Relationship between Respiratory Microbiome and Systemic Inflammatory Markers in COPD: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Features of Participants
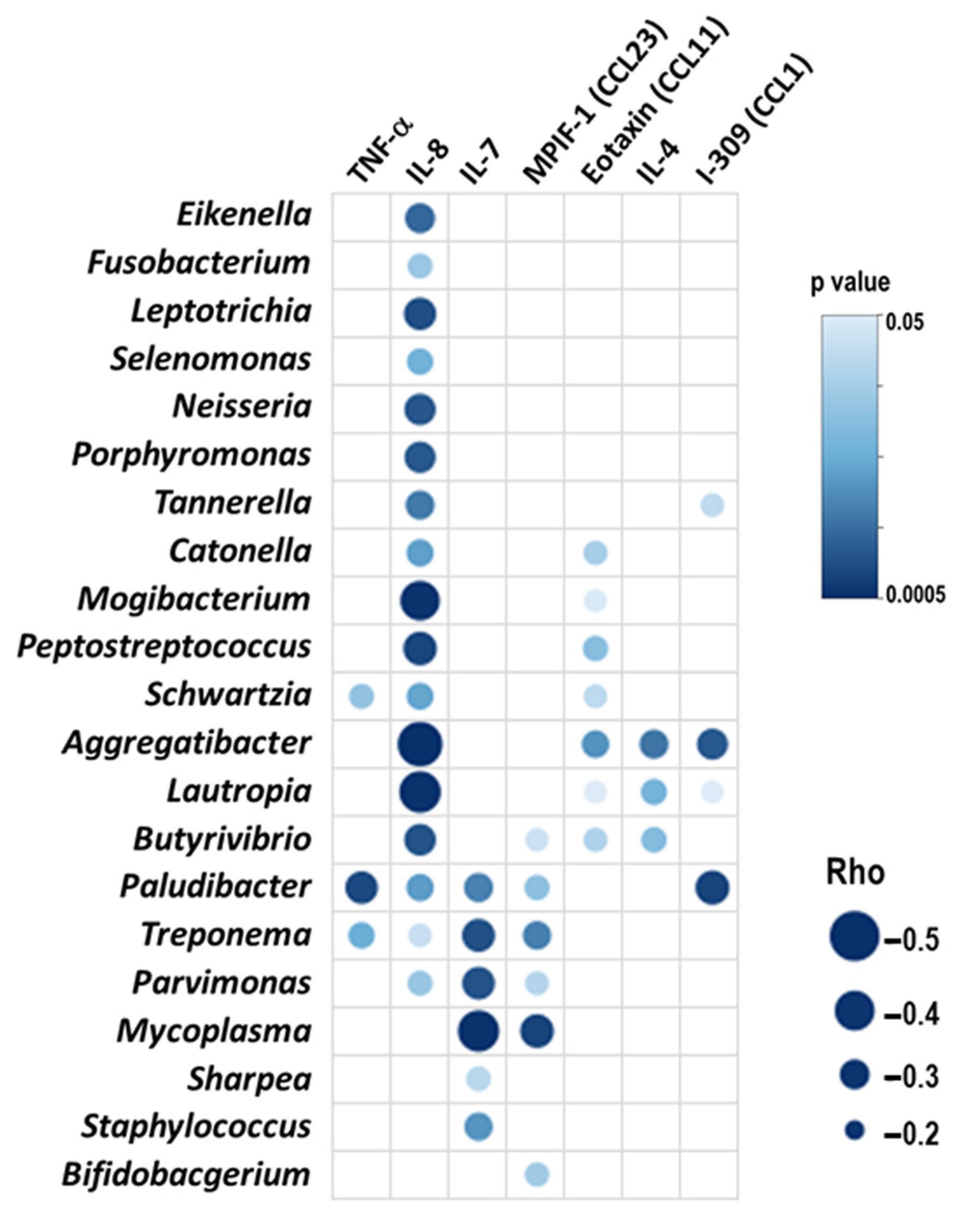
2.2. All Patients: Blood Inflammatory Mediators and Microbiome Interactions
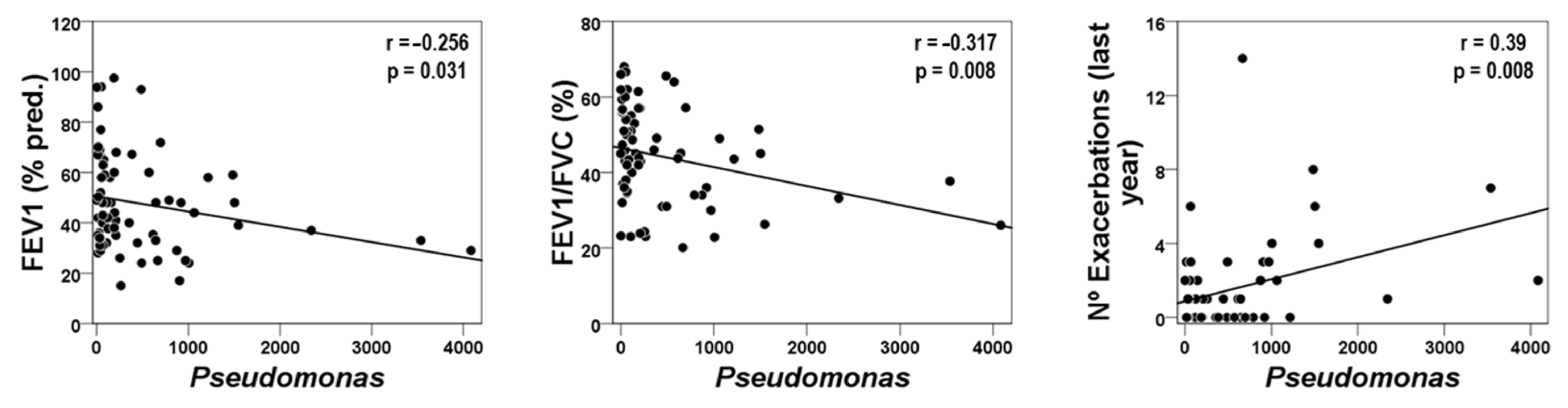
2.3. Blood Inflammatory Mediators and Host–Microbiome Interactions in the FE Phenotype
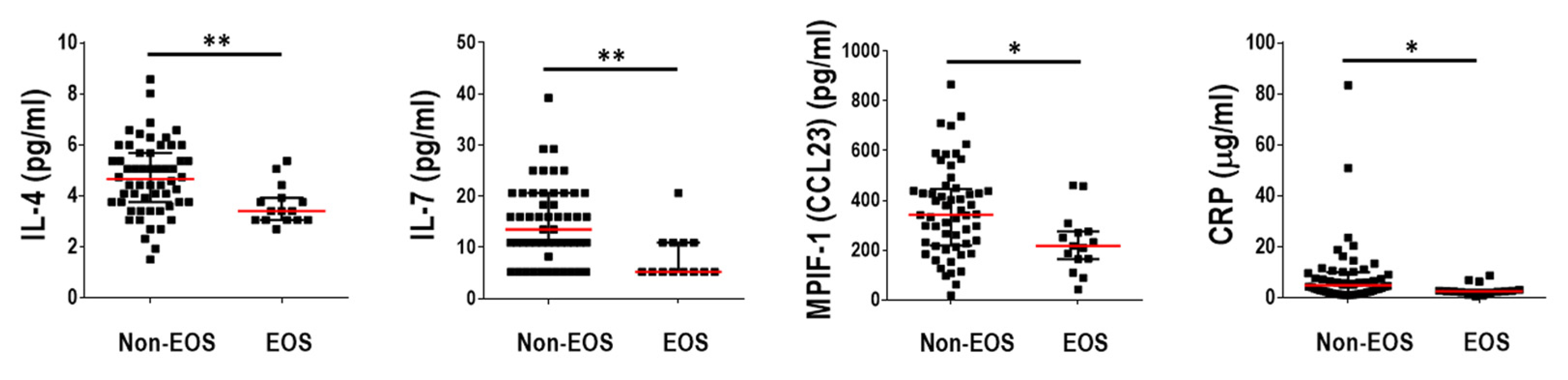
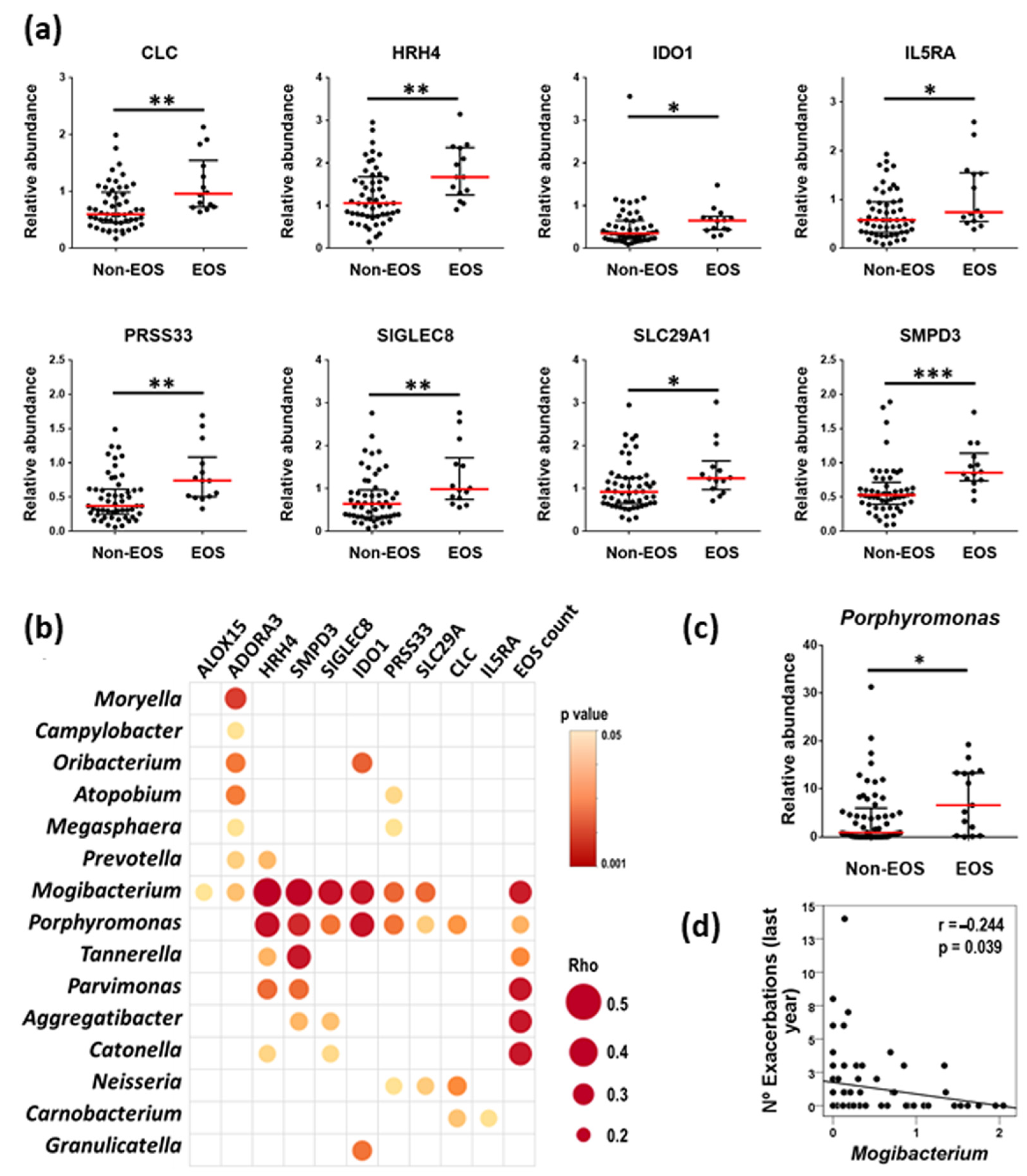
2.4. Blood Inflammatory Mediators and Host–Microbiome Interactions in the EOS Pheno/Endotype
3. Discussion
3.1. Microbiome and Inflammation in COPD
3.2. The FE Phenotype
3.3. The EOS Phenotype/Endotype
4. Materials and Methods
4.1. Study Design and Population
4.2. Samples Processing
4.3. 16S rRNA Gene Sequencing Analysis
4.4. Measurement of Systemic Inflammatory Mediators
4.5. RT-qPCR Assays
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1.
- Hospital del Mar Research Institute. Respiratory Medicine Department, Hospital del Mar. MELIS Dept., Universitat Pompeu Fabra (UPF). BRN. Barcelona. Spain.
- 2.
- Centro de Investigación Biomédica en Red, Área de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III. Madrid. Spain.
- 3.
- Servei de Pneumologia (Institut Clínic de Respiratori), Hospital Clínic-Fundació Clínic per la Recerca Biomèdica, Universitat de Barcelona. Barcelona. Spain.
- 4.
- Servicio de Neumología, Hospital 12 de Octubre. Madrid. Spain.
- 5.
- Dept. MELIS, Universitat Pompeu Fabra. Barcelona. Spain.
- 6.
- Servicio de Neumología, Hospital Son Espases-Instituto de Investigación Sanitaria de Palma (IdISBa), Universitat de les Illes Balears. Palma de Mallorca. Spain.
- 7.
- Hospital del Mar Research Institute. Barcelona. Spain.
- 8.
- Servicio de Neumología, Consorci Sanitari Parc Taulí, Universitat Autònoma de Barcelona. Sabadell. Spain.
- 9.
- Unidad Médico-Quirúrgica de Enfermedades Respiratorias, Hospital Universitario Virgen del Rocío, Universidad de Sevilla. Sevilla. Spain.
- 10.
- Servicio de Neumología, Fundación Jiménez Díaz, Universidad Autónoma de Madrid. Madrid. Spain
- 11.
- Servicio de Neumología, Fundación Jiménez Díaz, Universidad Autónoma de Madrid. Clínica Universidad de Navarra. Madrid. Spain.
- 12.
- Universitat Autònoma de Barcelona. Barcelona. Spain.
- 13.
- Laboratori de Referència de Catalunya. El Prat de Llobregat. Spain.
References
- Segal, L.N.; Martinez, F.J. Chronic obstructive pulmonary disease subpopulations and phenotyping. J. Allergy Clin. Immunol. 2018, 141, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Corlateanu, A.; Mendez, Y.; Wang, Y.; Garnica, R.d.J.A.; Botnaru, V.; Siafakas, N. Chronic obstructive pulmonary disease and phenotypes: A state-of-the-art. Pulmonology 2020, 26, 95–100. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Ramsheh, M.Y.; Haldar, K.; Esteve-Codina, A.; Purser, L.F.; Richardson, M.; Müller-Quernheim, J.; Greulich, T.; Nowinski, A.; Barta, I.; Stendardo, M.; et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: A bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021, 2, e300–e310. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Miller, B.E.; Tal-Singer, R.; Van Horn, S.; Tomsho, L.; Mackay, A.; Allinson, J.P.; Webb, A.J.; Brookes, A.J.; et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: An analysis of the COPDMAP study. Thorax 2018, 73, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maschera, B.; Lea, S.; Kolsum, U.; Michalovich, D.; Van Horn, S.; Traini, C.; Brown, J.R.; Hessel, E.M.; Singh, D. Airway host-microbiome interactions in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Li, S.W.; Chin, C.Y.; Hsu, C.W.; Lee, C.C.; Yeh, Y.M.; Wu, K.A. Association of exacerbation phenotype with the sputum microbiome in chronic obstructive pulmonary disease patients during the clinically stable state. J. Transl. Med. 2021, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.J.; Huang, J.T.J.; Lonergan, M.; Keir, H.R.; Fong, C.J.; Tan, B.; Cassidy, A.J.; Finch, S.; Mullerova, H.; Miller, B.E.; et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2021, 147, 158–167. [Google Scholar] [CrossRef]
- Wang, Z.; Locantore, N.; Haldar, K.; Ramsheh, M.Y.; Beech, A.S.; Ma, W.; Brown, J.R.; Tal-Singer, R.; Barer, M.R.; Bafadhel, M.; et al. Inflammatory Endotype-associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 1488–1502. [Google Scholar] [CrossRef]
- Millares, L.; Pascual, S.; Montón, C.; García-Núñez, M.; Lalmolda, C.; Faner, R.; Casadevall, C.; Setó, L.; Capilla, S.; Moreno, A.; et al. Relationship between the respiratory microbiome and the severity of airflow limitation, history of exacerbations and circulating eosinophils in COPD patients. BMC Pulm. Med. 2019, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, Y.; Gong, S.; Wei, M.; Ma, Y.; Feng, R.; Gao, J.; Liu, X.; Tu, F.; Ma, W.; et al. Airway dysbiosis accelerates lung function decline in chronic obstructive pulmonary disease. Cell Host Microbe 2023, 31, 1054–1070.e9. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Kang, Y.; Wang, X.; Cao, W.; Li, M.; He, Y.; Pan, X.; Ye, K.; Xu, D. Frequent exacerbators of chronic obstructive pulmonary disease have distinguishable sputum microbiome signatures during clinical stability. Front. Microbiol. 2022, 13, 1037037. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Pauletti, A.; Rossi, M.R.; Spanevello, A.; Casolari, P.; Marcellini, A.; Forini, G.; Gnesini, G.; Marku, B.; Barnes, N.; et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur. Respir. J. 2017, 50, 1700451. [Google Scholar] [CrossRef]
- Filho, F.S.L.; Alotaibi, N.M.; Ngan, D.; Tam, S.; Yang, J.; Hollander, Z.; Chen, V.; FitzGerald, J.M.; Nislow, C.; Leung, J.M.; et al. Sputum Microbiome Is Associated with 1-Year Mortality after Chronic Obstructive Pulmonary Disease Hospitalizations. Am. J. Respir. Crit. Care Med. 2019, 199, 1205–1213. [Google Scholar] [CrossRef]
- Xue, Q.; Xie, Y.; He, Y.; Yu, Y.; Fang, G.; Yu, W.; Wu, J.; Li, J.; Zhao, L.; Deng, X.; et al. Lung microbiome and cytokine profiles in different disease states of COPD: A cohort study. Sci. Rep. 2023, 13, 5715. [Google Scholar] [CrossRef]
- Yun, J.H.; Chase, R.; Parker, M.M.; Saferali, A.; Castaldi, P.J.; Silverman, E.K.; Hersh, C.P. Peripheral Blood Gene Expression Signatures of Eosinophilic Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2019, 61, 398–401. [Google Scholar] [CrossRef]
- Agusti, A.; Sin, D.D. Biomarkers in COPD. Clin. Chest Med. 2014, 35, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, C. The Significance of Serum Interleukin-8 in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Tanaffos 2018, 17, 13–21. [Google Scholar] [PubMed]
- Li, S.; Zhao, S.; Wu, Z.; Wang, F.; Li, W. Alteration of immune profiles is associated with pulmonary function and symptoms in patients with chronic obstructive pulmonary disease. Mol. Med. Rep. 2021, 24, 742. [Google Scholar] [CrossRef]
- 202 GOLD Report—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 10 January 2024).
- Pragman, A.A.; Knutson, K.A.; Gould, T.J.; Isaacson, R.E.; Reilly, C.S.; Wendt, C.H. Chronic obstructive pulmonary disease upper airway microbiota alpha diversity is associated with exacerbation phenotype: A case-control observational study. Respir. Res. 2019, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Tiew, P.Y.; Jaggi, T.K.; Chan, L.L.Y.; Chotirmall, S.H. The airway microbiome in COPD, bronchiectasis and bronchiectasis-COPD overlap. Clin. Respir. J. 2021, 15, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Jalalvand, F.; Thegerström, J.; Riesbeck, K. The Interplay between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae. Front. Immunol. 2018, 9, 2530. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.M.; Ochs-Balcom, H.M.; Zhao, J.; Murphy, T.F.; Sethi, S. Lower Airway Bacterial Colonization Patterns and Species-Specific Interactions in Chronic Obstructive Pulmonary Disease. J. Clin. Microbiol. 2018, 56, e00330-18. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Van Der Gast, C.J.; Serisier, D.J. Predominant pathogen competition and core microbiota divergence in chronic airway infection. ISME J. 2015, 9, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Q.; Ren, Z.; Li, H.; Ma, Q. Different Airway Inflammatory Phenotypes Correlate with Specific Fungal and Bacterial Microbiota in Asthma and Chronic Obstructive Pulmonary Disease. J. Immunol. Res. 2022, 2022, 2177884. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; McDonald, V.M.; Baines, K.J.; Wark, P.A.B.; Simpson, J.L.; Jones, P.W.; Gibson, P.G. Peripheral blood eosinophils: A surrogate marker for airway eosinophilia in stable COPD. Int. J. Chron. Obstruct Pulmon Dis. 2016, 11, 1495–1504. [Google Scholar] [CrossRef]
- Antus, B.; Paska, C.; Barta, I. Predictive Value of Exhaled Nitric Oxide and Blood Eosinophil Count in the Assessment of Airway Eosinophilia in COPD. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 2025–2035. [Google Scholar] [CrossRef]
- Baines, K.J.; Negewo, N.A.; Gibson, P.G.; Fu, J.J.; Simpson, J.L.; Wark, P.A.B.; Fricker, M.; McDonald, V.M. A Sputum 6 Gene Expression Signature Predicts Inflammatory Phenotypes and Future Exacerbations of COPD. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 1577–1590. [Google Scholar] [CrossRef]
- Kerkhof, M.; Sonnappa, S.; Postma, D.S.; Brusselle, G.; Agustí, A.; Anzueto, A.; Jones, R.; Papi, A.; Pavord, I.; Pizzichini, E.; et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur. Respir. J. 2017, 50, 1700761. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e10. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Lee, K.S.; Hong, Y.; Hwang, S.C.; Kim, J.Y.; Kim, D.K.; Yoo, K.H.; Lee, J.H.; Kim, T.H.; Lim, S.Y.; et al. Blood eosinophil count as a prognostic biomarker in COPD. Int. J. Chron. Obstruct Pulmon Dis. 2018, 13, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Monteagudo, M.; Solntseva, I.; Alcázar, B. Blood Eosinophil Counts and Their Variability and Risk of Exacerbations in COPD: A Population-Based Study. Arch. Bronconeumol. 2021, 57, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.F.; Pop, C.M. Correlations between neutrophil to lymphocyte ratio, blood eosinophils and clinical characteristics in chronic obstructive pulmonary disease. Med. Pharm. Rep. 2020, 93, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Lee, S.H.; Kim, S.Y.; Leem, A.Y.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Chang, J.; et al. Relationship between airway obstruction and C-reactive protein levels in a community-based population of Korea. Int. J. Tuberc. Lung Dis. 2019, 23, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, A.; Marras, V.; Harlander, M.; Mekov, E.; Esquinas, C.; Turel, M.; Lestan, D.; Petkov, R.; Yanev, N.; Pirina, P.; et al. Association between Routine Blood Biomarkers and Clinical Phenotypes and Exacerbations in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 681–690. [Google Scholar] [CrossRef]
- Martínez-Gestoso, S.; García-Sanz, M.-T.; Carreira, J.-M.; Nieto-Fontarigo, J.-J.; Calvo-Álvarez, U.; Doval-Oubiña, L.; Camba-Matos, S.; Peleteiro-Pedraza, L.; Roibás-Veiga, I.; González-Barcala, F.J. Prognostic Usefulness of Basic Analytical Data in Chronic Obstructive Pulmonary Disease Exacerbation. Open Respir. Arch. 2023, 5, 100271. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Galeone, C.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. C-reactive protein level predicts mortality in COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 2017, 26, 160070. [Google Scholar] [CrossRef]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| All (N = 72) | IE COPD (N = 52) | FE COPD (N = 20) | Non-EOS COPD (N = 57) | EOS COPD (N = 15) | |
|---|---|---|---|---|---|
| Age, years median (IQR) | 70 (62–73) | 69 (62–73) | 70 (66–74) | 70 (62–73) | 69 (62–74) |
| Sex (male), n (%) | 64 (89) | 46 (89) | 18 (90) | 50 (88) | 14 (93) |
| BMI, kg/m2 median (IQR) | 27.4 (24.2–30.1) | 27.4 (25.1–29.9) | 27.5 (23.5–30.7) | 27.8 (24.1–30.5) | 27.3 (24.7–28.4) |
| FEV1, (% pred.) median (IQR) | 44 (33–60) | 48 (35–67) | 34 (26–47) ** | 43 (32–60) | 48 (35–67) |
| FEV1/FVC, (%) median (IQR) | 44 (35–55) | 46 (37–57) | 35 (30–45) ** | 45 (34–54.3) | 43 (37–56) |
| DLco, (% pred.) median (IQR) | 51 (40–68) | 51 (45–71) | 40 (32–60) * | 49 (40–66) | 65 (40–82) |
| Nº of Exacerbations in the previous year, median (IQR) | 1 (0–2) | 0 (0–1) | 3 (2–6) *** | 1 (0–2) | 0 (0–1) |
| Blood leucocytes (cells/nL), median (IQR) | 8 (6.7–9.3) | 7.6 (6.7–8.7) | 8.4 (7–10.6) | 8 (6.7–10) | 7.1 (6.5–8.5) |
| Blood eosinophils (cells/nL), median (IQR) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.2) | 0.3 (0.2–0.3) ‡‡‡ |
| Blood neutrophils (cells/nL), median (IQR) | 4.6 (3.9–6.1) | 4.5 (3.8–5.3) | 5.7 (4–4.8) | 4.8 (4.1–6.3) | 3.8 (3.4–4.6) ‡‡‡ |
| GOLD stages, n (%) | |||||
| 1 | 6 (8) | 6 (12) | 0 (0) | 4 (7) | 2 (13) |
| 2 | 20 (28) | 17 (32) | 3 (15) | 15 (26) | 5 (33) |
| 3 | 35 (49) | 26 (50) | 9 (45) | 28 (49) | 7 (47) |
| 4 | 11 (15) | 3 (6) | 8 (40) *** | 10 (18) | 1 (7) |
| FEV1 (% Pred) | DLco (% Pred) ‡ | Nº EXACERBATIONS (Previous Year) | |
|---|---|---|---|
| Aggregatibacter | 0.252 * | 0.282 * | −0.364 ** |
| Butyrivibrio | 0.248 * | 0.320 * | −0.311 ** |
| Treponema | 0.436 *** | 0.384 ** | −0.305 ** |
| Parvimonas | 0.250 * | 0.335 * | - |
| EOSINOPHIL COUNTS | |
|---|---|
| ADORA3 | 0.517 *** |
| ALOX15 | 0.572 *** |
| CLC | 0.710 *** |
| HRH4 | 0.547 *** |
| IDO1 | 0.508 *** |
| IL5RA | 0.513 *** |
| PRSS33 | 0.703 *** |
| SIGLEC8 | 0.712 *** |
| SLC29A1 | 0.632 *** |
| SMPD3 | 0.618 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadevall, C.; Quero, S.; Millares, L.; Faner, R.; Cosío, B.G.; Peces-Barba, G.; Castro-Acosta, A.; Montón, C.; Palou, A.; Pascual-Guardia, S.; et al. Relationship between Respiratory Microbiome and Systemic Inflammatory Markers in COPD: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 8467. https://doi.org/10.3390/ijms25158467
Casadevall C, Quero S, Millares L, Faner R, Cosío BG, Peces-Barba G, Castro-Acosta A, Montón C, Palou A, Pascual-Guardia S, et al. Relationship between Respiratory Microbiome and Systemic Inflammatory Markers in COPD: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(15):8467. https://doi.org/10.3390/ijms25158467
Chicago/Turabian StyleCasadevall, Carme, Sara Quero, Laura Millares, Rosa Faner, Borja G. Cosío, Germán Peces-Barba, Ady Castro-Acosta, Concepción Montón, Alexandre Palou, Sergi Pascual-Guardia, and et al. 2024. "Relationship between Respiratory Microbiome and Systemic Inflammatory Markers in COPD: A Pilot Study" International Journal of Molecular Sciences 25, no. 15: 8467. https://doi.org/10.3390/ijms25158467





