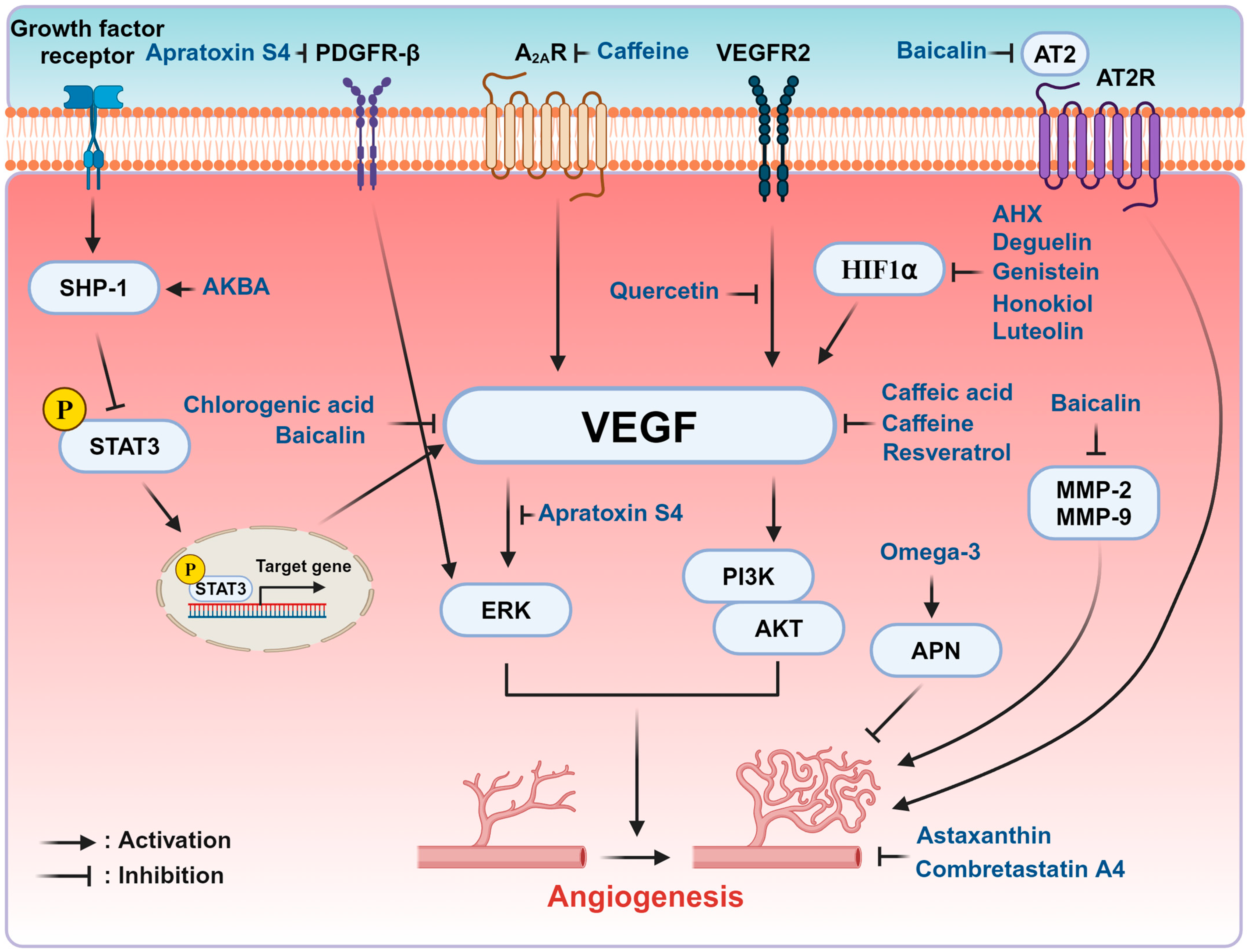
3. Natural Compounds in the Modulation of ROP
| Natural Compounds | Effect of Eye | Cellular Model | Animal Model | Major Findings | References |
|---|---|---|---|---|---|
| 2-Azahypoxanthine | Anti-angiogenic | CoCl2-induced ARPE-19 and 661 W cell line
| OIR mice 300 mg/kg/day PO at P12-P16 |
| [65] |
| Acetyl-11-keto-β-boswellic acid | Anti-angiogenic | VEGF-induced HRMECs
| OIR mice
|
| [66] |
| Apratoxin S4 | Anti-angiogenic | VEGF-induced HRECs, human retinal pericyte
| OIR mice
|
| [69] |
| Astaxanthin | Anti-angiogenic, antiproliferative | N/A | Hyperoxia-induced retinopathy (HIR) mouse model:
|
| [70] |
| Baicalin | Anti-angiogenic | N/A | OIR mice
|
| [71] |
| Caffeic acid | Anti-angiogenic antioxidant | VEGF-or H2O2- induced HRMECs
| OIR mice
|
| [72] |
| Caffeine | Anti-angiogenic | N/A | A2AR receptor and A1R knockout OIR mice
|
| [73] |
| Chlorogenic acid | Anti-angiogenic | VEGF-induced HUVECs
| OIR mice
|
| [74] |
| Combretastatin-A4 | Anti-angiogenic | N/A | OIR mice
|
| [75] |
| Deguelin | Anti-angiogenic | Hypoxia-induced HRECs
| OIR mice
|
| [76] |
| Genistein | Anti-angiogenic | N/A | OIR mice
|
| [77] |
| Honokiol | Anti-angiogenic | Hypoxia-induced human retinal pigment epithelial cells
| OIR mice,
|
| [80] |
| Luteolin | Anti-angiogenic, anti-oxidative | VEGF or t-BH induced HRMECs 1–50 μM | OIR mice
|
| [81] |
| Omega-3 LCPFA | Anti-angiogenic | tunicamycin-induced 3T3-L1 adipocytes
| Apn-knockout OIR mice
|
| [82] |
| Omega-3 and -6 LCPFA | Omega-3: promote retinal vascular development Omega-6: essential for neuronal development and metabolism | N/A | Hyperglycemia-associated phase I ROP mice model (streptozocin-induced mice)
|
| [83] |
| Quercetin | Anti-angiogenic | VEGF-A or CM-stimulated HRECs 25–100 μM | N/A |
| [84] |
| Resveratrol | Anti-angiogenic, anti-oxidative | N/A | OIR mice 5, 25, and 50 mg/kg/day IVT or gtts twice a day at P12-P16 |
| [85] |
4. Therapeutic Application of Natural Compounds in the Treatment of ROP
5. Challenges and Strategies for Development of Natural Product-Based Drugs
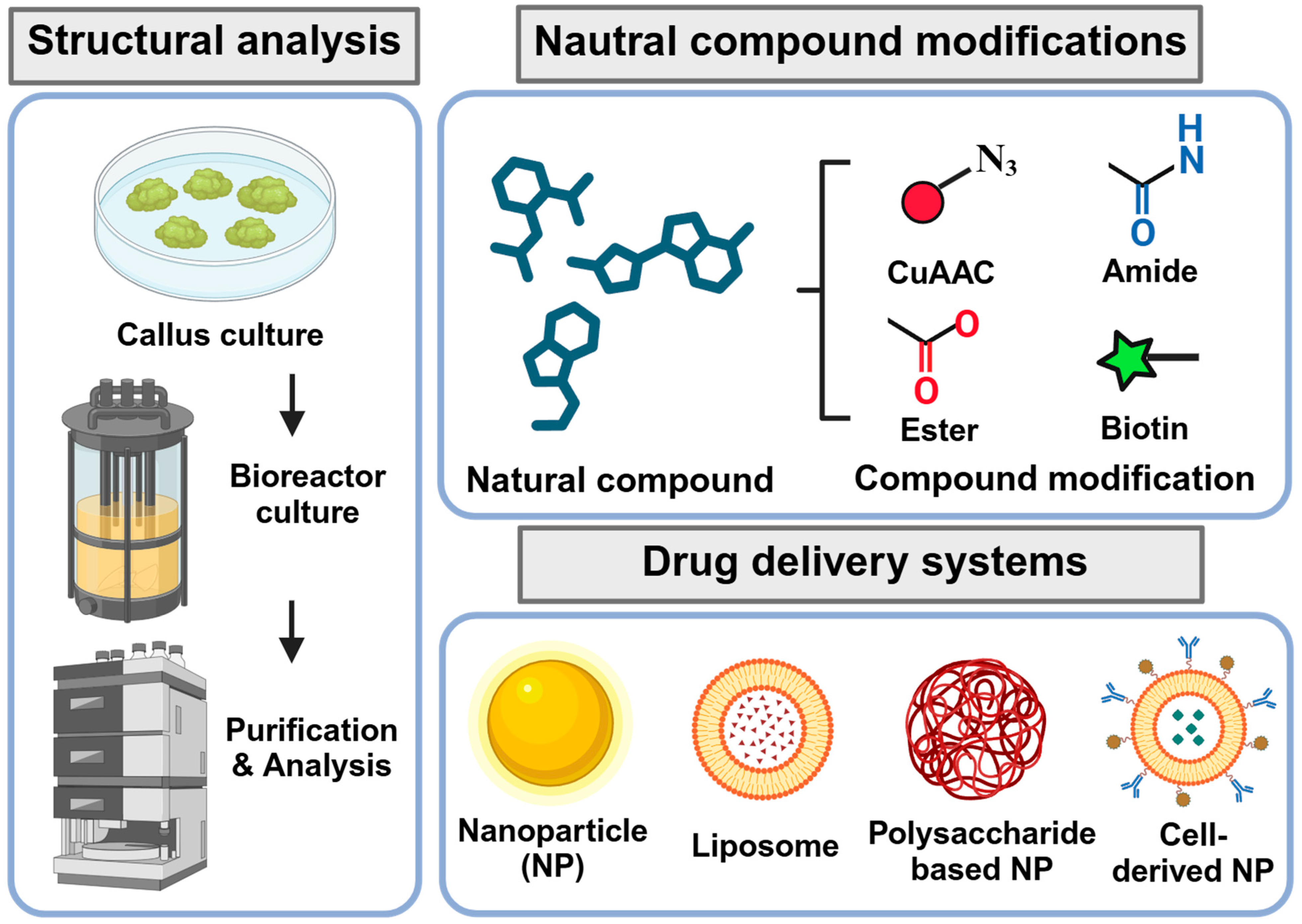
6. Application of Advanced Technologies for Identification and Development of Natural Products
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryu, J. New Aspects on the Treatment of Retinopathy of Prematurity: Currently Available Therapies and Emerging Novel Therapeutics. Int. J. Mol. Sci. 2022, 23, 8529. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Zhang, M.; Chen, X.; Han, S.; Zhu, J. Incidence and Risk Factors for Retinopathy of Prematurity in a Tertiary Hospital in China. Clin. Ophthalmol. 2023, 17, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; El Ballushi, R.; Anklesaria, B.Z.; Kamali, M.; Talat, M.; Watts, T. A Review on the Incidence and Related Risk Factors of Retinopathy of Prematurity Across Various Countries. Cureus 2022, 14, e32007. [Google Scholar] [CrossRef] [PubMed]
- Yucel, O.E.; Eraydin, B.; Niyaz, L.; Terzi, O. Incidence and risk factors for retinopathy of prematurity in premature, extremely low birth weight and extremely low gestational age infants. BMC Ophthalmol. 2022, 22, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Zhang, X.; Liu, Y.; Li, J.; Wang, H.; Luo, X.; Liu, S.; Liu, L.; Zhang, J. Global, regional and national burden of retinopathy of prematurity among childhood and adolescent: A spatiotemporal analysis based on the Global Burden of Disease Study 2019. BMJ Paediatr. Open 2024, 8, e002267. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Greven, K.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Visual acuity, amblyopia, and vision-related quality of life in preterm adults with and without ROP: Results from the Gutenberg prematurity eye study. Eye 2023, 37, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Buonfiglio, F.; Fieß, A.; Pfeiffer, N.; Gericke, A. Retinopathy of Prematurity—Targeting Hypoxic and Redox Signaling Pathways. Antioxidants 2024, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Joyal, J.S.; Rivera, J.C.; Kermorvant-Duchemin, E.; Sennlaub, F.; Hardy, P.; Lachapelle, P.; Chemtob, S. Retinopathy of prematurity: Understanding ischemic retinal vasculopathies at an extreme of life. J. Clin. Investig. 2010, 120, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Discovering Mechanisms in the Changing and Diverse Pathology of Retinopathy of Prematurity: The Weisenfeld Award Lecture. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1286–1297. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Marques-Neves, C.; Guimarães, H.; Bicho, M. Retinopathy of prematurity: A review of pathophysiology and signaling pathways. Surv. Ophthalmol. 2023, 68, 175–210. [Google Scholar] [CrossRef]
- Chan, E.C.; van Wijngaarden, P.; Liu, G.S.; Jiang, F.; Peshavariya, H.; Dusting, G.J. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7061–7067. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Jiang, Y.; Hartnett, M.E. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol. Vis. 2014, 20, 231–241. [Google Scholar] [PubMed]
- Poggi, C.; Giusti, B.; Vestri, A.; Pasquini, E.; Abbate, R.; Dani, C. Genetic polymorphisms of antioxidant enzymes in preterm infants. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 4), 131–134. [Google Scholar] [CrossRef]
- Gu, X.; El-Remessy, A.B.; Brooks, S.E.; Al-Shabrawey, M.; Tsai, N.T.; Caldwell, R.B. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am. J. Physiol. Cell Physiol. 2003, 285, C546–C554. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Ryu, J. Noncoding RNAs as a novel approach to target retinopathy of prematurity. Front. Pharmacol. 2022, 13, 1033341. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Penn, J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes. Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15 Pt 1, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Ramshekar, A.; Hartnett, M.E. Vascular Endothelial Growth Factor Signaling in Models of Oxygen-Induced Retinopathy: Insights Into Mechanisms of Pathology in Retinopathy of Prematurity. Front. Pediatr. 2021, 9, 796143. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Martiniuk, D.; Byfield, G.; Geisen, P.; Zeng, G.; Bautch, V.L. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: Relevance to plus disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3107–3114. [Google Scholar] [CrossRef]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J. Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E. IGF-1 and retinopathy of prematurity in the preterm infant. Biol. Neonate 2005, 88, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Presta, M.; Vacca, A.; Ria, R.; Giuliani, R.; Dell’Era, P.; Nico, B.; Roncali, L.; Dammacco, F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 1999, 93, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Koyama, S.; Seike, H.; Oh, H.; Otani, A.; Matsumura, M.; Honda, Y. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Fu, Y.-K.; Lien, R.-I.; Chiang, M.-C.; Lee, C.-C.; Chen, H.-C.; Hsueh, Y.-J.; Chen, K.-J.; Wang, N.-K.; Liu, L.; et al. Systemic Cytokines in Retinopathy of Prematurity. J. Pers. Med. 2023, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, S. Hypoxia inducible factor-1α/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J. Cell Biochem. 2018, 119, 7707–7718. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liang, D.; Liu, D.K.; Lin, L.; Zhang, L.; Yang, W.Q. The role of the ERK signaling pathway in promoting angiogenesis for treating ischemic diseases. Front. Cell Dev. Biol. 2023, 11, 1164166. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Luciano, A.K.; Ackah, E.; Rodriguez-Vita, J.; Bancroft, T.A.; Eichmann, A.; Simons, M.; Kyriakides, T.R.; Morales-Ruiz, M.; Sessa, W.C. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc. Natl. Acad. Sci. USA 2014, 111, 12865–12870. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.L.; Ziv, K.; Dabydeen, D.; Eyiah-Mensah, G.; Riveros, M.; Perruzzi, C.; Sun, J.; Monahan-Earley, R.A.; Shiojima, I.; Nagy, J.A.; et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 2006, 10, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.; Hoffhines, A. The discovery of aspirin’s antithrombotic effects. Tex. Heart Inst. J. 2007, 34, 179–186. [Google Scholar] [PubMed]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal Medicine for the Treatment of Cardiovascular Disease: Clinical Considerations. Arch. Intern. Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol. Eye Dis. 2014, 6, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lv, M.; Hu, J.; Huang, K.; Xu, H. Glycosylation and Activities of Natural Products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Chordia, P.; MacArthur, R.D. Crofelemer, a novel agent for treatment of non-infectious diarrhea in HIV-infected persons. Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Choi, H.; Kim, J.; Kim, J.; Kim, W.; Nurkolis, F.; Kim, B. Effects of natural products on polycystic ovary syndrome: From traditional medicine to modern drug discovery. Heliyon 2023, 9, e20889. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.; Zhang, J.; Zhang, Y.; He, W.; Ju, J.; Wu, Y.; Wang, Y. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci. Rep. 2023, 13, 13278. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Lei, Y.H.; Zhang, W.H.; Luo, X.Q. Antioxidant and Anti-inflammatory Properties of Resveratrol in Diabetic Nephropathy: A Systematic Review and Meta-analysis of Animal Studies. Front. Pharmacol. 2022, 13, 841818. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Miao, X.; Yang, F.J.; Cao, J.F.; Liu, X.; Fu, J.L.; Su, G.F. Therapeutic potential of curcumin in diabetic retinopathy (Review). Int. J. Mol. Med. 2021, 47, 75. [Google Scholar] [CrossRef] [PubMed]
- Kaabi, Y.A. Potential Roles of Anti-Inflammatory Plant-Derived Bioactive Compounds Targeting Inflammation in Microvascular Complications of Diabetes. Molecules 2022, 27, 7352. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.; Shameera, R.; Devarajan, N.; Perumal, E. Role of berberine on angiogenesis and blood flow hemodynamics using zebrafish model. J. Appl. Toxicol. 2023, 44, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Maci, S.; Santos, R. The beneficial role of lutein and zeaxanthin in cataracts. Nutrafoods 2015, 14, 63–69. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Agron, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y.; Areds; Groups, A.R. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow Geographic Atrophy Progression to the Fovea in Age-Related Macular Degeneration. Ophthalmology 2024. [Google Scholar] [CrossRef] [PubMed]
- Alugoju, P.; Krishna Swamy, V.K.D.; Anthikapalli, N.V.A.; Tencomnao, T. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10709–10774. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Miwa, Y.; Nimura, K.; Okamoto, K.; Yamagami, S.; Tsubota, K.; Kurihara, T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients 2020, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Lee, D.; Miwa, Y.; Yamagami, S.; Nakashizuka, H.; Nimura, K.; Okamoto, K.; Kawagishi, H.; Negishi, K.; Kurihara, T. Inhibition of hypoxia-inducible factors suppresses subretinal fibrosis. FASEB J. 2024, 38, e23792. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Lin, S. Ginkgo biloba and its potential role in glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Li, J.; Wu, E.; Tang, T.; Singla, R.K.; Shen, B.; Zhang, M. Natural Products for the Treatment of Age-Related Macular Degeneration. Phytomedicine 2024, 130, 155522. [Google Scholar] [CrossRef]
- Fanaro, G.B.; Marques, M.R.; Calaza, K.D.C.; Brito, R.; Pessoni, A.M.; Mendonça, H.R.; Lemos, D.E.A.; de Brito Alves, J.L.; de Souza, E.L.; Cavalcanti Neto, M.P. New Insights on Dietary Polyphenols for the Management of Oxidative Stress and Neuroinflammation in Diabetic Retinopathy. Antioxidants 2023, 12, 1237. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C.; Xu, Y.; Tan, H.Y.; Chen, H.; Feng, Y. Berberine improves insulin-induced diabetic retinopathy through exclusively suppressing Akt/mTOR-mediated HIF-1α/VEGF activation in retina endothelial cells. Int. J. Biol. Sci. 2021, 17, 4316–4326. [Google Scholar] [CrossRef]
- Lou, Y.; Oberpriller, J.C.; Carlson, E.C. Effect of hypoxia on the proliferation of retinal microvessel endothelial cells in culture. Anat. Rec. 1997, 248, 366–373. [Google Scholar] [CrossRef]
- Pierce, E.A.; Foley, E.D.; Smith, L.E.H. Regulation of Vascular Endothelial Growth Factor by Oxygen in a Model of Retinopathy of Prematurity. Arch. Ophthalmol. 1996, 114, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Busoy, J.M.; Zaman, B.A.A.; Tan, Q.S.W.; Tan, G.S.W.; Barathi, V.A.; Cheung, N.; Wei, J.J.; Hunziker, W.; Hong, W.; et al. A novel model of persistent retinal neovascularization for the development of sustained anti-VEGF therapies. Exp. Eye Res. 2018, 174, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Lulli, M.; Cammalleri, M.; Fornaciari, I.; Casini, G.; Dal Monte, M. Acetyl-11-keto-β-boswellic acid reduces retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Exp. Eye Res. 2015, 135, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, K.W.; Kim, J.Y.; Kim, K.D. Consideration of SHP-1 as a Molecular Target for Tumor Therapy. Int. J. Mol. Sci. 2024, 25, 331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, Z.C. STAT3: A critical transcription activator in angiogenesis. Med. Res. Rev. 2008, 28, 185–200. [Google Scholar] [CrossRef]
- Qiu, B.; Tan, A.; Veluchamy, A.B.; Li, Y.; Murray, H.; Cheng, W.; Liu, C.; Busoy, J.M.; Chen, Q.Y.; Sistla, S.; et al. Apratoxin S4 Inspired by a Marine Natural Product, a New Treatment Option for Ocular Angiogenic Diseases. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3254–3263. [Google Scholar] [CrossRef]
- Küçüködük, A.; Helvacioglu, F.; Haberal, N.; Dagdeviren, A.; Bacanli, D.; Yilmaz, G.; Akkoyun, I. Antiproliferative and anti-apoptotic effect of astaxanthin in an oxygen-induced retinopathy mouse model. Can. J. Ophthalmol. 2019, 54, 65–74. [Google Scholar] [CrossRef]
- Jo, H.; Jung, S.H.; Yim, H.B.; Lee, S.J.; Kang, K.D. The effect of baicalin in a mouse model of retinopathy of prematurity. BMB Rep. 2015, 48, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, B.J.; Kim, J.H.; Yu, Y.S.; Kim, K.W. Anti-angiogenic effect of caffeic acid on retinal neovascularization. Vasc. Pharmacol. 2009, 51, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, R.; Li, B.; Li, H.; Wang, Y.; Gu, X.; Tang, L.; Wang, C.; Zhong, D.; Ge, Y.; et al. Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J. 2017, 31, 3334–3348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Jung, W.; Park, S.B.; Kim, C.S.; Kim, J.S. Aster koraiensis Extract and Chlorogenic Acid Inhibit Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Evid. Based Complement. Altern. Med. 2018, 2018, 6402650. [Google Scholar] [CrossRef] [PubMed]
- Griggs, J.; Skepper, J.N.; Smith, G.A.; Brindle, K.M.; Metcalfe, J.C.; Hesketh, R. Inhibition of proliferative retinopathy by the anti-vascular agent combretastatin-A4. Am. J. Pathol. 2002, 160, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Shin, J.Y.; Lee, H.Y.; Kim, K.W. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1alpha in oxygen-induced retinopathy. J. Cell Mol. Med. 2008, 12, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zou, Y.; Li, H.; Yan, H.; Pan, J.S.; Yuan, Z.L. Genistein inhibited retinal neovascularization and expression of vascular endothelial growth factor and hypoxia inducible factor 1alpha in a mouse model of oxygen-induced retinopathy. J. Ocul. Pharmacol. Ther. 2005, 21, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Majji, A.B.; Hayashi, A.; Kim, H.C.; Grebe, R.R.; de Juan, E., Jr. Inhibition of choriocapillaris regeneration with genistein. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1477–1486. [Google Scholar]
- Joussen, A.M.; Rohrschneider, K.; Reichling, J.; Kirchhof, B.; Kruse, F.E. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp. Eye Res. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Vavilala, D.T.; O’Bryhim, B.E.; Ponnaluri, V.K.; White, R.S.; Radel, J.; Symons, R.C.; Mukherji, M. Honokiol inhibits pathological retinal neovascularization in oxygen-induced retinopathy mouse model. Biochem. Biophys. Res. Commun. 2013, 438, 697–702. [Google Scholar] [CrossRef]
- Park, S.W.; Cho, C.S.; Jun, H.O.; Ryu, N.H.; Kim, J.H.; Yu, Y.S.; Kim, J.S.; Kim, J.H. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7718–7726. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Lofqvist, C.A.; Shao, Z.; Sun, Y.; Joyal, J.S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; SanGiovanni, J.P.; et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Yan, W.; Chen, C.T.; Nilsson, A.K.; Bull, E.; Allen, W.; Yang, J.; Ko, M.; SanGiovanni, J.P.; Akula, J.D.; et al. Omega-3/Omega-6 Long-Chain Fatty Acid Imbalance in Phase I Retinopathy of Prematurity. Nutrients 2022, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.; Cambria, M.T.; Olivieri, M.; Rocco, C.; Caporarello, N.; Longo, A.; Zanghì, G.; Salmeri, M.; Foti, M.C.; Anfuso, C.D. Anti-angiogenic effect of quercetin and its 8-methyl pentamethyl ether derivative in human microvascular endothelial cells. J. Cell Mol. Med. 2019, 23, 6565–6577. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Zhang, X.Y.; Leung, K.W.; Duan, R.; Dong, T.T.; Qin, Q.W.; Tsim, K.W. Resveratrol, an Inhibitor Binding to VEGF, Restores the Pathology of Abnormal Angiogenesis in Retinopathy of Prematurity (ROP) in Mice: Application by Intravitreal and Topical Instillation. Int. J. Mol. Sci. 2022, 23, 6455. [Google Scholar] [CrossRef] [PubMed]
- Pertl, L.; Steinwender, G.; Mayer, C.; Hausberger, S.; Poschl, E.M.; Wackernagel, W.; Wedrich, A.; El-Shabrawi, Y.; Haas, A. A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity. PLoS ONE 2015, 10, e0129383. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Stahl, A. Laser versus Anti-VEGF: A Paradigm Shift for Treatment-Warranted Retinopathy of Prematurity. Ophthalmol. Ther. 2023, 12, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, T.S.; Zackula, R.E.; Hartnett, M.E. Aflibercept to treat retinopathy of prematurity: Need for more research. J. Perinatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Azuma, N.; Wu, W.-C.; Lepore, D.; Sukgen, E.; Nakanishi, H.; Mazela, J.; Leal, S.; Pieper, A.; Schlief, S.; et al. Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: Results from the FIREFLEYE randomized phase 3 study. Eye 2024, 38, 1444–1453. [Google Scholar] [CrossRef]
- Heo, J.I.; Ryu, J. Exosomal noncoding RNA: A potential therapy for retinal vascular diseases. Mol. Ther. Nucleic Acids 2024, 35, 102128. [Google Scholar] [CrossRef]
- Dogra, M.R.; Vinekar, A. Role of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) in the Treatment of Retinopathy of Prematurity: A Narrative Review in the Context of Middle-Income Countries. Pediatr. Health Med. Ther. 2023, 14, 59–69. [Google Scholar] [CrossRef]
- Cota, F.; Costa, S.; Giannantonio, C.; Purcaro, V.; Catenazzi, P.; Vento, G. Lutein supplementation and retinopathy of prematurity: A meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 175–180. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Diggikar, S.; Aradhya, A.S.; Swamy, R.S.; Namachivayam, A.; Chandrasekaran, M. Effect of Enteral Long-Chain Polyunsaturated Fatty Acids on Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Neonatology 2022, 119, 547–557. [Google Scholar] [CrossRef]
- Najm, S.; Lofqvist, C.; Hellgren, G.; Engstrom, E.; Lundgren, P.; Hard, A.L.; Lapillonne, A.; Savman, K.; Nilsson, A.K.; Andersson, M.X.; et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 17–23. [Google Scholar] [CrossRef]
- Karlinski Vizentin, V.; Madeira de Sá Pacheco, I.; Fahel Vilas Bôas Azevêdo, T.; Florêncio de Mesquita, C.; Alvim Pereira, R. Early versus Late Caffeine Therapy Administration in Preterm Neonates: An Updated Systematic Review and Meta-Analysis. Neonatology 2024, 121, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kua, K.P.; Lee, S.W. Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br. J. Clin. Pharmacol. 2017, 83, 180–191. [Google Scholar] [CrossRef]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Therapeutic Potential of Polyphenols and Other Micronutrients of Marine Origin. Mar. Drugs 2023, 21, 323. [Google Scholar] [CrossRef]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C. Patenting natural products just got harder. Nat. Biotechnol. 2014, 32, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Son, J.; Min, S.; Lee, H.; Park, M.S. Natural Resources Conflicts on Borderlands by the Five Spheres of Earth System. Land 2023, 12, 389. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Paine, M.F.; McCune, J.S.; Oberlies, N.H.; Cech, N.B. Selection and characterization of botanical natural products for research studies: A NaPDI center recommended approach. Nat. Prod. Rep. 2019, 36, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Tamura, S.; Wang, T. Editorial: Discovery and Total Synthesis of Bio-functional Natural Products From Traditional Medicinal Plants. Front. Chem. 2020, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Dhawan, D.; Jain, G.K.; Yerer, M.B.; Collignon, T.E.; Tewari, D.; Bishayee, A. Novel Strategies for the Bioavailability Augmentation and Efficacy Improvement of Natural Products in Oral Cancer. Cancers 2022, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Fraietta, I.; Gasparri, F. The development of high-content screening (HCS) technology and its importance to drug discovery. Expert. Opin. Drug Discov. 2016, 11, 501–514. [Google Scholar] [CrossRef]
- Dueñas, M.E.; Peltier-Heap, R.E.; Leveridge, M.; Annan, R.S.; Büttner, F.H.; Trost, M. Advances in high-throughput mass spectrometry in drug discovery. EMBO Mol. Med. 2023, 15, e14850. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Dara, S.; Dhamercherla, S.; Jadav, S.S.; Babu, C.M.; Ahsan, M.J. Machine Learning in Drug Discovery: A Review. Artif. Intell. Rev. 2022, 55, 1947–1999. [Google Scholar] [CrossRef]
- Simoben, C.V.; Babiaka, S.B.; Moumbock, A.F.A.; Namba-Nzanguim, C.T.; Eni, D.B.; Medina-Franco, J.L.; Günther, S.; Ntie-Kang, F.; Sippl, W. Challenges in natural product-based drug discovery assisted with in silico-based methods. RSC Adv. 2023, 13, 31578–31594. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Saldívar-González, F.I.; Aldas-Bulos, V.D.; Medina-Franco, J.L.; Plisson, F. Natural product drug discovery in the artificial intelligence era. Chem. Sci. 2022, 13, 1526–1546. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Horowitz, L.F.; Rodriguez, A.D.; Ray, T.; Folch, A. Microfluidics for interrogating live intact tissues. Microsyst. Nanoeng. 2020, 6, 69. [Google Scholar] [CrossRef]
- Dodson, K.H.; Echevarria, F.D.; Li, D.; Sappington, R.M.; Edd, J.F. Retina-on-a-chip: A microfluidic platform for point access signaling studies. Biomed. Microdevices 2015, 17, 114. [Google Scholar] [CrossRef]
- Alarautalahti, V.; Ragauskas, S.; Hakkarainen, J.J.; Uusitalo-Järvinen, H.; Uusitalo, H.; Hyttinen, J.; Kalesnykas, G.; Nymark, S. Viability of Mouse Retinal Explant Cultures Assessed by Preservation of Functionality and Morphology. Investig. Opthalmology Vis. Sci. 2019, 60, 1914. [Google Scholar] [CrossRef]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A Review of the Microbial Production of Bioactive Natural Products and Biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Hirschi, K.D. Genetically Modified Plants: Nutritious, Sustainable, yet Underrated. J. Nutr. 2020, 150, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.-I.; Ryu, J. Natural Products in the Treatment of Retinopathy of Prematurity: Exploring Therapeutic Potentials. Int. J. Mol. Sci. 2024, 25, 8461. https://doi.org/10.3390/ijms25158461
Heo J-I, Ryu J. Natural Products in the Treatment of Retinopathy of Prematurity: Exploring Therapeutic Potentials. International Journal of Molecular Sciences. 2024; 25(15):8461. https://doi.org/10.3390/ijms25158461
Chicago/Turabian StyleHeo, Jong-Ik, and Juhee Ryu. 2024. "Natural Products in the Treatment of Retinopathy of Prematurity: Exploring Therapeutic Potentials" International Journal of Molecular Sciences 25, no. 15: 8461. https://doi.org/10.3390/ijms25158461
APA StyleHeo, J.-I., & Ryu, J. (2024). Natural Products in the Treatment of Retinopathy of Prematurity: Exploring Therapeutic Potentials. International Journal of Molecular Sciences, 25(15), 8461. https://doi.org/10.3390/ijms25158461






