Exogenous Application of Amino Acids Alleviates Toxicity in Two Chinese Cabbage Cultivars by Modulating Cadmium Distribution and Reducing Its Translocation
Abstract
:1. Introduction
2. Results
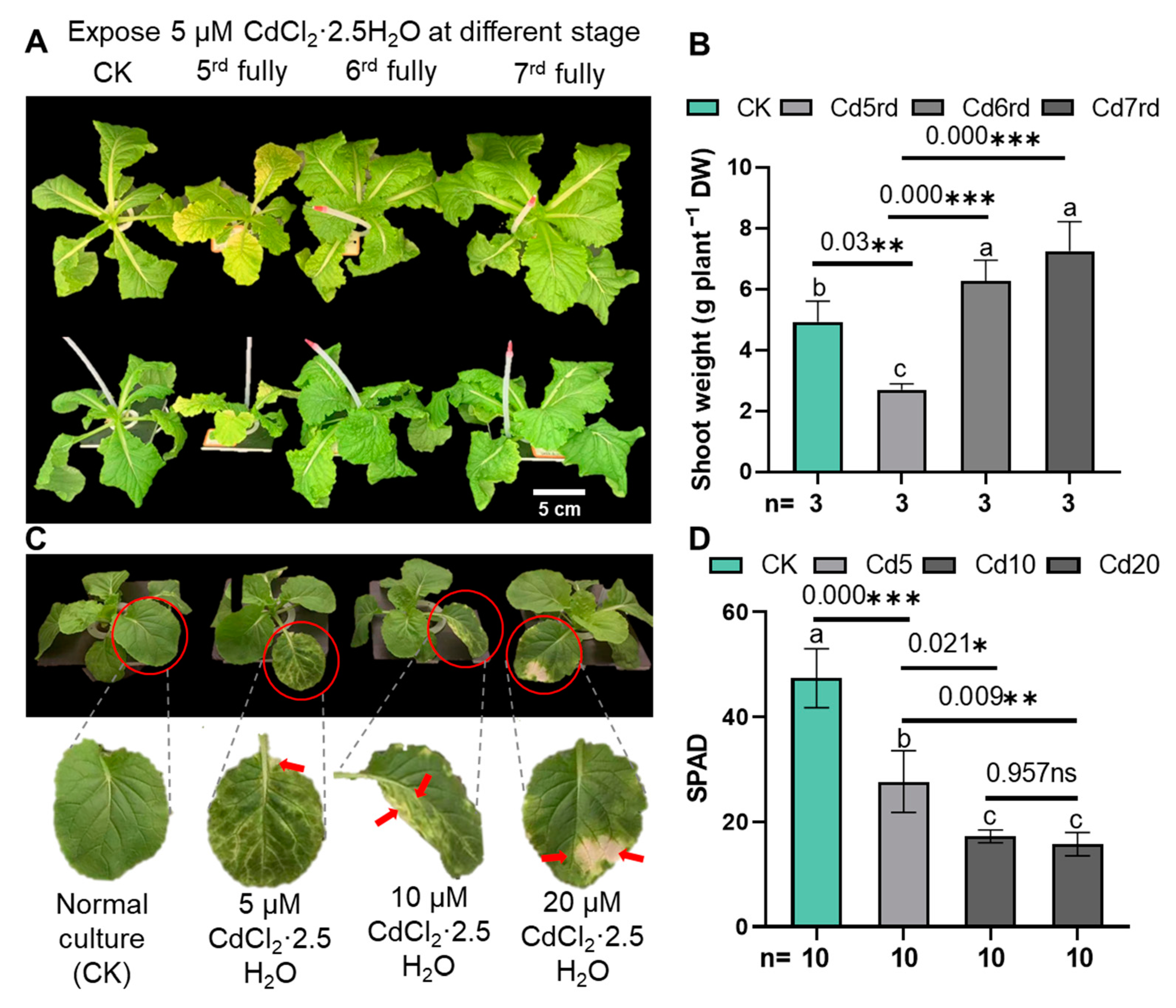
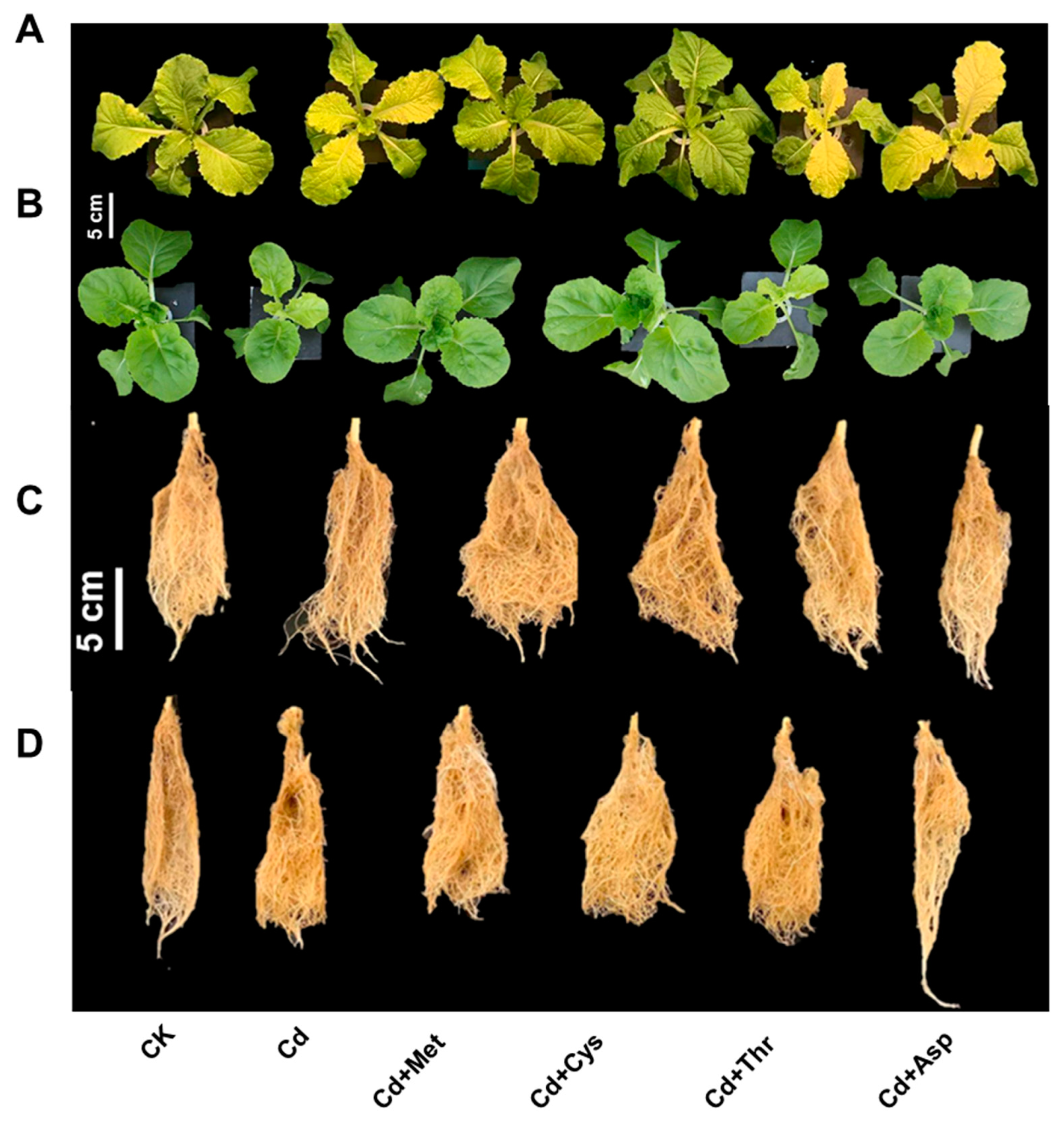
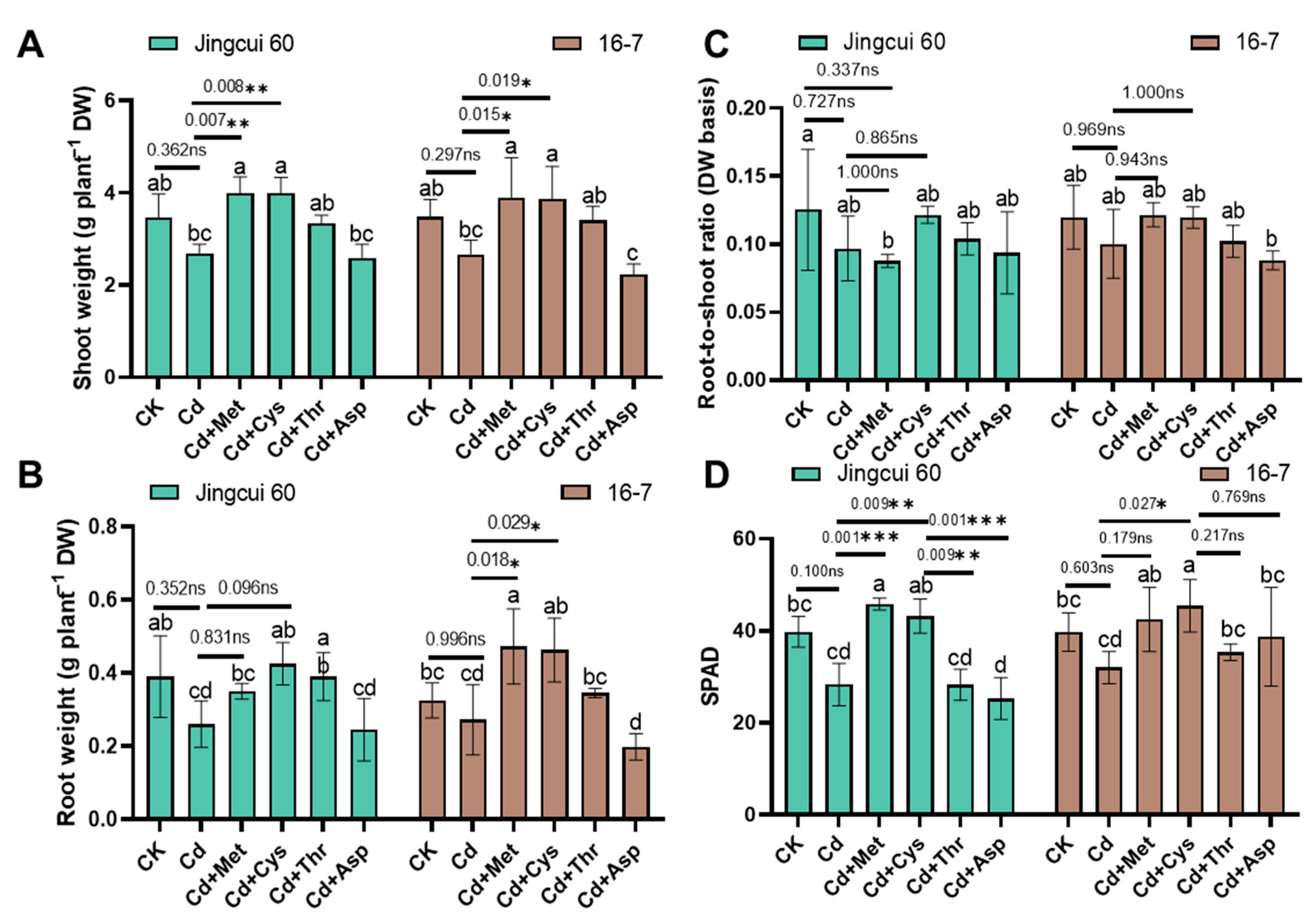
2.1. Growth and Photosynthetic Activity
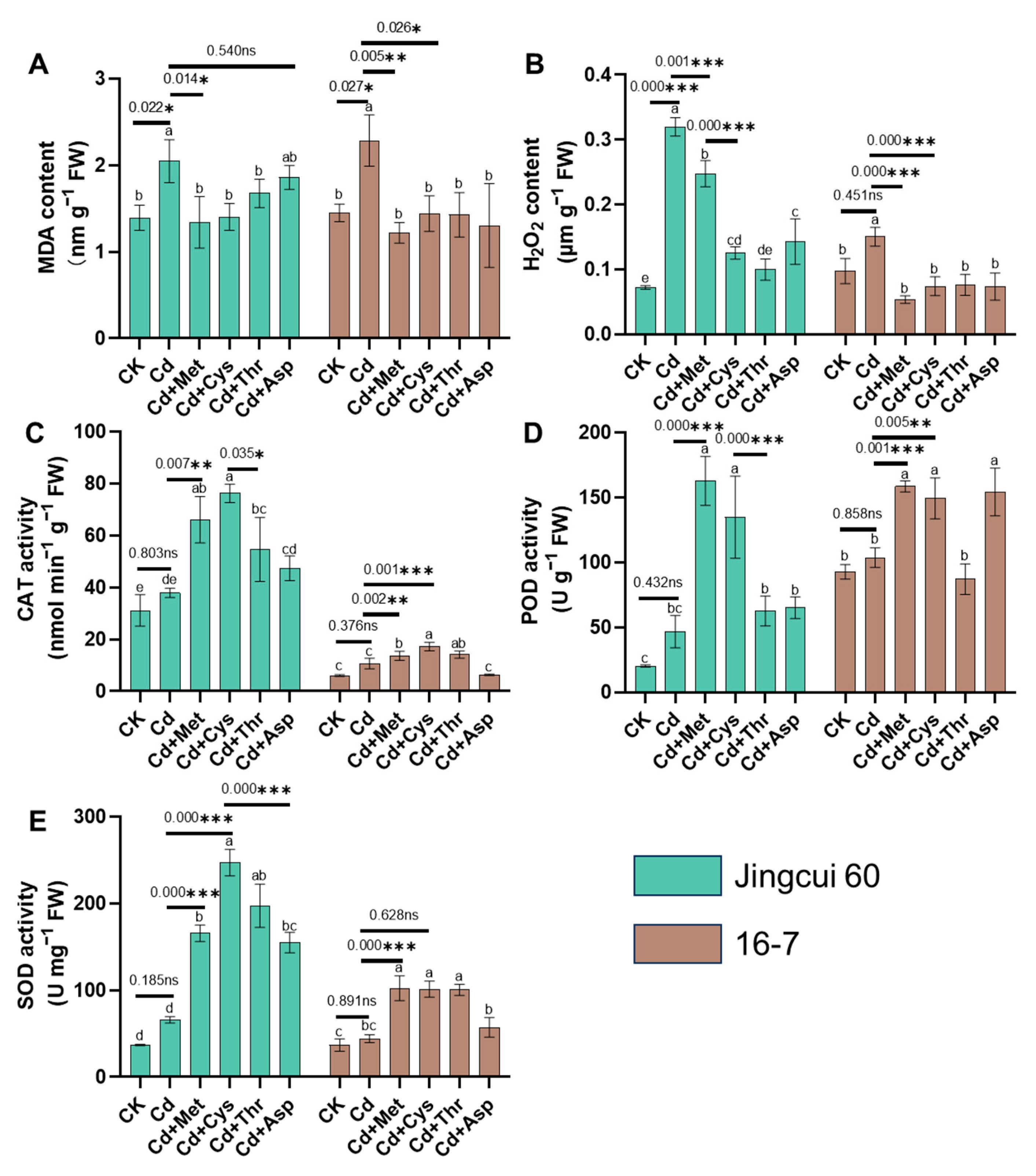
2.2. Oxidative Damage in Chinese Cabbage
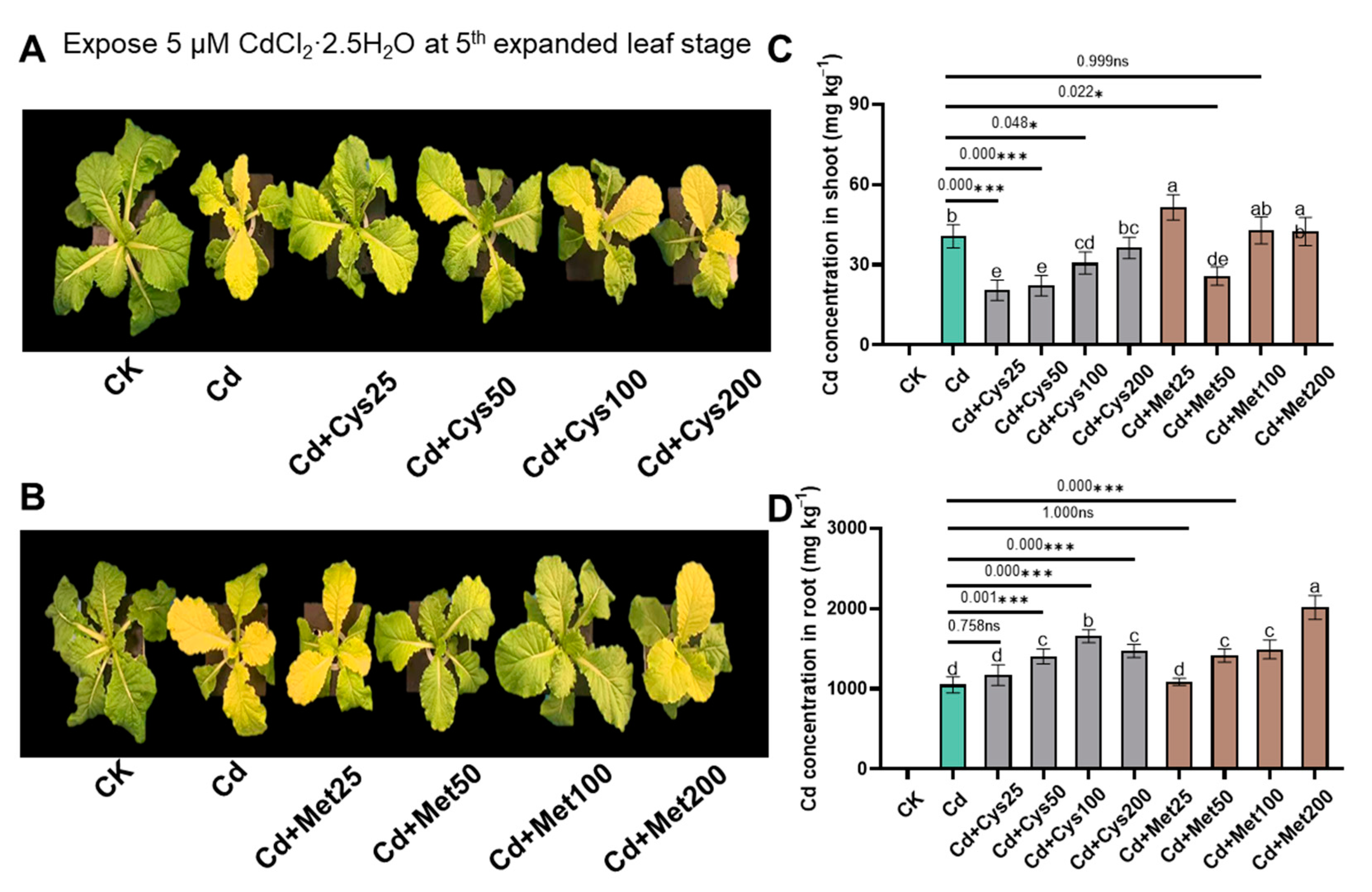
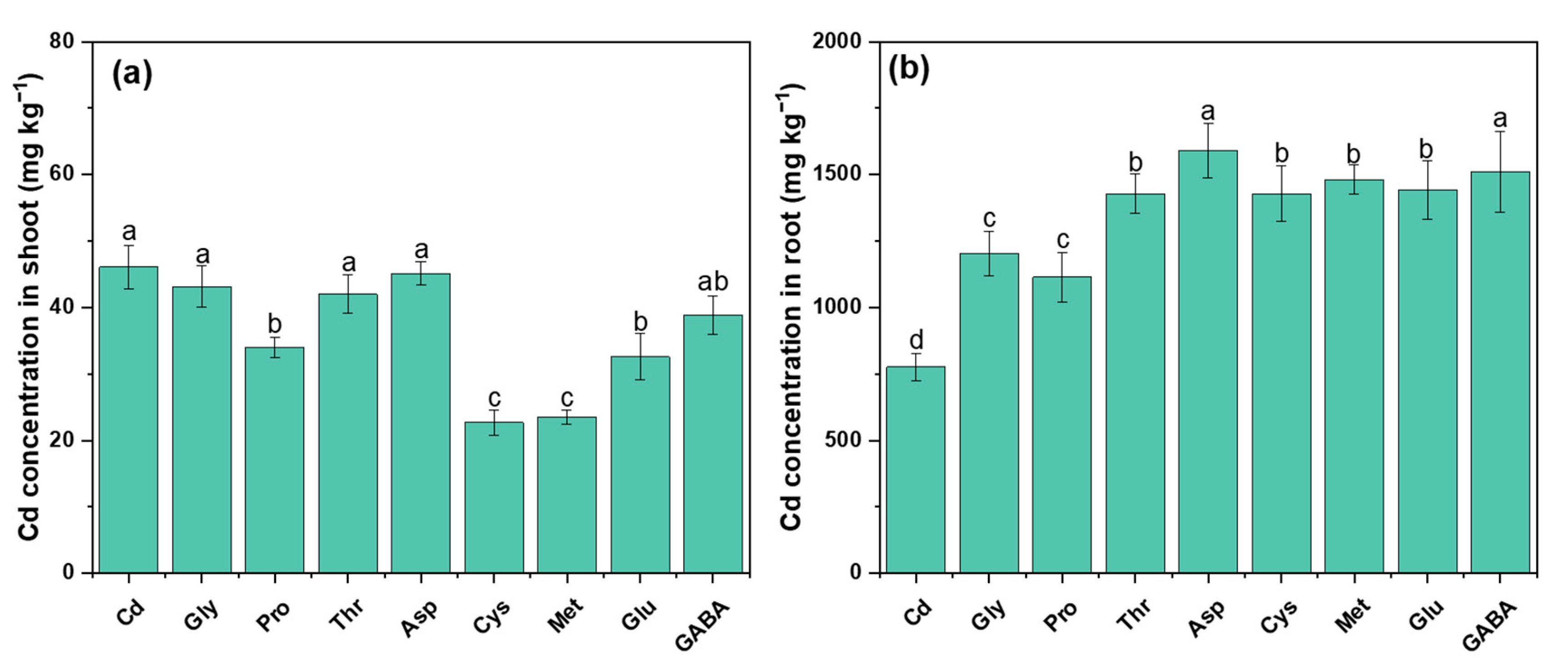
2.3. Cd Uptake and Translocation
2.4. Cd Subcellular Distribution
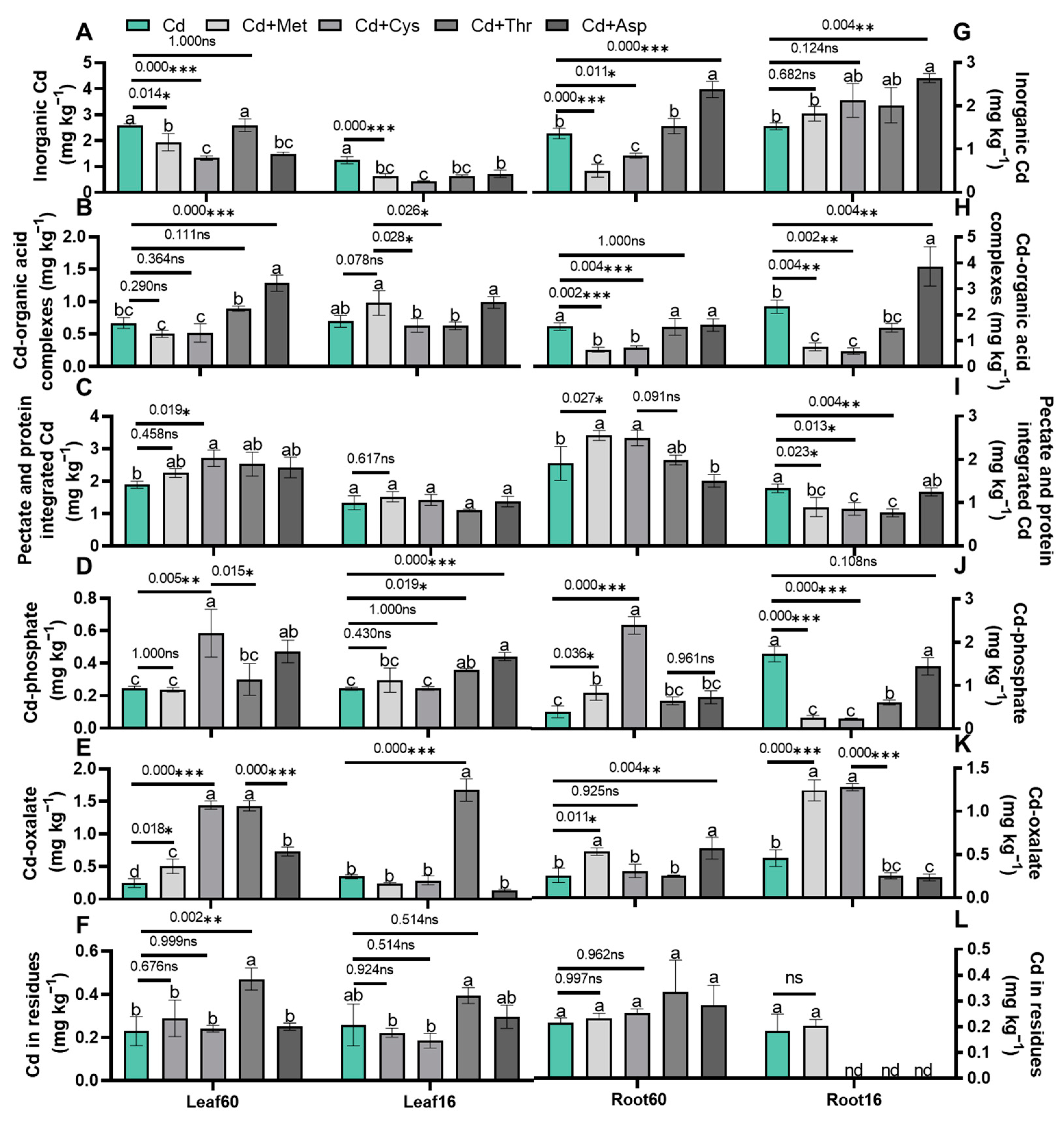
2.5. Chemical Forms of Cd
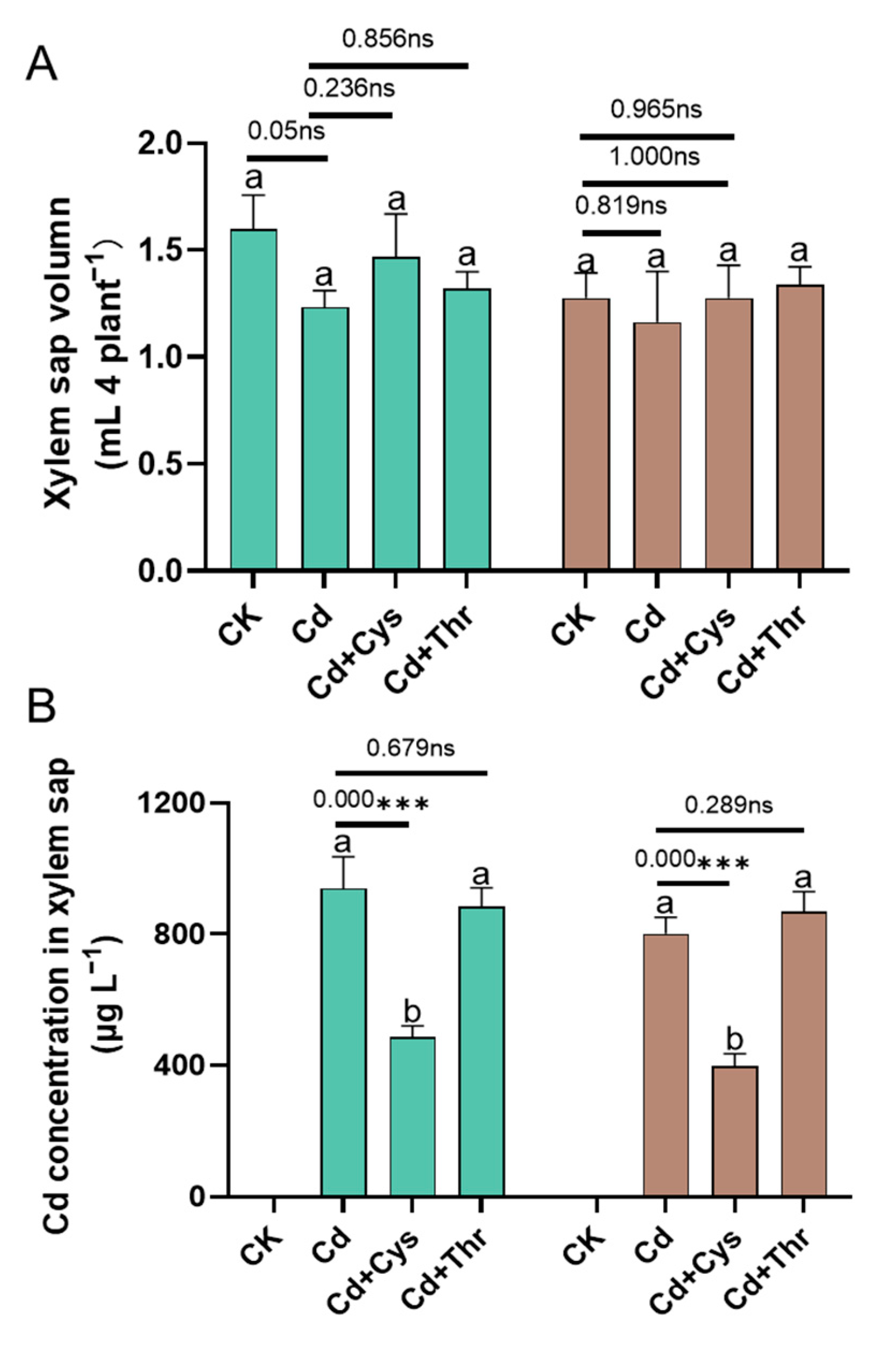
2.6. Cd Concentration in the Xylem and Relative Expression of Metal Transport Genes
3. Discussion
3.1. Effect of Exogenous AAs on the Growth and Photosynthetic Activity in Chinese Cabbage
3.2. Mitigation of Cd Toxicity by Modifying the Subcellular Distribution and Chemical Speciation of Cd
3.3. Alleviation of Cd Toxicity by Regulating the Expression of Metal Transporter Genes and Cd Translocation into the Xylem
4. Materials and Methods
4.1. Treatments and Conditions
4.2. Chlorophyll Contents, Dry Weight, and Cd Accumulation Pattern
4.3. Various Subcellular Cd Fractionations
4.4. Chemical Speciation of Cd
4.5. Cd Concentration in the Xylem
4.6. Quantitative RT-PCR of Chinses Cabbage MRNA
4.7. Determination of Malondialdehyde, Hydrogen Peroxide (H2O2), and Antioxidant Enzymes
4.8. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene ID | cDNA Primer | Primer Forward | Primer Reverse |
|---|---|---|---|
| Actin | 5′-GGAGCTGAGAGATTCCGTTG-3’ | 5’-GAACCACCACTGAGGACGAT-3’ | |
| Bra009399 | HMA2 | 5’-GAGGATGCCACATGGTTGGA-3’ | 5’-CTTTGGTACGGCGGAAGAGT-3’ |
| Bra032640 | HMA4 | 5’-TTCCCCACAAGAATCGCTCC-3’ | 5’-CACTCGAACCTTCCACGTCA-3’ |
| Bra037319 | HMA3 | 5’-AACCTCGACGCTATGCACAA-3’ | 5’-GCTTGCCACGTCATCATTGG-3’ |
| Bra013419 | IRT1 | 5’-TGGCATTCTTTTTCGCGGTG-3’ | 5’-GCCGAGCATGCATTGAGAAG-3’ |
| Bra013422 | IRT2 | 5’-CTCGTCGACCTTCTGGCTAC-3’ | 5’-ACTTGGCGACGACAGACATT-3’ |
| Bra010773 | ABCC1 | 5’-GTTGACGTTAGAACCGATGT-3’ | 5’-TTGAGACGATGAGCGATG-3’ |
| Bra032385 | ABCC2 | 5’-CTGTTGATGTTAGGACTGATG-3’ | 5’-GTGAGCGATGATGAGCAT-3’ |
| Bra036010 | PCS1 | 5’-CACAGACATGGTCAGGGAT-3’ | 5’-AAGCATAGTTGGGAGGGA-3’ |
References
- Liu, J.; Wang, Y.; Liu, X.; Xu, J. Occurrence and health risks of heavy metals in plastic-shed soils and vegetables across China. Agric. Ecosyst. Environ. 2021, 321, 107632. [Google Scholar] [CrossRef]
- Hu, N.-W.; Yu, H.-W.; Deng, B.-L.; Hu, B.; Zhu, G.-P.; Yang, X.-T.; Wang, T.-Y.; Zeng, Y.; Wang, Q.-Y. Levels of heavy metal in soil and vegetable and associated health risk in peri-urban areas across China. Ecotoxicol. Environ. Saf. 2023, 259, 115037. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Q.; Zhou, Q.; Ma, L.; Wu, Y.; Liu, Q.; Wang, S.; Feng, Y. Cadmium uptake from soil and transport by leafy vegetables: A meta-analysis. Environ. Pollut. 2020, 264, 114677. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Q.; An, J.; Sun, Y.; Liu, R. Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J. Hazard. Mater. 2010, 173, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Luo, J.; Du, Y.; Li, J.; Liu, Y.; Liang, Y.; Li, T. Coordination between root cell wall thickening and pectin modification is involved in cadmium accumulation in Sedum alfredii. Environ. Pollut. 2021, 268, 115665. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.S.; Wang, Y.J.; Ding, G.; Ma, H.L.; Zhang, Y.J.; Gong, J.M. A Pivotal Role of Cell Wall in Cadmium Accumulation in the Crassulaceae hyperaccumulator Sedum plumbizincicola. Mol. Plant 2017, 10, 771–774. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, P.; Li, H.; Xu, C.; Cohen, H.; Aharoni, A.; Wu, S. Developmental programs interact with abscisic acid to coordinate root suberization in Arabidopsis. Plant J. 2020, 104, 241–251. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.-P.; Giehl, R.F.H.; Pitann, B.; Mühling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Liu, S.; Du, Y.; Li, F.; Du, R.; Wen, D.; Zhao, J. Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2016, 131, 173–180. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2011, 69, 278–288. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2008, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.F.; Valdes, B.; Duke, M.; Peaston, K.A.; Lahner, B.; Salt, D.E.; Williams, L.E. Functional significance of AtHMA4 C-terminal domain in planta. PLoS ONE 2010, 5, e13388. [Google Scholar] [CrossRef]
- Zheng, P.; Cao, L.; Zhang, C.; Pan, W.; Wang, W.; Yu, X.; Li, Y.; Fan, T.; Miao, M.; Tang, X.; et al. MYB43 as a novel substrate for CRL4(PRL1) E3 ligases negatively regulates cadmium tolerance through transcriptional inhibition of HMAs in Arabidopsis. New Phytol. 2022, 234, 884–901. [Google Scholar] [CrossRef]
- Chen, H.; Yang, R.; Zhang, X.; Chen, Y.; Xia, Y.; Xu, X. Foliar application of gibberellin inhibits the cadmium uptake and xylem transport in lettuce (Lactuca sativa L.). Sci. Hortic. 2021, 288, 110410. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Y.; Wang, S.; Shang, X. Ca alleviated Cd-induced toxicity in Salix matsudana by affecting Cd absorption, translocation, subcellular distribution, and chemical forms. J. Plant Physiol. 2023, 281, 153926. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Z.; Song, J.; Long, A.; Cao, M.; Luo, J. Cadmium subcellular distribution and chemical form in Festuca arundinacea in different intercropping systems during phytoremediation. Chemosphere 2021, 276, 130137. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.Y.; Zhang, H.H.; Pan, W.; Jin, C.W.; Lin, X.Y. Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci. Total Environ. 2018, 627, 663–670. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Kang, Y.; Liu, J.; Wang, Y.; Sun, H.; Ao, T.; Chen, W. Selenium alleviates toxicity in Amaranthus hypochondriacus by modulating the synthesis of thiol compounds and the subcellular distribution of cadmium. Chemosphere 2022, 291, 133108. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Zhao, S.; Li, S.; Lei, X.; Qin, L.; Sun, X.; Chen, S. Manganese facilitates cadmium stabilization through physicochemical dynamics and amino acid accumulation in rice rhizosphere under flood-associated low pe+pH. J. Hazard. Mater. 2021, 416, 126079. [Google Scholar] [CrossRef]
- Fu, H.; Yu, H.; Li, T.; Zhang, X. Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 150, 168–175. [Google Scholar] [CrossRef]
- Wang, W.; Cang, L.; Zhou, D.-M.; Yu, Y.-C. Exogenous amino acids increase antioxidant enzyme activities and tolerance of rice seedlings to cadmium stress. Environ. Prog. Sustain. Energy 2017, 36, 155–161. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Khoshgoftarmanesh, A.H.; Maibody, S.A.M.M. Root uptake and xylem transport of cadmium in wheat and triticale as affected by exogenous amino acids. Crop Pasture Sci. 2017, 68, 415–420. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- Zhang, C.; He, Q.; Wang, M.; Gao, X.; Chen, J.; Shen, C. Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol. Environ. Saf. 2020, 190, 110090. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Andresen, E.; Küpper, H. Cadmium Toxicity in Plants. In Cadmium: From Toxicity to Essentiality; Springer: Berlin/Heidelberg, Germany, 2013; pp. 395–413. [Google Scholar] [CrossRef]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Jannat, R.; Banu, M.N.A.; Jahan, M.S.; Nakamura, Y.; Murata, Y. Proline and Glycinebetaine Confer Cadmium Tolerance on Tobacco Bright Yellow-2 Cells by Increasing Ascorbate-Glutathione Cycle Enzyme Activities. Biosci. Biotechnol. Biochem. 2014, 73, 2320–2323. [Google Scholar] [CrossRef]
- Xu, J.; Yin, H.; Li, X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2008, 28, 325–333. [Google Scholar] [CrossRef]
- Dominguez-Solis, J.R.; Lopez-Martin, M.C.; Ager, F.J.; Ynsa, M.D.; Romero, L.C.; Gotor, C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol. J. 2004, 2, 469–476. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R. Cysteine–jack of all glutathione-based plant stress defense trades. In Plant Adaptation to Environmental Change: Significance of Amino Acids and Their Derivatives; CABI Digital Library: Wallingford, UK, 2014; pp. 35–52. [Google Scholar]
- Cai, Y.; Cao, F.; Cheng, W.; Zhang, G.; Wu, F. Modulation of Exogenous Glutathione in Phytochelatins and Photosynthetic Performance Against Cd Stress in the Two Rice Genotypes Differing in Cd Tolerance. Biol. Trace Element Res. 2010, 143, 1159–1173. [Google Scholar] [CrossRef]
- Oloumi, H.; Maleki, M.; Habibipour, L.; Lotfi, S. Foliar application of NaHS alleviates Cd toxicity in soybean plants through regulation of Glutathione metabolism. Plant Stress 2024, 11, 100363. [Google Scholar] [CrossRef]
- Vadas, T.M.; Ahner, B.A. Cysteine- and glutathione-mediated uptake of lead and cadmium into Zea mays and Brassica napus roots. Environ. Pollut. 2009, 157, 2558–2563. [Google Scholar] [CrossRef]
- Erdal, S.; Turk, H. Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ. Exp. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Kong, X.; Chen, Y.; Li, J. Exogenous tryptophan application improves cadmium tolerance and inhibits cadmium upward transport in broccoli (Brassica oleracea var. italica). Front. Plant Sci. 2022, 13, 969675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Ali, A.A.; Baig, M.A.; Iqbal, M.; Haq, I.; Irfan Qureshi, M. Role of Phytochelatins in Cadmium Stress Tolerance in Plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–212. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Dalir, N.; Rahnemaie, R.; Schulin, R. Phosphate and methionine affect cadmium uptake in valerian (Valeriana officinalis L.). Plant Physiol. Biochem. 2021, 158, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Dalir, N.; Rahimi, M.; Schulin, R. Methionine application at low phosphate level promotes root-to-shoot cadmium translocation by mobilizing weakly bound apoplastic cadmium in valerian (Valeriana officinalis L.). Rhizosphere 2023, 25, 100672. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Sun, Y.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, H.; Liu, W.; Liu, P. Integrative study of subcellular distribution, chemical forms, and physiological responses for understanding manganese tolerance in the herb Macleaya cordata (papaveraceae). Ecotoxicol. Environ. Saf. 2019, 181, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef]
- Lai, H.Y. Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere 2015, 138, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Weiss, D.J.; Liu, J.; Lu, H.; Yan, C. Kandelia obovata (S., L.) Yong tolerance mechanisms to Cadmium: Subcellular distribution, chemical forms and thiol pools. Mar. Pollut. Bull. 2012, 64, 2453–2460. [Google Scholar] [CrossRef]
- Sela, M.; Fritz, E.; Huttermann, A.; Tel-Or, E. Studies on cadmium localization in the water fern Azolla. Physiol. Plant. 1990, 79, 547–553. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in Cd detoxification for the production of low Cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef]
- Van Belleghem, F.; Cuypers, A.; Semane, B.; Smeets, K.; Vangronsveld, J.; d’Haen, J.; Valcke, R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007, 173, 495–508. [Google Scholar] [CrossRef]
- Akhter, M.F.; Omelon, C.R.; Gordon, R.A.; Moser, D.; Macfie, S.M. Localization and chemical speciation of cadmium in the roots of barley and lettuce. Environ. Exp. Bot. 2014, 100, 10–19. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of Cadmium Accumulation in Plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Wu, X.; Su, N.; Yue, X.; Fang, B.; Zou, J.; Chen, Y.; Shen, Z.; Cui, J. IRT1 and ZIP2 were involved in exogenous hydrogen-rich water-reduced cadmium accumulation in Brassica chinensis and Arabidopsis thaliana. J. Hazard. Mater. 2021, 407, 124599. [Google Scholar] [CrossRef]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239–240, 302–307. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. 2020, 28, 15394–15405. [Google Scholar] [CrossRef]
- Li, F.; Deng, Y.; Liu, Y.; Mai, C.; Xu, Y.; Wu, J.; Zheng, X.; Liang, C.; Wang, J. Arabidopsis transcription factor WRKY45 confers cadmium tolerance via activating PCS1 and PCS2 expression. J. Hazard. Mater. 2023, 460, 132496. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. Int. 2020, 27, 8719–8731. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, X.; Lv, J.; Tang, Z.; Hu, L.; Luo, S.; Wang, R.; Ali, B.; Yu, J. Physiological Mechanism of Exogenous 5-Aminolevulinic Acid Improved the Tolerance of Chinese Cabbage (Brassica pekinensis L.) to Cadmium Stress. Front. Plant Sci. 2022, 13, 845396. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, W.; Lin, H.; Zhou, L.; Zhang, D.; Ishfaq, M.; Zhong, Y.; Li, B.; Peng, Y.; Wu, X.; et al. Amino acid application inhibits root-to-shoot cadmium translocation in Chinese cabbage by modulating pectin methyl-esterification. Plant Physiol. Biochem. 2024, 207, 108401. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Zeng, X.; Zhang, L.; Zhou, Y.; Anastopoulos, I.; Wang, A.; Zeng, Q.; Xiao, Z. Enhancing cadmium extraction potential of Brassica napus: Effect of rhizosphere interactions. J. Environ. Manag. 2021, 284, 112056. [Google Scholar] [CrossRef] [PubMed]
- Weigel, H.J.; Jäger, H.J. Subcellular Distribution and Chemical Form of Cadmium in Bean Plants. Plant Physiol. 1980, 65, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, T.; Xu, W.; Chai, Y. Distribution of cadmium in subcellular fraction and expression difference of its transport genes among three cultivars of pepper. Ecotoxicol. Environ. Saf. 2021, 216, 112182. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Iwashita, T.; Zhao, F.J.; Ma, J.F. Characterization of Cd Translocation and Identification of the Cd Form in Xylem Sap of the Cd-Hyperaccumulator Arabidopsis halleri. Plant Cell Physiol. 2008, 49, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C.; et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef]
- Ding, Y.; Jian, H.; Wang, T.; Di, F.; Wang, J.; Li, J.; Liu, L. Screening of candidate gene responses to cadmium stress by RNA sequencing in oilseed rape (Brassica napus L.). Environ. Sci. Pollut. Res. Int. 2018, 25, 32433–32446. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, Q.; Cui, S.; Ishfaq, M.; Zhou, L.; Zhou, X.; Liu, Y.; Peng, Y.; Yu, Y.; Wu, W. Exogenous Application of Amino Acids Alleviates Toxicity in Two Chinese Cabbage Cultivars by Modulating Cadmium Distribution and Reducing Its Translocation. Int. J. Mol. Sci. 2024, 25, 8478. https://doi.org/10.3390/ijms25158478
Li L, Chen Q, Cui S, Ishfaq M, Zhou L, Zhou X, Liu Y, Peng Y, Yu Y, Wu W. Exogenous Application of Amino Acids Alleviates Toxicity in Two Chinese Cabbage Cultivars by Modulating Cadmium Distribution and Reducing Its Translocation. International Journal of Molecular Sciences. 2024; 25(15):8478. https://doi.org/10.3390/ijms25158478
Chicago/Turabian StyleLi, Longcheng, Qing Chen, Shihao Cui, Muhammad Ishfaq, Lin Zhou, Xue Zhou, Yanli Liu, Yutao Peng, Yifa Yu, and Wenliang Wu. 2024. "Exogenous Application of Amino Acids Alleviates Toxicity in Two Chinese Cabbage Cultivars by Modulating Cadmium Distribution and Reducing Its Translocation" International Journal of Molecular Sciences 25, no. 15: 8478. https://doi.org/10.3390/ijms25158478






